Believe it or not: how much can we rely on published data on potential drug targets? (original) (raw)
- Correspondence
- Published: 31 August 2011
Nature Reviews Drug Discovery volume 10, page 712 (2011)Cite this article
- 136k Accesses
- 1000 Altmetric
- Metrics details
Subjects
A recent report by Arrowsmith noted that the success rates for new development projects in Phase II trials have fallen from 28% to 18% in recent years, with insufficient efficacy being the most frequent reason for failure (Phase II failures: 2008–2010. Nature Rev. Drug Discov. 10, 328–329 (2011))1. This indicates the limitations of the predictivity of disease models and also that the validity of the targets being investigated is frequently questionable, which is a crucial issue to address if success rates in clinical trials are to be improved.
Candidate drug targets in industry are derived from various sources, including in-house target identification campaigns, in-licensing and public sourcing, in particular based on reports published in the literature and presented at conferences. During the transfer of projects from an academic to a company setting, the focus changes from 'interesting' to 'feasible/marketable', and the financial costs of pursuing a full-blown drug discovery and development programme for a particular target could ultimately be hundreds of millions of Euros. Even in the earlier stages, investments in activities such as high-throughput screening programmes are substantial, and thus the validity of published data on potential targets is crucial for companies when deciding to start novel projects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
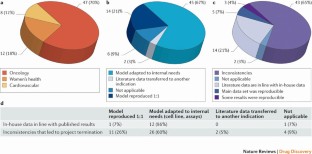
Figure 1: Analysis of the reproducibility of published data in 67 in-house projects.
References
- Arrowsmith, J. Phase II failures: 2008–2010. Nature Rev. Drug Discov. 10, 328–329 (2011).
Article CAS Google Scholar - Lehrer, J. The truth wears off: is there something wrong with the scientific method? The New Yorker [online], (2010).
Google Scholar - Freeman, D. H. Lies, damned lies, and medical science. The Atlantic [online], (2010).
Google Scholar - Osherovich, L. Hedging against academic risk. SciBX 14 Apr 2011 (doi:10.1038/scibx.2011.416).
Article Google Scholar - Barrows, N. J., Le Sommer, C., Garcia-Blanco, M. A. & Pearson, J. L. Factors affecting reproducibility between genome-scale siRNA-based screens. J. Biomol. Screen. 15, 735–747 (2010).
Article CAS PubMed PubMed Central Google Scholar - Ioannidis, J. P. Why most published research findings are false. PLoS Med. 2, e124 (2005).
Article PubMed PubMed Central Google Scholar - Schroter, S, et al. What errors do peer reviewers detect, and does training improve their ability to detect them? J. R. Soc. Med. 101, 507–514 (2008).
Article PubMed PubMed Central Google Scholar - Nemery, B. What happens to the manuscripts that have not been accepted for publication in Occupational and Environmental Medicine? Occup. Environ. Med. 58, 604–607 (2001).
Article CAS PubMed PubMed Central Google Scholar - McDonald, R. J., Cloft, H. J. & Kallmes, D. F. Fate of submitted manuscripts rejected from the American Journal of Neuroradiology: outcomes and commentary. Am. J. Neuroradiol. 28, 1430–1434 (2007).
Article CAS PubMed PubMed Central Google Scholar
Acknowledgements
We would like to thank B. Kreft and T. Zollner for their valuable contributions to this project, S. Schoepe for support in the data analysis and S. Decker for support with bioinformatics analysis of the results.
Author information
Authors and Affiliations
- Florian Prinz is at Target Research Berlin, Bayer HealthCare, Müllerstraße 178, 13342 Berlin, Germany.,
Florian Prinz - Thomas Schlange is at Target Research Wuppertal, Bayer HealthCare, Aprather Weg 18a, 42096 Wuppertal, Germany.,
Thomas Schlange - Khusru Asadullah is Vice President and Head of Target Discovery at Bayer HealthCare, Müllerstraße 178, 13342 Berlin, Germany.,
Khusru Asadullah
Authors
- Florian Prinz
You can also search for this author inPubMed Google Scholar - Thomas Schlange
You can also search for this author inPubMed Google Scholar - Khusru Asadullah
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toKhusru Asadullah.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Prinz, F., Schlange, T. & Asadullah, K. Believe it or not: how much can we rely on published data on potential drug targets?.Nat Rev Drug Discov 10, 712 (2011). https://doi.org/10.1038/nrd3439-c1
- Published: 31 August 2011
- Issue Date: September 2011
- DOI: https://doi.org/10.1038/nrd3439-c1