Modulation of human Vα24+Vβ11+ NKT cells by age, malignancy and conventional anticancer therapies (original) (raw)
Main
Natural killer T (NKT) cells are a unique lymphocyte subset, which in humans are characterized by the presence of an invariant T-cell receptor (TCR) V_α_24+V_β_11+ associated with the natural killer (NK) receptor NKR-P1A (Dellabona et al, 1994). Unlike conventional T cells human NKT cells and their murine counterpart, V_α_14 NKT cells are activated by the glycolipid, _α_-galactosylceramide (_α_-GalCer) presented by a nonpolymorphic major histocompatability complex (MHC) class I-like molecule CD1d (Kawano et al, 1999b; Nieda et al, 1999). Natural killer T cells have been shown to have strong antitumour activity upon _α_-GalCer stimulation (Kawano et al, 1998; Nicol et al, 2000b; Kikuchi et al, 2001; Nieda et al, 2001). As a consequence, clinical trials aimed at activating and increasing NKT cell numbers are currently a major focus for immune therapy of cancer.
There are limited studies detailing the normal range of human peripheral blood (PB) NKT cells numbers and the effects of parameters such as age, malignant disease and associated treatment on their number and function. As the ageing process and immune dysfunction due to malignant disease have been shown to impact other cell types, these factors require consideration for NKT cells (Goto et al, 1999; Ninomiya et al, 1999; Hakim et al, 2004; Plackett et al, 2004).
The effect of the ageing process on NKT cell numbers has only been assessed in a single study, where the percentage of V_α_24+ T cells was shown to decrease with age (DelaRosa et al, 2002). The impact of age, however, on NKT cell expansion following _α_-GalCer stimulation has not yet been evaluated (DelaRosa et al, 2002). If NKT cells do contribute to the normal protective mechanisms against cancer, a reduction in NKT cell number or function as a result of the ageing process age may contribute to the higher incidence of cancer in this group of patients.
The effects of malignancy on NKT cell number and function remain unclear. Significant reductions in PB NKT cell numbers have been reported in patients with melanoma, prostate cancer and lung cancer (Kawano et al, 1999a; Tahir et al, 2001; Motohashi et al, 2002), however in other studies the numbers of NKT cells in advanced cancer patients are comparable to those in healthy individuals (Yanagisawa et al, 2002). Variations in NKT cell function have also been described. Decreased responsiveness of NKT cells to _α_-galactosylceramide (_α_-GalCer) stimulation has been demonstrated in prostate cancer patients and advanced cancer patients (Tahir et al, 2001); however, in contrast NKT cells of lung cancer and melanoma patients have been shown to have comparatively normal _α_-GalCer proliferative capacity (Kawano et al, 1999a; Motohashi et al, 2002).
Of note, previous studies investigated NKT cells of cancer patient's either pretreatment or did not take into account potential effects of chemotherapy and radiation therapy on NKT cell numbers and expansion capacity. This is important information due to the likelihood that NKT cell immunotherapy will be used secondary to conventional cancer therapies.
For interventions aimed at increasing NKT cells to be effective at treating malignancy, it is a prerequisite that NKT cells in cancer patients firstly exist and also retain the ability to respond to specific stimuli. This study evaluated the effects of age, malignancy and associated treatment on the numbers of NKT cells in 109 patients with solid organ cancer using 40 healthy individuals as controls. Responsiveness to _α_-GalCer stimulation was also assessed in 28 of the cancer patients and 37 of the healthy donors following assessment of NKT cell numbers.
Materials and methods
Subject groups and controls
The study group consisted of 109 subjects (64 males and 45 females) with solid organ cancer (median age 58 years, range 26–82). They were subclassified according to cancer type (colorectal, breast, melanoma, renal cell carcinoma (RCC), lung, others) and prior cancer treatment. The control healthy volunteer group comprised 40 healthy volunteers, 25 females and 15 males (median age 34 years, range 19–68) with no history of malignant disease. Natural killer T cell number was assessed in each of the cancer patients and healthy donor, however only 37 of the healthy donors and 28 of the cancer patients could be assessed for expansion capacity following _α_-GalCer stimulation.
Characteristics of the study group are presented in Table 1. Peripheral blood samples were collected after obtaining informed consent from the subjects, and the study was approved by The Royal Brisbane and Womens Hospital and the Health Services Districts Human Research Ethics Committee.
Table 1 Characteristics of each group of cancer subjects and normal donors
Phenotypic analysis
Fresh whole blood or peripheral blood mononuclear cells (PBMC), separated by Ficoll–Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) density centrifugation were stained with fluorescein isothiocyanate (FITC)-conjugated anti-V_α_24 (IgG1: Cl5), phycoerythrin (PE)-conjugated anti-V_β_11 (IgG2a: C21) and phycoerythrin–cyanin 5.1 (PC5)-conjugated anti-CD3 (IgG1, UCHT1) and analysed by three-colour flow cytometry using a FACSCalibur (Becton Dickinson, San Diego CA, USA). Appropriate isotype controls were used and all antibodies were purchased from Beckman Coulter (Sydney, Australia). Natural killer T cells were defined as V_α_24+V_β_11+CD3+ and to ensure accuracy of the flow cytometric evaluation up to 1 × 106 cells were assessed in order to acquire >100 NKT cell events.
Calculation of NKT cell numbers
Natural killer T cell numbers were expressed as a percentage of the CD3+ lymphocyte population as determined by flow cytometry. To determined the number of NKT cells per litre (l) of blood, the fraction of lymphocytes that were NKT cells according to flow cytometry was multiplied by the number of lymphocytes per litre as determined by an automated full blood count on the same sample.
Expansion of V_α_24+V_β_11+ NKT cells
Peripheral blood mononuclear cells were assessed for NKT cell numbers as described above (Day 0) and cultured at 1 × 106 cells ml−1 in AIM-V medium (Gibco BRL, Melbourne, Australia) supplemented with 10% normal human AB serum, 10Uml−1 interleukin-2 (IL-2) (R&D Systems, Sydney, Australia) and 100 ngml−1 KRN 7000 (a gift from Pharmaceutical Division, Kirin Brewery, Gumna, Japan) for 7 days. On Day 7, the cells were harvested, counted and analysed by flow cytometry as described above and the results are expressed in terms of fold expansion, as determined from the respective NKT numbers at Day 0 and following 7 days expansion.
Statistics
Results for NKT cell percentages and absolute numbers of NKT cells and CD3+ T cells have been presented as medians separately for the different cancer groups. Owing to the large number of zero NKT cell percentage counts, the distribution was extremely skewed to the right and thus standard normal distribution-based statistics were not appropriate. For crude analysis, Kruskal–Wallis nonparametric analysis of variance was used to compare median NKT cell percentages and NKT l−1 between cancer groups. Two-way comparisons between the various cancer groups and healthy volunteers were performed using Mann–Whitney _U_-tests. Both NKT cell percentage and absolute NKT cell numbers followed an approximately negative-binomial distribution, and thus negative-binomial regression was used to evaluate the statistical significance of its association with tumour status, after adjusting for age and sex differences. The degree of association between age and NKT cell percentage or NKT l−1, age and fold expansion and NKT percentage and fold expansion were calculated using a Spearman's nonparametric correlation coefficient.
Results
Effect of malignancy on PB NKT cell numbers
Natural killer T cells as a percentage of CD3+ T cells (NKT %) and the absolute number of NKT cells l−1 of PB were assessed in healthy donors (_n_=40) and cancer patients (_n_=109) with a broad range of malignancies. Natural killer T cells comprised up to 1.27% of CD3+ T cells (median value 0.113%) and 9.89 × 106 l−1) of PB in healthy donors, and up to 1.19% of CD3+ T cells (median value 0.02%) and 6.52 × 106 l−1 (median value 0.2 × 106 l−1) of PB in all cancer patients combined (Table 2). No significant difference was observed between the frequency (_P_=0.28) or number (_P_=0.06) of circulating NKT cells in cancer patients and those in healthy donors after adjusting for age and sex differences. Analysis of the individual cancer subgroups showed melanoma patients (_n_=17) to have a significantly lower NKT% (median 0.01%, _P_=0.04) and NKT l−1 (median 0.17 × 106 l−1, _P_=0.01), and breast cancer patients (_n_=10) to have a reduced frequency of NKT cells (median 0.01, _P_=0.02) but comparatively similar absolute numbers of NKT cells (_P_=0.13). Total CD3+ T cells l−1 of PB were significantly lower in all cancer groups with the exception of colorectal cancer and RCC, indicating that the reduced NKT% observed in melanoma and breast cancer patients could not be attributed to increased numbers of total PB T cells.
Table 2 NKT cell percentages, absolute number of NKT cells l−1 (× 106) and absolute number of CD3+ T cells l−1 (× 109) of PB for each study group in comparison to healthy volunteers
Impact of prior radiation and/or chemotherapy treatment on NKT cell numbers
Statistical comparison was conducted between the NKT cell numbers of all cancer patients who had not undergone previous chemotherapy or radiation treatment and those who had not undergone each treatment respectively. No significant difference was detected between the groups in either comparison, suggesting that neither radiation nor chemotherapy had an independent effect on NKT cell numbers in cancer patients when all cancers were considered collectively (Table 3).
Table 3 Effects of prior radiation and chemotherapy treatment on NKT cell percentages and absolute numbers of NKT cells and CD3+ T cells in cancer patients
To assess whether chemotherapy or radiation treatment modulated NKT cell numbers in the individual cancer subgroups, statistical analysis was conducted for each group following exclusion of these patients who had previous chemotherapy (Table 4) or previous radiation therapy (Table 5). As shown in Table 4, the frequency and absolute number of NKT cell numbers remained significantly lower in melanoma patients, indicating chemotherapy was not responsible for the reduction in NKT cells. However, it must be noted that this result is not unexpected as chemotherapy is not a common treatment modality for this cancer type. Conversely, following exclusion of those melanoma patients with a history of radiation treatment (Table 5), NKT cell numbers in the group were returned to comparatively healthy levels. This result suggests that radiation therapy may have a direct effect on NKT cell numbers in patients with melanoma.
Table 4 NKT cell percentages, absolute number of NKT cells l−1 (× 106) and absolute number of CD3+ T cells l−1 (× 109) of PB for each study group excluding subjects who have had prior chemotherapy in comparison to healthy volunteers
Table 5 NKT cell percentages, absolute number of NKT cells l−1 (× 106) and absolute number of CD3+ T cells l−1 (× 109) of PB for each study group excluding subjects who have had prior radiation therapy in comparison to healthy volunteers
In the instance of breast cancer, there were insufficient patients in this subgroup following exclusion to ascertain whether prior treatment was responsible for the decreased NKT % previously observed. Furthermore, while the significant difference detected between healthy donors and lung cancer patients with no history of chemotherapy treatment (_P_=0.01) may suggest chemotherapy-mediated modulation of NKT cell numbers; the low subject numbers (_n_=4) in this subgroup does not facilitate accurate analysis.
Effect of the ageing process on NKT cell numbers
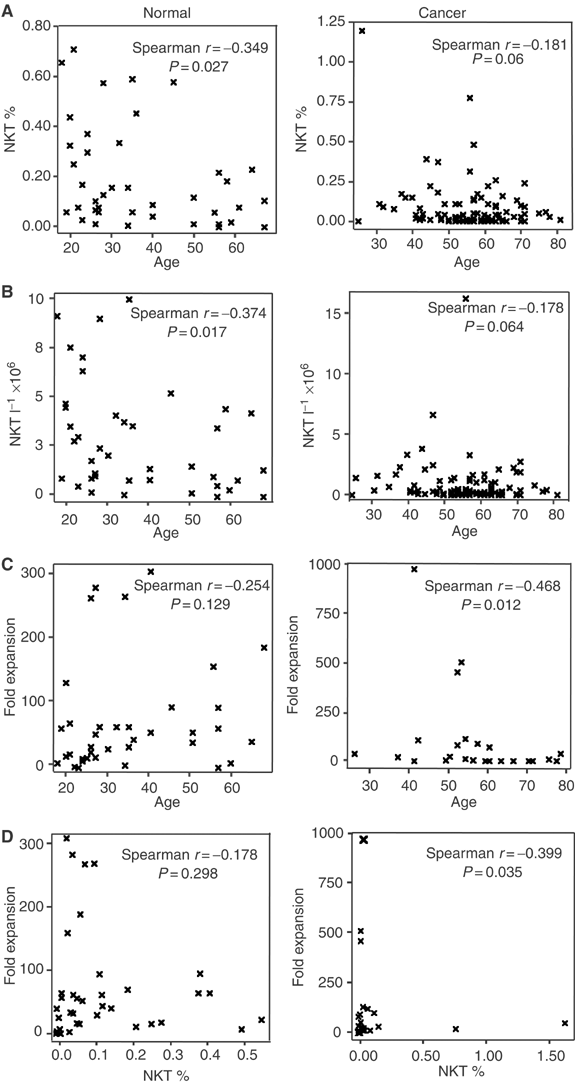
The percentage and absolute number of circulating NKT cells in healthy donors weakly but significantly correlated with age such that NKT % and NKT l−1 decreased with increasing age (Figure 1, panels A and B). While the number of NKT as a percentage and per litre of blood of cancer patients also appeared to decrease with increasing age, this correlation proved insignificant (Figure 1, panels A and B).
Figure 1
Association of age on proportion of NKT cells (NKT %; A), absolute numbers of circulating NKT cells (NKT l−1; B) and responsiveness to _α_-GalCer (C) in cancer patients and healthy donors. (D) represents the correlation between NKT cell numbers and NKT cell expansion capacity.
Expansion capability of NKT cells in cancer patients and healthy volunteers – the effect of age and malignancy
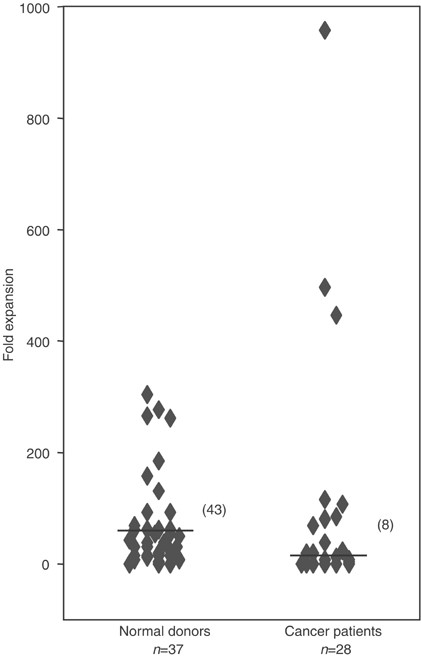
Stimulation by _α_-GalCer resulted in the rapid expansion of NKT cells from most cancer patients and healthy donors. Figure 2 displays the expansion capacity (fold expansion) of healthy donor (_n_=37) and cancer patient (_n_=28) NKT cells in response to _in vitro α_-GalCer stimulation. Natural killer T cells of healthy donors expanded between 0- and 303-fold and those of cancer patients expanded between 0- and 959-fold. While NKT cells of cancer patients had an overall reduced capacity to expand (median eight-fold) as compared to healthy donors (median 43-fold, _P_=0.02), some individual cancer patients were clearly better NKT cell expanders than healthy donors.
Figure 2
Expansion capacity of NKT cells in normal donors (_n_=37) and cancer patients (_n_=28) in response to _α_-GalCer stimulation (line indicates median of group). Natural killer T cells can rapidly expand in both groups, however overall to a significantly lower degree in cancer patients (_P_=0.02).
Contrary to the effects of age on NKT numbers in healthy donors, age did not significantly influence their fold expansion as displayed in Figure 1C. Although the proliferative potential of cancer patient NKT cells to _α_-GalCer appears to decrease significantly with age, conclusive analysis requires the assessment of increased numbers of younger cancer patients.
Although NKT cells expanded rapidly from most donors, there was no relationship between the NKT % in healthy donors and the degree to which the NKT cells expand. The results do suggest, however, that NKT % is a reliable indicator of fold expansion in cancer patients, although the highly skewed nature of the population towards low NKT % may make the spread of NKT % too low to determine the correlation accurately. It is of interest that the largest in vitro NKT cell expansion was observed from donors with relatively low NKT cell numbers.
Discussion
Clinical application of NKT cells as therapy for malignancy is critically dependent upon their activation and subsequent expansion in humans. With the development of therapeutic approaches with the potential to activate NKT cells in vivo (Nieda et al, 2003) and expand NKT cells in vitro (Nieda et al, 1999; Nicol et al, 2000a; Takahashi et al, 2000), it is important to more clearly define their applicability in the clinical setting. Previous data indicate that NKT cells may be decreased in some cancer patients (Kawano et al, 1999a; Tahir et al, 2001; Motohashi et al, 2002). It this is associated with or caused by decreased responsiveness to _α_-GalCer, then these therapies may have less than expected clinical utilities.
We have confirmed with considerably larger subject numbers in this study that the percentage of V_α_24+ NKT cells in cancer patients does not significantly differ to that in healthy donors when all cancer types are considered collectively (Yanagisawa et al, 2002). Our data also support previous reports that NKT cell numbers are significantly reduced in patients with melanoma (Kawano et al, 1999a); however, in contrast to Motohashi et al (2002) decreased frequency and absolute number of NKT cells was not seen in lung cancer patients. A likely explanation for this inconsistency is the inclusion of insufficient patients in this subgroup to detect a statistically significant difference, in view of the 60 lung cancer patients assessed in the previous study. As no studies to date have assessed the numbers of NKT cells in breast cancer patients we have, for the first time, demonstrated a significantly reduced percentage of NKT cells in this cancer group.
Reasons for the observed reductions in breast cancer and melanoma patients are unknown. It is evident that the results are not simply a consequence of increased total CD3+ T cell numbers in either disease (Table 2). Contrary to previous studies, which have in majority assessed NKT cell levels pretreatment or without indicating prior treatment, we have taken into consideration potential effects of chemotherapy or radiation treatment on NKT cell numbers. We have identified that radiation treatment may be directly responsible for the decreased NKT cell numbers in melanoma patients. It remains possible, however, that a disease-specific effect by melanoma may still contribute to the observed reduction and although not examined in this study, decreases in NKT cell numbers could also be due to an accumulation of NKT cells at tumour sites, thus decreasing their overall number in the circulating PB. This, together with longitudinal studies, aimed at ascertaining whether the low NKT cells numbers in these patients predated the manifestation of cancer, would help further the understanding of the relationship between NKT cell numbers and cancer.
There were insufficient subject numbers to ascertain treatment-related effects on NKT cells breast cancer patients, and indications that chemotherapy modulates NKT cell numbers in lung cancer patients cannot be conclusively determined with the subject numbers available in this subgroup.
The effect of the ageing process on NKT cell numbers has only been assessed in a singly study (DelaRosa et al, 2002). In accordance with that study, we showed a decreased percentage of V_α_24+ NKT cells in healthy elderly individuals. We have also demonstrated that absolute NKT cell numbers decrease significantly with age in healthy donors. A similar trend was observed in cancer patients, however the statistical significance (_P_=0.06) was potentially influenced by a concentration of middle- to late-aged cancer patients. Although greater numbers of younger cancer patients are required to assess this association accurately, the results do raise the possibility that low NKT cells may be a risk factor for cancer in young adults. They also confirm that in consideration of their intrinsically low NKT cell numbers, middle and older aged cancer patients could benefit greatly from therapeutic manipulations that boost their NKT cell numbers.
The magnitude of NKT cell activation and expansion may have critical consequences on the efficacy of NKT cell therapies. We and others have shown that NKT cells of cancer patients can be dramatically expanded by _α_-GalCer stimulation, however to levels lower that that of healthy donors (Tahir et al, 2001). Studies of melanoma and lung cancer patients have in contrast reported NKT cells with comparatively normal capacities to expand (Kawano et al, 1999a; Motohashi et al, 2002). As we could not assess in vitro NKT expansion on every cancer subject involved, we cannot determine whether NKT cell expansion is reduced in every cancer type, or whether the overall result is influenced by one or several subgroups of cancer patients.
Mechanisms of reduced responsiveness of NKT cells to _α_-GalCer stimulation have not been determined. Immune defects and dysfunction in T cells and the antigen presenting and stimulatory capacity of DC have previously been reported in cancer patients (Mizoguchi et al, 1992; Goto et al, 1999; Kiessling et al, 1999; Ninomiya et al, 1999). As in vitro expansion capacity is dependent upon both the number and stimulatory capacity of CDld antigen-presenting cells, reduced numbers of and/or functionally defective CDld APC may suppress NKT activation in cancer patients. Production of immunosuppressive factors by tumour cells or TGF-β by CD3+ T cells or intrinsic defects to the NKT cells themselves may also inhibit their expansion potential (Tada et al, 1991; Chouaib et al, 1997).
While functional impairment as a consequence of age has been reported to occur in other cell types such as T cells (Hakim et al, 2004; Plackett et al, 2004), there appears no association between the functional capacity of an NKT cell to respond to _α_-GalCer and the age of the healthy donor. Although fold expansion significantly decreased with age in cancer patients, this result was dependent upon three individuals with particularly high expansion. Confirmation of this latter observation would require the inclusion of an increased numbers of younger cancer patients in the subject group.
The precise relationship, if any, between the proportion of NKT cells in a donor and the NKT cell's functional capacity to expand has to date not been examined. While we have shown that there is no association between the percentage of NKT cells and fold expansion in normal donors, it is clearly evident that the largest degree of expansion was in donors with an NKT cell % at the lower end of the scale. Although fold expansion significantly increased with NKT % in cancer patients, the highly skewed nature of the population towards low NKT % makes the spread of this variable too narrow to accurately determine the correlation. NKT cells capable of dramatic expansion, some that exceeded values seen in normal donors were also prevalent within the cancer patient group. These data go a significant way to disproving the assumption held by some that people with low NKT cells will have NKT cells defective for in vitro expansion potential. It will be interesting to determine through clinical trials whether this translates to in vivo NKT cell expansion and whether this group of patients would benefit most from clinical interventions aimed at the in vivo activation and expansion of NKT cells.
In summary, our study demonstrates that NKT cell numbers and their activation and capacity can be modulated by both age and malignancy and in contrast to previous studies, conventional cancer treatment. Whilst further studies are required to determine both the stage at which treatment or malignant disease leads to decreased NKT cell numbers and the mechanism by which cancer affects impacts NKT cell function, our results provide important information for the clinical trials involving in vivo activation or large-scale in vitro expansion of NKT cells.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
- Chouaib S, Asselin-Paturel C, Mami-Chouaib F, Caignard A, Blay JY (1997) The host–tumor immune conflict: from immunosuppression to resistance and destruction. Immunol Today 18: 493–497
Article CAS Google Scholar - DelaRosa O, Tarazona R, Casado JG, Alonso C, Ostos B, Pena J, Solana R (2002) Valpha24+ NKT cells are decreased in elderly humans. Exp Gerontol 37: 213–217
Article CAS Google Scholar - Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A (1994) An invariant V alpha 24-J alpha Q/V_β_11 T cell receptor is expressed in all individuals by clonally expanded CD4-8- T cells. J Exp Med 180: 1171–1176
Article CAS Google Scholar - Goto S, Sato M, Kaneko R, Itoh M, Sato S, Takeuchi S (1999) Analysis of Th1 and Th2 cytokine production by peripheral blood mononuclear cells as a parameter of immunological dysfunction in advanced cancer patients. Cancer Immunol Immunother 48: 435–442
Article CAS Google Scholar - Hakim FT, Flomerfelt FA, Boyiadzis M, Gress RE (2004) Aging immunity and cancer. Curr Opin Immunol 16: 151–156
Article CAS Google Scholar - Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T, Tanaka Y, Taniguchi M (1998) Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated V_α_14 NKT cells. Proc Natl Acad Sci USA 95: 5690–5693
Article CAS Google Scholar - Kawano T, Nakayama T, Kamada N, Kaneko Y, Harada M, Ogura N, Akutsu Y, Motohashi S, Iizasa T, Endo H, Fujisawa T, Shinkai Y, Taniguchi M (1999a) Antitumor cytotoxicity mediated by ligand-activated human V_α_24 NKT cells. Cancer Res 59: 5102–5105
CAS PubMed Google Scholar - Kawano T, Tanaka Y, Shimizu E, Kaneko Y, Kamata N, Sato H, Osada H, Sekiya S, Nakayama T, Taniguchi M (1999b) A novel recognition motif of human NKT antigen receptor for a glycolipid ligand. Int Immunol 11: 881–887
Article CAS Google Scholar - Kiessling R, Wasserman K, Horiguchi S, Kono K, Sjoberg J, Pisa P, Petersson M (1999) Tumor-induced immune dysfunction. Cancer Immunol Immunother 48: 353–362
Article CAS Google Scholar - Kikuchi A, Nieda M, Schmidt C, Koezuka Y, Ishihara S, Ishikawa Y, Tadokora K, Durrant S, Boyd A, Juji T, Nicol A (2001) In vitro anti-tumour activity of _α_-galactosylceramide-stimulated human invariant V_α_24+NKT cells against melanoma. Br J Cancer 85: 741–746
Article CAS Google Scholar - Mizoguchi J, O’Shea JJ, Longo DL, Loeffler CM, McVicar DW, Ochoa AC (1992) Alterations in signal transduction moledules in T lymphocytes from tumor-bearing mice. Science 258: 1795–1798
Article CAS Google Scholar - Motohashi S, Kobayashi S, Ito t, Magara KK, Mikuni O, Kamada N, Iizasa T, Nakayama T, Fujisawa T, Taniguchi M (2002) Preserved IFN-α production of circulating V_α_24 NKT cells in primary lung cancer patients. Int J Cancer 102: 159–165
Article CAS Google Scholar - Nicol A, Nieda M, Koezuka Y, Porcelli S, Suzuki K, Tadokoro K, Durrant S Juji T (2000a) Dendritic cells are targets for human invariant V_α_24+ natural killer T-cell cytotoxic activity: an important immune regulatory function. Exp Hematol 28: 276–282
Article CAS Google Scholar - Nicol A, Nieda M, Koezuka Y, Porcelli S, Suzuki K, Tadokoro K, Durrant S, Juji T (2000b) Human invariant v_α_24+ natural killer T cells activated by _α_- galactosylceramide (KRN7000) have cytotoxic anti-tumour activity through mechanisms distinct from T cells and natural killer cells. Immunology 99: 229–234
Article CAS Google Scholar - Nieda M, Nicol A, Koezuka Y, Kikuchi A, Lapteva N, Tanaka Y, Tokunaga K, Suzuki K, Kayagaki N, Yagita H, Hirai H Juji T (2001) TRAIL expression by activated human CD4(+)V_α_24NKT cells induces in vitro and in vivo apoptosis of human acute myeloid leukemia cells. Blood 97: 2067–2074
Article CAS Google Scholar - Nieda M, Nicol A, Koezuka Y, Kikuchi A, Takahashi T, Nakamura H, Furukawa H, Yabe T, Ishikawa Y, Tadokoro K, Juji T (1999) Activation of human V_α_24NKT cells by _α_-glycosylceramide in a CDld-restricted and V_α_24TCR-mediated manner. Hum Immunol 60: 10–19
Article CAS Google Scholar - Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, Juji T, Macfarlane DJ, Nicol AJ (2003) Therapeutic activation of V{α}24+V{β}11+NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood 25: 25
Google Scholar - Ninomiya T, Akbar SM, Masumoto T, Horiike N, Onji M (1999) Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol 31: 323–331
Article CAS Google Scholar - Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ (2004) Aging and innate immune cells. J Leukoc Biol 23: 23
Google Scholar - Tada T, Ohzeki S, Utsumi K, Takiuchi H, Muramatsu M, Li XF, Shimizu J, Fujiwara H, Hamaoka T (1991) Transforming growth factor-beta-induced inhibition of T cell function. Susceptibility difference in T cells of various phenotypes and functions and its relevance to immunosuppression in the tumor-bearing state. J Immunol 146: 1077–1082
CAS PubMed Google Scholar - Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, Balk SP, Exley MA (2001) Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol 167: 4046–4050
Article CAS Google Scholar - Takahashi T, Nieda M, Koezuka Y, Nicol A, Porcelli SA, Ishikawa Y, Tadokoro K, Hirai H, Juji T (2000) Analysis of human V_α_24+ CD4+ NKT cells activated by _α_-glycosylceramide-pulsed monocyte-derived dendritic cells. J Immunol 164: 4458–4464
Article CAS Google Scholar - Yanagisawa K, Seino K, Ishikawa Y, Nozue M, Todoroki T, Fukao K (2002) Impaired proliferative response of V_α_24 NKT cells from cancer patients against _α_-galactosylceramide. J Immunol 168: 6494–6499
Article CAS Google Scholar
Acknowledgements
We thank Nirmala Pondeja for assistaance with statistical analyses.
Author information
Authors and Affiliations
- Department of Medicine, University of Queensland, Brisbane, Australia
T Crough & A J Nicol - The Queensland Institute of Medial Research, Brisbane, Australia
T Crough, D M Purdie, M Okai, A Maksoud & A J Nicol - Bone Marrow Transplant Unit, Royal Brisbane Hospital, Brisbane, Australia
M Nieda - Yokohama City University School of Medicine, Japan
A J Nicol
Authors
- T Crough
- D M Purdie
- M Okai
- A Maksoud
- M Nieda
- A J Nicol
Corresponding author
Correspondence toA J Nicol.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Crough, T., Purdie, D., Okai, M. et al. Modulation of human V_α_24+V_β_11+ NKT cells by age, malignancy and conventional anticancer therapies.Br J Cancer 91, 1880–1886 (2004). https://doi.org/10.1038/sj.bjc.6602218
- Received: 09 August 2004
- Accepted: 13 September 2004
- Published: 02 November 2004
- Issue date: 29 November 2004
- DOI: https://doi.org/10.1038/sj.bjc.6602218