Induction of Egr-1 expression by the retinoid AHPN in human lung carcinoma cells is dependent on activated ERK1/2 (original) (raw)
Introduction
Retinoids play an important role in the regulation of morphogenesis during lung development and in the control of proliferation, differentiation, and apoptosis of airway epithelial cells in vivo as well as in in vitro cell systems.1,2,3 It has been demonstrated that retinoids regulate surfactant protein synthesis in alveolar Type II cells4,5 and control cellular proliferation, differentiation, and the synthesis of mucins in tracheobronchial epithelial cells.6,7,8,9 The therapeutic value of retinoids in lung disease has been indicated by several studies. Retinoids have been shown to reverse squamous metaplasia and elastase-induced emphysema,3,10 promote differentiation in lungs of early infants,11 and inhibit the formation of second primary tumors in the lung.12
Many of the biological and molecular effects of retinoids are mediated by nuclear retinoid receptors.13 These receptors comprise two subfamilies, the retinoic acid receptors (RARs) and retinoid X receptors (RXRs), each consisting of three different genes (α, β and γ). RARs and RXRs alter gene expression by binding to specific response elements, RAREs or RXREs, respectively, in the regulatory regions of target genes. RARs and RXRs have been identified in many normal lung and airway epithelial cells as well as in lung carcinoma cells.8,14,15,16,17 The induction of the mucosecretory phenotype in normal tracheobronchial epithelial cells by retinoic acid is associated with an increase in the levels of RARβ, suggesting a regulatory role for this receptor in this process.8,15 In many lung carcinoma cell lines, the inability of retinoic acid to induce RARβ is related to defects in the retinoid signaling pathway and may contribute to the malignant phenotype of these cells.14,15,18,19,20 RARβ has been shown to mediate the growth-inhibitory effects of RA. The resistance of many lung carcinoma cells to the growth-inhibitory effects of retinoic acid may be due in part to defects in the retinoid signaling pathway.19,21,22
The actions of a number of retinoids, including 4-(hydroxyphenyl)retinamide and 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (AHPN/CD437), do not involve RARE- or RXRE-dependent transactivation and are not mediated by nuclear retinoid receptors.18,23,24,25,26,27,28 AHPN has been shown to be a very effective inhibitor of cell proliferation and to be able to induce apoptosis in a variety of cell lines, including melanoma, lymphoma, mammary and lung carcinoma cell lines.25,27,28,29,30,31,32,33,34,35,36,37,38,39 AHPN has been demonstrated to inhibit tumor formation in mice and is able to induce apoptosis in leukemia cells at low concentrations.29,33,40 These results may indicate a potential usefulness of AHPN in chemotherapy. Although AHPN can selectively bind to and activate the RARγ receptor,41 several studies have provided evidence showing that its action is mediated through a unique, yet unknown, mechanism that is independent of the RAR/RXR signaling pathway.25,28,29,30
In order to identify genes that are important in the AHPN-induced growth arrest and apoptosis, we employed cDNA-array screening. This screening identified several AHPN-inducible genes most of which are early response genes and genes induced by DNA-damage agents or growth arrest. Egr-1 (Early-growth response gene 1; also named NGFI-A, Krox 24, Zif/268 or TIS8)42,43,44,45,46 is one of the AHPN-inducible genes identified in this study. Egr-1 is a nuclear phosphoprotein and a member of a C2H2-type zinc-finger family of transcription factors that control gene transcription by binding to GC-rich regulatory elements in the promoter region of target genes. Egr-1 has been reported to regulate numerous growth regulatory genes, such as c-myc and TGF-β1, and to have an important function in negative growth control.47 We report that AHPN induces Egr-1 mRNA and protein in several carcinoma cell lines. The growth suppressor function of Egr-1 likely cooperates with other growth-inhibitory proteins in AHPN-induced growth arrest. The induction of Egr-1 by AHPN appears to be regulated at the transcriptional level and occurs through a mechanism that does not involve retinoid nuclear receptors. AHPN caused an activation of MAPK p38 but did not have any effect on the activation of the ERK1/2 signaling pathway. However, we demonstrated that the induction of Egr-1 by AHPN was dependent on activated ERK1/2 but did not require activated p38.
Results
cDNA-array screening
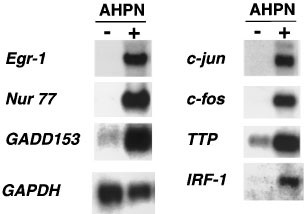
To obtain insight into the mechanism by which AHPN induces growth arrest and apoptosis, we used cDNA array screening to identify AHPN-mediated changes in gene expression. Human lung carcinoma H460 cells were treated with AHPN (2.5 μM) or vehicle. After 8 h incubation, poly(A)+ RNA was isolated and used in cDNA-array screening. Egr-1, Nur77, GADD45, MyD118, GAD153, c-jun, c-fos, interferon response factor (IRF)-1, tristetraproline (TTP), and the cdk-inhibitor p21WAF1/Cip1 where among the genes identified by cDNA array screening. Differential expression of the genes was confirmed by Northern blot analysis (Figure 1). Induction of MyD118, GADD45, Nur77, and cdk-inhibitor p21WAF1/Cip1 by AHPN have been reported previously.27,31,34,48 It is interesting to note that many of the genes up-regulated by AHPN represent immediate-early response genes and genes induced after DNA damage.49,50 Moreover, many of these proteins have been implicated in the regulation of cell growth and apoptosis.34,47,51,52,53 In this study, we focus particularly on the characterization of AHPN-induced expression of Egr-1.
Figure 1
Northern blot analysis of several AHPN-inducible genes identified by cDNA array screening. Human lung carcinoma H460 cells were treated with 2.5 μM AHPN (+) or vehicle (−). After 8 h, cells were collected and total and poly(A)+ RNA isolated. RNA was examined by Northern blot analysis using radiolabeled probes for GAPDH and several differentially-expressed genes (Egr-1, Nur77, GADD153, c-jun, c-fos, TTP, and IRF-1) identified by cDNA array analysis
Induction of Egr-1 mRNA occurs in many cell lines
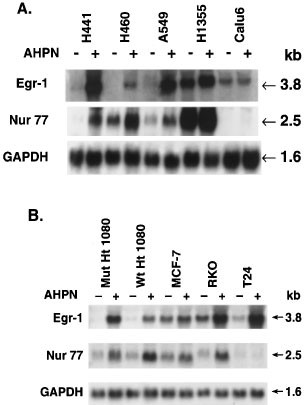
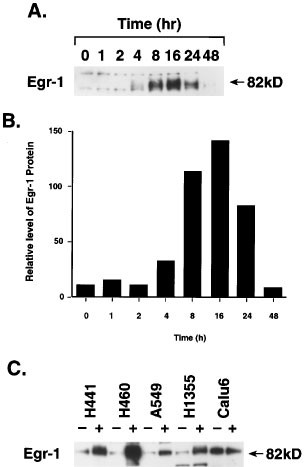
The induction of Egr-1 mRNA by AHPN was not limited to lung carcinoma H460 cells. AHPN increased the level of Egr-1 mRNA in lung carcinoma H441 and A549 cells (Figure 2A), human colorectal carcinoma RKO, and bladder carcinoma T24 cells (Figure 2B). In contrast to H460, H441, and A549 cells, untreated lung carcinoma H1355 cells expressed relatively high levels of Egr-1 mRNA that were increased only slightly by AHPN treatment. Egr-1 mRNA levels did not change significantly in Calu-6 cells and mammary carcinoma MCF-7 cells. In H460 cells, AHPN induced Egr-1 mRNA expression at concentrations as low as 0.3 μM (not shown).
Figure 2
Comparison of the induction of Egr-1 and Nur77 mRNA by AHPN in several human carcinoma cell lines. Cells, treated with 2.5 μM AHPN or vehicle, were collected and total RNA was isolated. RNA (30 μg) was examined by Northern blot analysis using radiolabeled probes for Egr-1 and Nur77. A probe for GAPDH was used as a control. (A) Human lung carcinoma cell lines H441, H460, A549, H1355 and Calu-6 were treated for 8 h with AHPN (+) or vehicle (−). (B) Human fibrosarcoma Ht1080(p53wt) and Ht1080(p53mut), mammary carcinoma MCF-7, colon carcinoma RKO, and bladder carcinoma T24 cells were treated for 16 h with AHPN
In several cell lines, AHPN treatment also increased the expression of Nur77 mRNA (Figure 2). As shown for Egr-1, untreated H1355 cells expressed relative high levels of Nur77 mRNA that increased only slightly after AHPN treatment. Calu-6 cells contained very low levels of Nur77 mRNA and its expression changed little after AHPN treatment. In T24 cells, AHPN increased expression of Egr-1 but not Nur77 mRNA. These results show that in the majority of the cell lines tested, there is a good qualitative correlation between Egr-1 and Nur77 expression; however, in some cell lines the expression of these two genes do not coincide suggesting different mechanisms of regulation in these cells.
Expression of p53 has been reported to enhance the sensitivity to the growth-inhibitory and apoptosis-inducing effects of AHPN.54 We therefore, examined the effect of p53 on the induction of Egr-1 and Nur77 mRNA by AHPN. As shown in Figure 2B, AHPN was able to induce the expression of these genes in both human fibrosarcoma Ht1080(p53wt) and Ht1080(p53mut) cells27 which contain wild-type or mutated p53, respectively (Figure 2B). These two cell lines are almost equally sensitive to AHPN-induced growth inhibition and cell death (not shown). These results suggest that the increase in Egr-1 and Nur77 expression, the induction cell death, and the inhibition of cell growth do not require p53wt. This is supported by observations showing that AHPN was able to induce Egr-1 and Nur77 mRNA very effectively in H441 cells containing a mutated p53 (Figure 2A).27 Comparison between the various parameters revealed that there is not a simple correlation between expression of p53wt, inhibition of proliferation, cell death and the expression of Egr-1 or Nur77 (Table 1).
Table 1 Comparison between the effect of AHPN on cell proliferation, cell death and the expression of p53, Egr-1 and Nur77 in several carcinoma cell lines
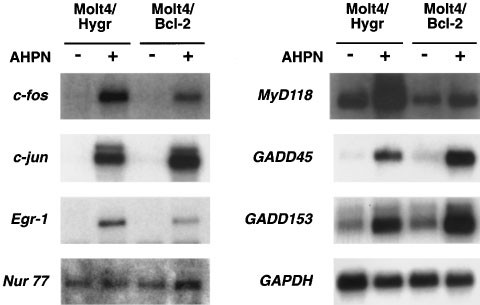
Bcl-2, which inhibits apoptosis but not growth-arrest, does not block induction of Egr-1 mRNA
Previously, we showed that AHPN induces apoptosis in human T cell lymphoma Molt-4 cells.30 Overexpression of Bcl-2 in these cells resulted in an inhibition of AHPN-induced apoptosis but did not prevent growth-arrest nor the accumulation of cells in the S-phase of the cell cycle. Therefore, we examined the effect of Bcl-2 on the induction of several AHPN-inducible genes. As demonstrated in Figure 3, AHPN was able to induce the expression of Egr-1, GADD45, GADD153, MyD118, c-fos, and c-jun in both Molt-4Hygr and Molt-4Bcl-2 cells. Although Bcl-2 expression had some minor effects on the magnitude of the increase of some mRNAs, the results indicate that their induction is largely independent of Bcl-2 expression and are upstream of Bcl-2. Expression of most of these genes may be involved in growth inhibition as well as cell death and therefore, likely contributes to AHPN-induced growth-arrest in Molt-4Bcl-2 cells. In contrast to H460 cells, Molt-4Hygr cells expressed very low levels of Nur77 mRNA that remained unaltered after AHPN treatment, suggesting that its induction is not required for AHPN-induced growth arrest or apoptosis in these cells.
Figure 3
Effect of Bcl-2 on the induction of Egr-1 and several other early response genes. Human T cell lymphoma Molt-4-Hygr cells and Molt-4-Bcl12, overexpressing Bcl-2, were treated with 2.5 μM AHPN for 8 h. RNA was isolated and examined by Northern blot analysis for the expression of Egr-1, Nur77, c-jun, c-fos, GADD45, GADD153, MyD118 and GAPDH mRNA
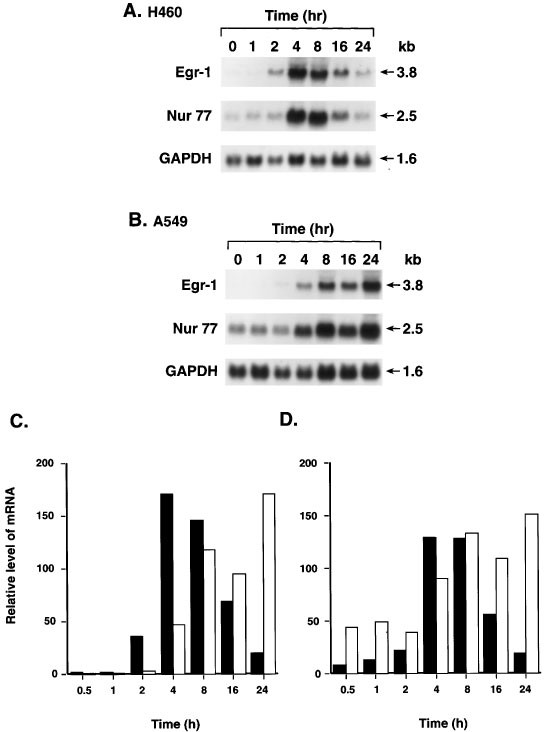
Time-dependent induction of Egr-1 mRNA and protein
The expression of Egr-1 mRNA was dependent on the length of AHPN treatment. In human lung carcinoma H460 cells, AHPN caused a transient induction of Egr-1 mRNA (Figure 4A). The level of Egr-1 mRNA increased within 2 h after the addition of AHPN, reached a maximum between 4 and 8 h, and decreased by 24 h to that of untreated cells (Figure 4C). A transient induction was also observed for the expression of Nur77 mRNA (Figure 4D). In contrast, in lung carcinoma A549 cells both Egr-1 and Nur77 mRNA expression steadily increased for up to 24 h after the addition of AHPN (Figure 4B–D). The similarities in the time courses of Egr-1 and Nur77 induction suggest similarities in the mechanism by which they are regulated by AHPN.
Figure 4
Time course of the induction of Egr-1 and Nur77 mRNA in human lung carcinoma H460 and A549 cells. (A,B) Cells were treated with 1 μM AHPN for the period indicated. Total RNA was isolated and examined by Northern blot analysis using radiolabeled probes for Egr-1, Nur77, and GAPDH. (C,D) Hybridization signals were quantitated with a PhosphorImage analyzer using ImageQuant software as described in Materials and Methods. The results are plotted as the relative level of Egr-1 (C) or Nur77 mRNA (D). Solid bars represent H460 and open bars A549
The induction of Egr-1 mRNA expression in H460 cells by AHPN was associated with an increase in Egr-1 protein (Figure 5A). As with Egr-1 mRNA, the induction of the protein was transient. The level of Egr-1 protein increased 4 h after AHPN addition, reached a maximum between 8 and 16 h, and after 24 h, returned back to that of control cells. The time course of the induction of Egr-1 protein followed the increase in Egr-1 mRNA; however, the level of Egr-1 protein was maintained for a longer period of time than that of Egr-1 mRNA, reflecting differences in their half-life (Compare Figures 4C and 5B). An increase in Egr-1 protein was also observed in H441, A549, and H1355 cells. This increase was not always proportional to the level of Egr-1 mRNA indicating possible differences in posttranscriptional control between cell lines (Figure 5C). As demonstrated for Egr-1 mRNA, little difference in the amount of Egr-1 protein was seen between AHPN-treated and untreated Calu-6 cells.
Figure 5
Induction of Egr-1 protein by AHPN. Cells were treated with 2.5 μM AHPN and at the times indicated cells were collected and proteins examined by Western blot analysis using an Egr-1 specific antibody. (A) Time course of the induction of Egr-1 in H460 cells. (B) Level of Egr-1 protein was quantitated with a PhosphorImage analyzer as described in Materials and Methods and the relative amount of Egr-1 protein plotted. (C) Induction of Egr-1 protein in several human lung carcinoma cell lines
Specificity of AHPN action
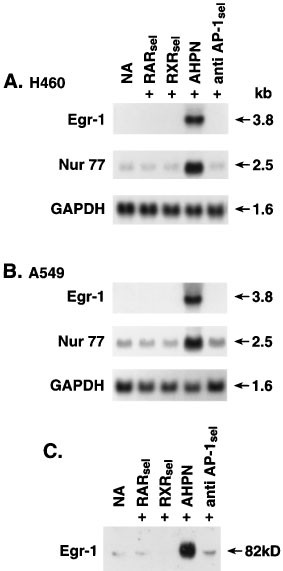
AHPN has been reported to selectively bind to and activate the RARγ receptor.41 To determine whether the effect of AHPN is mediated through retinoid receptors, the effect of AHPN was compared with those of several other retinoids. As shown in Figure 6, Egr-1 protein in H460 cells did not increase upon treatment with the retinoid TTAB, which selectively binds to and activates all three RAR receptors, nor did treatment with the RXR-selective retinoid SR11217. These results demonstrate that the induction of Egr-1 and Nur77 mRNA expression is specific for AHPN and suggest that this action is not mediated through RAR/RXR receptors but through a novel mechanism in agreement with previous studies.25,29,30,34 Many retinoids have been shown to exhibit anti-AP-1 activity. Our results suggest that the increase in Egr-1 expression by AHPN does not involve anti-AP-1 activity since the reportedly anti-AP-1 selective retinoid SR11302 had little effect on Egr-1 protein levels. This was further supported by the demonstration that the RAR- and RXR-selective retinoids, which also exhibit anti-AP-1 activity, were inactive as well.
Figure 6
Specificity of the Egr-1 and Nur77 induction by AHPN. (A,B) H460 and A549 cells were treated with vehicle (0.1% DMSO; NA) the RAR-selective retinoid TTAB (RARse1), the RXR selective retinoid SR12117 (RXRse1, AHPN, and the reported anti-AP-1-selective retinoid SR11302 (anti AP-1se1). After 8 h, cells were collected and RNA was isolated. RNA was examined by Northern blot analysis using a radiolabeled probe for Egr-1, Nur77, and GAPDH. (C) H460 cells were treated as described under A,B. After 16 h treatment, cells were collected and protein examined by Western blot analysis using an antibody specific for Egr-1
AHPN regulates Egr-1 mRNA expression at the transcriptional level
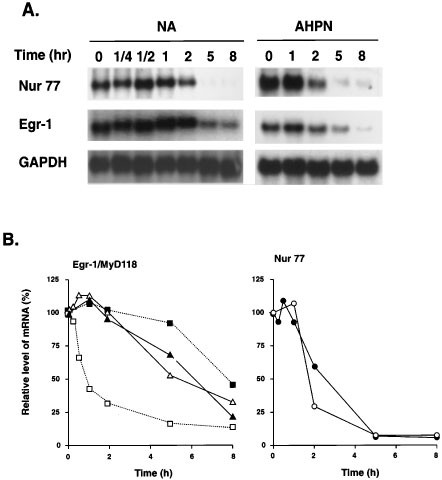
Previous studies have reported that the AHPN-induced expression of several genes is related to increased RNA stability.27,34,36,48 However, in contrast to MyD118, GADD45, and p21, AHPN had little effect on the stability of Egr-1 and Nur77 mRNA in H460 cells (Figure 7). These observations are in agreement with the concept that the increase in the expression of these genes is regulated at the transcriptional level.
Figure 7
Effect of AHPN on the stability of Egr-1 and Nur77 mRNAs. (A) H460 cells were treated with AHPN for 8 h and subsequently with actinomycin D (5 μg/ml). At the indicated times, RNA was isolated and examined by Northern blot analysis with radiolabeled probes for Egr-1, Nur77 and GAPDH. Poly(A)+ RNA was used in the case of untreated H460 cells and total RNA for AHPN treated cells. (B) Hybridization signals were quantitated with a PhosphorImage analyzer using ImageQuant software as described in Materials and Methods. The results are plotted as the relative level of Egr-1 (triangles) or Nur77 (circles) mRNA (relative to the level of GAPDH and as the percentage of the signal at time 0 of actinomycin D addition). Experiment was performed twice with similar results. Results with MyD118 (squares) were shown for comparison and obtained from ref. 34. Open symbols, control cells; closed symbols, AHPN-treated cells
AHPN-induced activation of MAPK
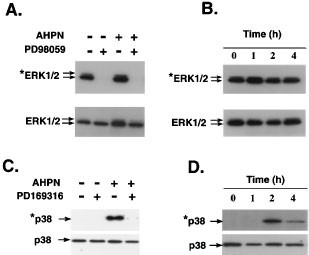
Several studies have reported a role for MAPKs in the regulation of Egr-1.55,56,57,58,59 To determine whether the induction of Egr-1 by AHPN involves any of the MAPK signaling pathways, we determined the effect of AHPN on the activation of the ERK1/2 and p38 MAPKs. Figure 8 shows that control H460 cells contained relatively low levels of activated (phosphorylated) p38 and high levels of activated ERK1/2. Treatment with AHPN caused a rapid increase in the phosphorylation of p38 indicating that it is able to activate the p38 MAPK signaling pathway. In contrast, AHPN had little effect on the activation of ERK1/2. AHPN has also been demonstrated to cause activation of p38 MAPK in human myeloid leukemia HL60 cells.60
Figure 8
Effect of AHPN on the activation of ERK1/2 and p38 MAPKs in H460 cells. (A,C) H460 cells were treated with 2.5 μM AHPN in the presence or absence of the MEK1/2 inhibitor PD98059 or the p39 MAPK inhibitor PD169316. After 2 h incubation, cells were collected and proteins examined by Western blot analysis using antibodies specific for total ERK1/2, phosphorylated (activated) ERK1/2 (*ERK1/2), total p38, and phosphorylated (activated) p38 (*p38) as described in Materials and Methods. (B,D) H460 cells were treated with 2.5 μM AHPN and at the times indicated cells were collected and proteins examined by Western blot analysis for the level of total p38 and ERK1/2, or phosphorylated p38 and ERK1/2 protein
Induction of Egr-1 by AHPN requires active ERK1/2
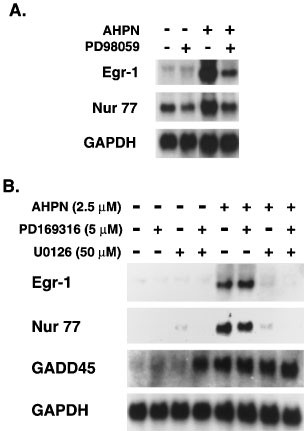
To determine the role of p38 and ERK1/2 in the upregulation of Egr-1, H460 cells were treated with AHPN in the presence and absence of the p38 MAPK inhibitor PD169316 or the MEK1 inhibitors PD98059 or U0126. As shown in Figure 9, inhibition of MEK-1 by either U0126 or PD98059 reduced Egr-1 mRNA expression in both AHPN-treated and control cells. In contrast, inhibition of p38 had little effect on the induction of Egr-1. The MAPK inhibitors had similar effects on AHPN-induced expression of Nur77 mRNA. These observations suggest that activation of the p38 signaling pathway is not involved in the AHPN-mediated increase in Egr-1 and Nur77 expression but that active ERK1/2 is needed for this induction. Neither PD98059 nor PD169316 affected the AHPN-induced increase in GADD45 mRNA expression which was reported to be regulated at the level of RNA stability.34,36 However, simultaneous treatment of H460 with PD169316 and U0126 increased the basal level of GADD45 mRNA expression in untreated cells. These results suggest that the induction of GADD45 mRNA by AHPN is independent of ERK1/2 and p38 and appears to involve a mechanism that is different from that regulating Egr-1.
Figure 9
Effect of p38 MAPK and MEK1/2 inhibitors on the induction of Egr-1, Nur77 and GADD45 mRNA by AHPN. H460 cells were treated with 2.5 μM AHPN in the presence or absence of the p38 MAPK inhibitor PD169316 (5 μM) or the MEK1/2 inhibitors U0126 (50 μM) (A) or PD98059 (50 μM) (B). After 8 h, RNA was isolated and examined by Northern blot analysis for the expression of Egr-1, Nur77, GADD45, and GAPDH mRNA
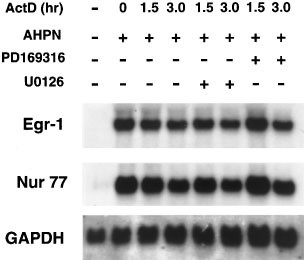
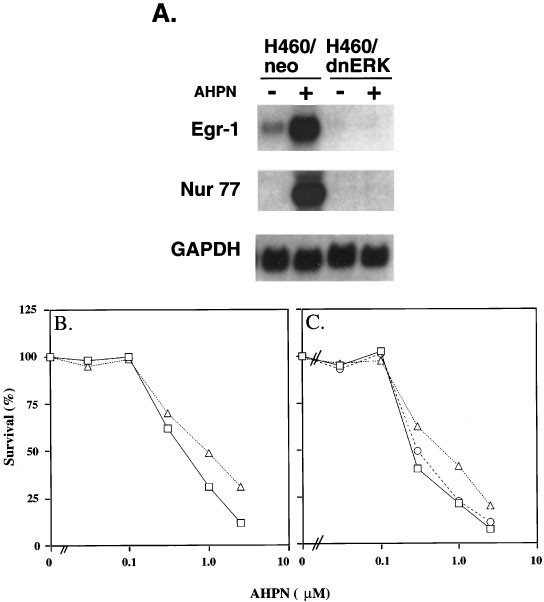
It was intriguing that although AHPN did not enhance the phosphorylation of ERK1/2, inhibition of its activation by U0126 or PD98059 completely abolished the increase in Egr-1 mRNA indicating the importance of activated ERK1/2 in the upregulation of Egr-1. Active ERK1/2 could play a role in the control of Egr-1 at either the transcriptional or posttranscriptional level. Several studies have reported that MAPKs can regulate mRNA stability61 (and references therein). Similarly, active ERK1/2 may be needed to maintain the stability of Egr-1 mRNA. However, Figure 10 demonstrates that inhibition of ERK1/2 activation by U0126 had little effect on the stability of Egr-1 mRNA, indicating that active ERK1/2 is likely not required for maintaining the stability of Egr-1 mRNA. These results suggest that active ERK1/2 may be required for the transcriptional activation of Egr-1 by AHPN. A role for ERK1/2 in AHPN-induced Egr-1 expression is further supported by experiments examining the effect of dominant-negative ERK1 expression of the induction of Egr-1 in H460 cells. Figure 11A shows that expression of dominant-negative ERK1 (dn-ERK1) strongly inhibited the induction of Egr-1 and Nur77 mRNA. Expression of dn-ERK1 enhanced the survival of AHPN-treated H460 cells (Figure 11B). Likewise, the presence of PD98059 increased survival of AHPN (2.5 μM)-treated cells from 7.2 to 17.3% (Figure 11C). The presence of PD169316 had little effect. These results suggest that ERK1/2 can modulate the sensitivity to AHPN.
Figure 10
Effect of the inhibition of ERK1/2 and p38 MAPK on the stability of Egr-1 and Nur77 mRNA. H460 cells were treated with AHPN (2.5 μM) for 8 h before actinomycin D (5 μg/ml) was added. After 30 min cells were treated with the p38 MAPK inhibitor PD169316 (5 μM) or the MEK1/2 inhibitor U0126 (50 μM). At the times indicated RNA was isolated and examined by Northern blot analysis
Figure 11
Effect of dominant-negative ERK1 on the induction of Egr-1 and Nur77 expression by AHPN-in lung carcinoma H460 cells. RNA was isolated from H460-neo and H460-dnERK cells treated for 8 h with or without AHPN (2.5 μM). RNA was examined by Northern blot analysis for expression of Egr-1, Nur77, and GAPDH mRNA. (B) Effect of dominant-negative ERK1 on AHPN-induced cell death by AHPN. H460-neo (□) and H460-dnERK (▵) cells were treated with and without AHPN at the concentrations indicated. After 2 days, the per cent surviving cells was determined. (C) Effect of MAPK inhibitors on AHPN-induced cell death in H460 cells. Cells were treated with different concentrations of AHPN in the presence or absence of the p38 MAPK inhibitor PD169316 (5 μM) (○) or the MEK1/2 inhibitor PD98059 (50 μM) (▵). □, control H460 cells. The per cent surviving cells was determined after 30 h
Discussion
In this study, we identified by cDNA array screening several AHPN-inducible genes encoding proteins that control cell growth and apoptosis. Tristetraprolin (TTP),62 c-jun, and c-fos belong to a group of immediate-early genes that are induced by a variety of stimuli.63 TTP binds to AU-rich elements and promotes deadenylation and destabilization of specific RNAs.64 MyD118, GADD45, Nur77, and cdk-inhibitor p21 have been previously reported to be induced by AHPN.25,27,28,34,36 MyD118, GADD45, and GADD153 are induced by a variety of conditions, including DNA-damaging agents, stress, and during terminal differentiation in a number of cell systems.50,65 All three genes have been shown to be important modulators of growth arrest and apoptosis. IRF-1 is a transcriptional regulator of IFN-stimulated genes and plays a role in immune response and cell growth control by interferons. It can inhibit cell growth and induce apoptosis and loss of IRF-1 function has been reported to lead to malignant transformation, indicating that IRF-1 acts as a tumor suppressor.51,52 These and previous results suggest that AHPN-induced growth inhibition results from the increased expression of many growth-suppressor genes.25,27,28,30,31,34,36,54,66 Therefore, these growth-suppressor proteins may cooperate with each other in the inhibition of cell proliferation and the induction of apoptosis by AHPN.
In this study, we have been focusing largely on the regulation of Egr-1 by AHPN. Evidence showing that Egr-1 acts as a suppressor of cell proliferation is accumulating.47 Egr-1 has been reported to inhibit transformation in a variety of carcinoma cell lines; overexpression of Egr-1 has been demonstrated to reduce DNA synthesis and cell growth, and to induce apoptosis in some cell systems.47,67,68,69 In addition, Egr-1 is induced during terminal differentiation in embryonal carcinoma P19 cells, neuronal differentiation in pheochromocytoma PC12 cells, and differentiation in myoblastic leukemia HL-60 cells. The induction of Egr-1 mRNA and protein by AHPN in a number of carcinoma cell lines correlates with its growth-inhibitory effects and is in agreement with the growth-suppressive function of Egr-1. Egr-1 has been reported to be significantly repressed in non-small cell lung carcinomas compared to normal tissue and a role for Egr-1 down-regulation in the pathogenesis of lung cancer has been suggested.70 Although loss of chromosomal region 5q31 containing the Egr-1 locus has been observed in small cell lung carcinoma, no gross alterations (deletions or rearrangements) in the Egr-1 gene were found, suggesting that the decreased expression could be due to changes in the transcriptional control of Egr-1 expression.70,71 In this study we show that Egr-1 mRNA and protein were expressed at relatively low levels in most lung carcinoma cell lines but are greatly induced by AHPN. Calu-6 cells are an exception and expressed Egr-1 constitutively while AHPN treatment did not cause a further increase in Egr-1 mRNA or protein levels. These observations suggest an altered mechanism of Egr-1 regulation in Calu-6 cells.
Egr-1 belongs to a group of zinc-finger proteins with specificity for GC-rich DNA-response elements.47 This family further includes Egr-2 to -4 and members of the Sp-1 subfamily. In a variety of gene promoters, Egr-1 and Sp-1 interact with overlapping binding sites and therefore are able to compete for binding to these DNA elements. In several instances, Sp-1 activates transcription whereas binding of Egr-1 inhibits transactivation by Sp-1. Sp-1 increases expression of many growth-stimulatory genes whereas expression of Egr-1 inhibits cell growth, possibly by interfering at least in part with Sp-1 transactivation. The induction of Egr-1 by AHPN would result in changes in the ratio of these proteins and contribute to the mechanism by which AHPN causes growth arrest. It is interesting to note in this context that Sp-1 protein is rapidly degraded in lung carcinoma cells after treatment with AHPN.72 Therefore, the increase in Egr-1 protein and decrease in Sp-1 may have a synergistic effect on the expression of certain genes and inhibition of cell growth.
The induction of Egr-1 expression can be mediated by p53-dependent and -independent mechanisms. The increase in Egr-1 expression by thapsigargin in melanoma cells was shown to depend on p53.67 In contrast, ionizing radiation was able to induce Egr-1 and apoptosis in prostate carcinoma PC3 cells lacking p53.73 Our results show that Egr-1 mRNA is highly induced in human lung carcinoma H441 containing p53mt. Moreover, induction of Egr-1 in fibrosarcoma Ht1080(p53mut) cells containing p53mt was slightly higher than in Ht1080(p53wt) cells. These results suggest that p53 is not required for AHPN-dependent induction of Egr-1 and that Egr-1 is controlled by a different mechanism.
Both the p38 and the ERK1/2 MAPK signaling pathways have been implicated in the regulation of Egr-1.55,59 Since AHPN induces activation of p38 MAPK in H460 cells, this activation could be causally related to the induction of Egr-1. However, the p38 MAPK inhibitor PD169316 did not prevent the induction of Egr-1 by AHPN indicating that activation of p38 MAPK is not required for the induction of Egr-1 (Figure 12). In contrast, while AHPN treatment did not cause activation of ERK1/2, inhibitors of the activation of the ERK-signaling pathway blocked the increase in Egr-1 and Nur77 mRNA, suggesting that active ERK1/2 is required for the induction of Egr-1. This conclusion is supported by observations showing that expression of a dominant-negative ERK1 also blocked the induction of Egr-1 mRNA. MAPKs have been reported to regulate gene expression at the transcriptional level through activation (phosphorylation) of transcription factors or by affecting the stability of specific mRNAs61 (and references therein). Our results show that inhibition of ERK1/2 activation does not effect the stability of Egr-1 mRNA, indicating that active ERK1/2 is not needed to maintain the stability of Egr-1 mRNA but is likely required for the transcriptional regulation of Egr-1 by AHPN. Possibly, both transcriptional factors activated by ERK1/2, such as Elk-1, as well as signals generated by AHPN are essential for the AHPN-dependent induction of Egr-1 mRNA and affect the sensitivity of cells to AHPN growth inhibition (Figure 12). It is interesting to note that the ERK1/2 signaling pathway has been implicated in the induction of Egr-1 in several other cell systems, including hypoxia-exposed monocytes,57 nitric oxide-treated endothelial cells,74 and growth hormone-treated preadipocytes.58
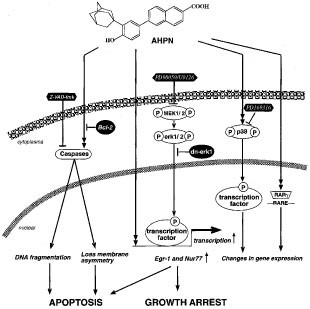
Figure 12
Schematic representation of the Egr-1 regulation by AHPN. AHPN action can be mediated by several signaling pathways, including activation of the RARγ receptor and activation of p38 MAPK. The induction of Egr-1 appears to be controlled at the transcriptional level but does not involve nuclear retinoid receptors or p38 activation and is independent of Bcl-2 expression. Inhibition of the ERK signaling pathway either by MEK1 inhibitors PD98059 or U0126, or by expression of dnERK1 block Egr-1 induction by AHPN. Presence of PD98059 or expression of dnERK1 increase survival of AHPN-treated H460 cells. We hypothesize that the induction of Egr-1 expression by AHPN requires the cooperation between an ERK1/2-dependent transcription factor and an AHPN-dependent signal. Although growth-inhibition can occur independently of apoptosis,30 ERK1/2 activity and growth-regulatory proteins, such as Egr-1, likely influence both AHPN-induced growth arrest and apoptosis
Several studies have demonstrated that AHPN acts through multiple mechanisms.25,28,34,35,36,72 AHPN was originally reported as a synthetic retinoid that selectively binds and activates the nuclear retinoid receptor RARγ75 and at least some of AHPN-dependent changes in gene expression are likely mediated through this signaling pathway (Figure 12). However, AHPN-induced growth arrest, apoptosis, and the induction of several immediate-early genes, including Egr-1 and Nur77, appear not to involve nuclear retinoid receptors. This is supported by observations showing the lack of a correlation between the expression of RARγ and the growth-inhibitory effects of AHPN.15,27,28,29,30,31 The growth of small cell lung carcinoma and leukemia cells that do not express RARγ is effectively inhibited by AHPN. In addition, RAR and RXR-selective retinoids do not induce growth arrest or Egr-1 and Nur77 expression indicating that these effects are highly specific for AHPN. These results are in agreement with previous studies indicating that the action of AHPN is mediated by a novel mechanism. However, it cannot be ruled out that in certain cell lines binding and activation of RARγ either contributes to or modulates the growth-inhibitory action of AHPN.
Recent studies have demonstrated that AHPN can have a dramatic effect on the stability of certain mRNAs. AHPN causes an 8–10-fold increase in the stability of MyD118 and GADD45 mRNA34,36 and the induction of p21 mRNA by AHPN has also been shown to be largely controlled at the level of its stability.48 However, in H460 cells AHPN had little effect on the stability of Egr-1 and Nur77 mRNA suggesting that the AHPN-induced expression of Egr-1 and Nur77 is at least in part regulated at the transcriptional level. Our results also demonstrate that up-regulation of Egr-1 and Nur77 by AHPN does not always occur in parallel. For example, AHPN induced Egr-1 expression in T24 and Molt-4Hygr cells while it did not have any effect on Nur77 mRNA expression, suggesting that in these cells Nur77 and Egr-1 expression are regulated differently. As shown in Table 1, there are no simple correlations between the expression of p53, Egr-1, and Nur77 and the induction of apoptosis and growth arrest.
Induction of apoptosis by AHPN is associated with increased caspase-3 activity.28,34 Expression of Bcl-2 greatly inhibits caspase activation and apoptosis in T cell lymphoma Molt-4 cells but has only little effect on the growth-inhibitory action of AHPN. In this study, we show that expression of Bcl-2 inhibits neither the induction of Egr-1 nor that of several other immediate-early genes (Figure 12). The caspase inhibitor ZVAD.fmk greatly inhibits DNA-fragmentation but affects neither AHPN-induced growth inhibition34 nor Egr-1 and Nur77 expression (Sakaue M, and Jetten AM, not shown). These results demonstrate that induction of Egr-1 occurs independently of caspase activation and Bcl-2 expression. It is difficult to determine the precise role of Egr-1 in growth arrest and apoptosis since AHPN induces many genes that with similar functions. Previous studies indicated possible differences in the mechanism by which AHPN causes growth arrest and apoptosis.30 A recent study demonstrated that AHPN can disrupt mitochondrial potential in isolated mitochondria, suggesting that the induction of apoptosis by AHPN may not require de novo protein synthesis.33,76 Another study reported that translocation of Nur77 from the nucleus to mitochondria plays a critical role in the dissipation of mitochondrial potential induced by a variety of apoptotic signals, including AHPN.77 Possibly immediate-early proteins are not an absolute requirement but influence the sensitivity to AHPN induced cell death and growth arrest, as has been demonstrated for p53.54
In summary, we demonstrate that AHPN induces a cascade of changes in immediate-early genes that resembles those reported for DNA damage agents and stress. AHPN-induced growth arrest, and in many cell lines apoptosis, is associated with an increase in the expression of many genes, including Egr-1 and Nur77, that regulate proliferation and apoptosis. Table 1 suggests that there is no simple correlation between cell death, inhibition of cell proliferation, and expression of Egr-1, Nur77 and p53. It is likely that all these proteins are involved in some degree in AHPN-induced growth arrest and apoptosis, however, their importance and contribution may vary among cell lines and be obscured by the expression of other genes. The induction of Egr-1 and Nur77 by AHPN appears to be transcriptionally regulated and mediated by a novel mechanism that does not require retinoid nuclear receptors (Figure 12). Although we demonstrate that AHPN induces activation of p38 but not ERK1/2 MAPK, the induction of Egr-1 and nur77 requires activated ERK1/2 and not activated p38. Expression of dnERK or the presence of PD98059 enhances the survival of AHPN-treated H460 cells. This reduced responsiveness may in part be due to the diminished induction of growth suppressor genes, such as Egr-1. These results suggest that ERK1/2 can modulate the cell's sensitivity to AHPN.
Materials and Methods
Materials
The retinoid 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (AHPN, also referred to as CD437) was described previously75 and synthesized by Dr. M Dawson (MMRI, Mountain View, CA, USA). The RAR-selective (SRI-6751-84/TTAB, 4-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-anthracenyl)-benzoic acid), RXR-selective (SR11217, 4-[1-(5,6,7,8-tetrahydro-5,5,8,8,-tetramethyl-2-naphthalenyl)-2-methyl-propenyl]-benzoic acid), and anti-AP1-selective (SR11302, (E)-3-methyl-9-(2,6,6-trimethylcyclohexenyl)-7-(4-methyl-phenyl)-2,4,6,8-nonatetraenoic acid) retinoids78,79 were provided by Dr. M Dawson. All-trans retinoic acid was obtained from Hoffmann-La Roche, (Nutley, NJ, USA). Retinoids were dissolved in DMSO. Control cells received DMSO only. Actinomycin D was purchased from Sigma (St. Louis, MO, USA). The selective p38 MAP kinase inhibitor PD169316 and the MEK-1 inhibitor PD98059 were purchased from Calbiochem (San Diego, CA, USA). The MEK-1 inhibitor U0126 was purchased from Promega (Madison, WI, USA).
Cell culture
The human lung adenocarcinoma cell lines NCI-H441 and NCI-H460, alveolar carcinoma A549, anaplastic carcinoma Calu-6 and the human mammary cancer cell line MCF-7 were obtained from American Type Culture Collection (Rockville, MD, USA). The human lung adenocarcinoma NCI-H1355 cells were provided by Dr. A Gazdar (Austin, TX, USA). Human T cell lymphoma Molt-4Hygr and Molt-4-Bcl2 were described previously.30,80 The human fibrosarcoma cell line Ht1080, a variant Ht1080 containing a mutant p53, and the human colorectal cancer cell line RKO were obtained from Dr. Gloria Preston (NIEHS). The human bladder cancer cell line T24 was obtained from Dr. T Eling (NIEHS). All cell lines, except for Ht1080 and RKO, were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 units penicillin and 100 μg streptomycin. The Ht1080 and the RKO cells were grown in Dulbecco's modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum. All cells were Mycoplasma free and were cultured under the condition of 5% CO2 humid air at 37°C.
Expression of dominant-negative ERK
The expression vector pCMV-dnERK, encoding a dominant-negative form of ERK1 MAP kinase, was obtained from Dr. R Khosravi-Far. H460 cells were plated in 6-well dishes (2×105 cells per well) 16 h prior to transfection. PCMV-dnERK or the neomycin phosphotransferase (neo) gene expression plasmid (pCB6) was transfected into cells using 1,3-di-oleoyloxy-2-(6-carboxy-spermyl)-propylamide (DOSPER, Roche) as directed by the manufacturer's protocol. Twenty-four hours after transfection, cells were treated with G418 (Gibco, 500 μg/ml) to select for neomycin-resistant cells. H460 cells expressing dnERK or neo gene only are referred to as H460-dnERK and H460-neo, respectively. Detailed characterization of these cell lines will be described elsewhere.
cDNA-array analysis
Human lung carcinoma H460 cells were treated with 2.5 μM AHPN or vehicle (DMSO) for 8 h. Total RNA was isolated using Tri-Reagent (Sigma, St. Louis, MO, USA) according to the manufacturer's protocol. Poly(A)+ RNA was subsequently prepared by PolyAtract (Promega) and treated with DNase I. Analysis of the Atlas cDNA Expression Arrays (Clontech) was carried out according to the manufacturer's directions. Briefly, RNAs were reverse-transcribed using MMLV-reverse transcriptase in the presence of [α-32P]dATP. The radiolabeled cDNA probes were hybridized overnight to Atlas cDNA Array membranes in ExpressHyb hybridization solution (Clontech). After washing in 0.1×SSC plus 0.5% SDS at 68°C, membranes were subjected to autoradiography at −70°C. Differentially expressed cDNAs were confirmed by Northern blot analysis.
Northern blot analysis
Total RNA (30 μg) or poly(A)+ RNA (2 μg) was separated by electrophoresis on a formaldehyde 1.2% agarose gel, blotted to a Nytran Plus membrane (Schleicher & Schuell, Keene, NH, USA), and UV-crosslinked as described previously.30 Hybridizations were carried out for 1–2 h at 68°C using QuikHyb™ reagent (Stratagene, La Jolla, CA, USA), blots were washed twice with 2×SSC, 0.05% SDS for 15 min at room temperature and subsequently with 0.5×SSC, 0.1% SDS at 65°C for 30 min. Autoradiography was carried out with Hyperfilm-MP (Amersham) at −70°C using double intensifying screens. The cDNA probe for Egr-1 used in Northern analysis was obtained through differential display.34 The cDNA probe for Nur77 was obtained from Dr. C Chang, (Glaxo Wellcome, NC, USA). A 1.26 kb fragment of the chicken glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH) was used as a control probe.
RNA stability assay
H460 cells grown for 8 h in the presence or absence of 2.5 μM AHPN were treated with actinomycin D (5 μg/ml) and, at different time intervals, cells were collected and RNA isolated. For RNA stability assay, total RNA from AHPN-treated cells and poly(A)+ RNA from control H460 cells were examined by Northern blot analysis using radiolabeled probes for Egr-1, Nur77, or GAPDH. Hybridization signals were quantitated with a PhosphorImage analyzer (STORM 860; Molecular Dynamics, Sunnyvale, CA, USA) using ImageQuant software (Molecular Dynamics).
Western blot analysis
Cells were washed in PBS and then collected in sample buffer (60 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 10 mM DTT, 1 mM phenylmethylsulfonyl fluoride, aprotinin, and leupeptin) and phosphatase inhibitor mixture I and II (Sigma). Proteins were examined by Western blot analyses. Primary antibodies against Egr-1 and ERK1/2 were purchased from Santa Cruz Biotechnology (San Diego, CA, USA). Antibodies against phospho-p44/p42 ERK1/2, phospho-p38, and p38 were obtained from New England Biolabs (Beverly, MA, USA). Peroxidase-conjugated anti-goat or anti-rabbit IgG from Chemicon (Temecula, CA, USA) was used as secondary antibody. Antibodies were diluted in PBS containing 1 or 5% milk powder and 0.05% Tween 20. Detection was carried out using Super Signal Chemiluminescent Substrate; Luminol and Peroxide were purchased from Pierce (Rockford, IL, USA).
Abbreviations
AHPN:
6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid
Egr:
Early-growth response gene
ERK:
Extracellular signal-regulated kinase
MAPK:
mitogen-activated protein kinase
RAR:
retinoic acid receptor
RXR:
retinoid X receptor
References
- Zachman RD . 1995 Role of vitamin A in lung development J. Nutr. 125: 1634S–1638S
CAS PubMed Google Scholar - Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M . 1994 Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants Development 120: 2749–2771
CAS PubMed Google Scholar - Jetten AM, Nervi C, Vollberg TM . 1992 Control of squamous differentiation in tracheobronchial and epidermal epithelial cells: role of retinoids J. Natl. Cancer Inst. Monogr. 13: 93–100
Google Scholar - Grummer MA, Zachman RD . 1998 Retinoic acid and dexamethasone affect RAR-beta and surfactant protein C mRNA in the MLE lung cell line Am. J. Physiol. 274: L1–L7
Article CAS Google Scholar - Yan C, Ghaffari M, Whitsett JA, Zeng X, Sever Z, Lin S . 1998 Retinoic acid-receptor activation of SP-B gene transcription in respiratory epithelial cells Am. J. Physiol. 275: L239–L246
Article CAS Google Scholar - Rearick JI, Deas M, Jetten AM . 1987 Synthesis of mucous glycoproteins by rabbit tracheal cells in vitro. Modulation by substratum, retinoids and cyclic AMP Biochem. J. 242: 19–25
Article CAS Google Scholar - Koo JS, Yoon JH, Gray T, Norford D, Jetten AM, Nettesheim P . 1999 Restoration of the mucous phenotype by retinoic acid in retinoid-deficient human bronchial cell cultures: changes in mucin gene expression [In Process Citation] Am. J. Respir. Cell. Mol. Biol. 20: 43–52
Article CAS Google Scholar - Koo JS, Jetten AM, Belloni P, Yoon J-H, Kim Y-D, Nettesheim P . 1999 The role of nuclear retinoid receptors in the regulation of mucin gene expression in normal tracheobronchial epithelial cells J. Biochem. 338: 351–357
Article CAS Google Scholar - Sueoka N, Lee HY, Walsh GL, Hong WK, Kurie JM . 1999 Posttranslational mechanisms contribute to the suppression of specific cyclin: CDK complexes by all-trans retinoic acid in human bronchial epithelial cells Cancer Res. 59: 3838–3844
CAS PubMed Google Scholar - Massaro GD, Massaro D . 1997 Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats [see comments] [published erratum appears in Nat Med 1997 Jul; 3(7):805] Nat. Med. 3: 675–677
Article CAS Google Scholar - Shenai JP, Kennedy KA, Chytil F, Stahlman MT . 1987 Clinical trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia J. Pediatr. 111: 269–277
Article CAS Google Scholar - Lippman SM, Benner SE, Hong WK . 1994 Retinoid chemoprevention studies in upper aerodigestive tract and lung carcinogenesis Cancer Res. 54: 2025s–2028s
CAS PubMed Google Scholar - Giguere V . 1994 Retinoic acid receptors and cellular retinoid binding proteins: complex interplay in retinoid signaling Endocr. Rev. 15: 61–79
CAS PubMed Google Scholar - Gebert JF, Moghal N, Frangioni JV, Sugarbaker DJ, Neel BG . 1991 High frequency of retinoic acid receptor beta abnormalities in human lung cancer [published erratum appears in Oncogene 1992 Apr; 7(4):821] Oncogene 6: 1859–1868
CAS Google Scholar - Nervi C, Vollberg TM, George MD, Zelent A, Chambon P, Jetten AM . 1991 Expression of nuclear retinoic acid receptors in normal tracheobronchial cells and in lung carcinoma cells Exp. Cell Res. 195: 163–170
Article CAS Google Scholar - Haq R, Pfahl M, Chytil F . 1991 Differential effects of all-trans and 13-cis-retinoic acid on mRNA levels of nuclear retinoic acid receptors in rat lung and liver Biochem. Biophys. Res. Commun. 180: 1137–1144
Article CAS Google Scholar - Sun SY, Kurie JM, Yue P, Dawson MI, Shroot B, Chandraratna RA, Hong WK, Lotan R . 1999 Differential responses of normal, premalignant, and malignant human bronchial epithelial cells to receptor-selective retinoids Clin. Cancer Res. 5: 431–437
CAS PubMed Google Scholar - Sun SY, Yue P, Dawson MI, Shroot B, Michel S, Lamph WW, Heyman RA, Teng M, Chandraratna RA, Shudo K, Hong WK, Lotan R . 1997 Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells Cancer Res. 57: 4931–4939
CAS PubMed Google Scholar - Zhang XK, Liu Y, Lee MO, Pfahl M . 1994 A specific defect in the retinoic acid response associated with human lung cancer cell lines Cancer Res. 54: 5663–5669
CAS PubMed Google Scholar - Picard E, Seguin C, Monhoven N, Rochette-Egly C, Siat J, Borrelly J, Martinet Y, Martinet N, Vignaud JM . 1999 Expression of retinoid receptor genes and proteins in non-small-cell lung cancer [see comments] J. Natl. Cancer Inst. Monogr. 91: 1059–1066
Article CAS Google Scholar - Moghal N, Neel BG . 1995 Evidence for impaired retinoic acid receptor-thyroid hormone receptor AF-2 cofactor activity in human lung cancer Mol. Cell. Biol. 15: 3945–3959
Article CAS Google Scholar - Wu Q, Li Y, Liu R, Agadir A, Lee MO, Liu Y, Zhang X . 1997 Modulation of retinoic acid sensitivity in lung cancer cells through dynamic balance of orphan receptors nur77 and COUP-TF and their heterodimerization EMBO J. 16: 1656–1669
Article CAS Google Scholar - Geradts J, Chen JY, Russell EK, Yankaskas JR, Nieves L, Minna JD . 1993 Human lung cancer cell lines exhibit resistance to retinoic acid treatment Cell Growth Differ. 4: 799–809
CAS PubMed Google Scholar - Kalemkerian GP, Slusher R, Ramalingam S, Gadgeel S, Mabry M . 1995 Growth inhibition and induction of apoptosis by fenretinide in small-cell lung cancer cell lines [see comments] J. Natl. Cancer Inst. Monogr. 87: 1674–1680
Article CAS Google Scholar - Shao ZM, Dawson MI, Li XS, Rishi AK, Sheikh MS, Han QX, Ordonez JV, Shroot B, Fontana JA . 1995 p53 independent G0/G1 arrest and apoptosis induced by a novel retinoid in human breast cancer cells Oncogene 11: 493–504
CAS Google Scholar - Sun SY, Yue P, Shroot B, Hong WK, Lotan R . 1997 Induction of apoptosis in human non-small cell lung carcinoma cells by the novel synthetic retinoid CD437 J. Cell. Physiol. 173: 279–284
Article CAS Google Scholar - Adachi H, Preston G, Harvat B, Dawson MI, Jetten AM . 1998 Inhibition of cell proliferation and induction of apoptosis by the retinoid AHPN in human lung carcinoma cells Am. J. Respir. Cell Mol. Biol. 18: 323–333
Article CAS Google Scholar - Sun SY, Yue P, Wu GS, El-Deiry WS, Shroot B, Hong WK, Lotan R . 1999 Mechanisms of apoptosis induced by the synthetic retinoid CD437 in human non-small cell lung carcinoma cells Oncogene 18: 2357–2365
Article CAS Google Scholar - Hsu CA, Rishi AK, Su-Li X, Gerald TM, Dawson MI, Schiffer C, Reichert U, Shroot B, Poirer GC, Fontana JA . 1997 Retinoid induced apoptosis in leukemia cells through a retinoic acid nuclear receptor-independent pathway Blood 89: 4470–4479
CAS PubMed Google Scholar - Adachi H, Adams A, Hughes FM, Zhang J, Cidlowski JA, Jetten AM . 1998 Induction of apoptosis by the novel retinoid AHPN in human T-cell lymphoma cells involves caspase-dependent and independent pathways Cell Death Differ. 5: 973–983
Article CAS Google Scholar - Lin Y, Lin B, Agadir A, Liu R, Dawson MI, Reed JC, Fontana JA, Bost F, Hobbs PD, Zheng Y, Chen GQ, Shroot B, Mercola D, Zhang XK . 1998 Molecular determinants of AHPN (CD437)-induced growth arrest and apoptosis in human lung cancer cell lines Mol. Cell. Biol. 18: 4719–4731
Article Google Scholar - Zou CP, Kurie JM, Lotan D, Zou CC, Hong WK, Lotan R . 1998 Higher potency of N-(4-hydroxyphenyl)retinamide than all-trans-retinoic acid in induction of apoptosis in non-small cell lung cancer cell lines Clin. Cancer Res. 4: 1345–1355
CAS PubMed Google Scholar - Mologni L, Ponzanelli I, Bresciani F, Sardiello G, Bergamaschi D, Gianni M, Reichert U, Rambaldi A, Terao M, Garattini E . 1999 The novel synthetic retinoid 6-[3-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (CD437) causes apoptosis in acute promyelocytic leukemia cells through rapid activation of caspases Blood 93: 1045–1061
CAS PubMed Google Scholar - Sakaue M, Adachi H, Jetten AM . 1999 Post-transcriptional regulation of MyD118 and GADD45 in human lung carcinoma cells during 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid-induced apoptosis Mol. Pharmacol. 55: 668–676
CAS PubMed Google Scholar - Schadendorf D, Kern MA, Artuc M, Pahl HL, Rosenbach T, Fichtner I, Nurnberg W, Stuting S, von Stebut E, Worm M, Makki A, Jurgovsky K, Kolde G, Henz BM . 1996 Treatment of melanoma cells with the synthetic retinoid CD437 induces apoptosis via activation of AP-1 in vitro, and causes growth inhibition in xenografts in vivo J. Cell Biol. 135: 1889–1898
Article CAS Google Scholar - Rishi AK, Sun RJ, Gao Y, Hsu CK, Gerald TM, Sheikh MS, Dawson MI, Reichert U, Shroot B, Fornace Jr AJ, Brewer G, Fontana JA . 1999 Post-transcriptional regulation of the DNA damage-inducible gadd45 gene in human breast carcinoma cells exposed to a novel retinoid CD437 Nucleic Acids Res. 27: 3111–3119
Article CAS Google Scholar - Oridate N, Higuchi M, Suzuki S, Shroot B, Hong WK, Lotan R . 1997 Rapid induction of apoptosis in human C33A cervical carcinoma cells by the synthetic retinoid 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (CD437) Int. J. Cancer 70: 484–487
Article CAS Google Scholar - Liang JY, Fontana JA, Rao JN, Ordonez JV, Dawson MI, Shroot B, Wilber JF, Feng P . 1999 Synthetic retinoid CD437 induces S-phase arrest and apoptosis in human prostate cancer cells LNCaP and PC-3 Prostate 38: 228–236
Article CAS Google Scholar - Harvat B, Jetten AM . 1999 Growth control by retinoids: regulation of cell cycle progression and apoptosis Handbook Exp Pharmacol 139: 239–276
Article CAS Google Scholar - Lu XP, Fanjul A, Picard N, Pfahl M, Rungta D, Nared-Hood K, Carter B, Piedrafita J, Tang S, Fabbrizio E . 1997 Novel retinoid-related molecules as apoptosis inducers and effective inhibitors of human lung cancer cells in vivo Nat. Med. 3: 686–690
Article CAS Google Scholar - Bernard BA, Bernardon JM, Delescluse C, Martin B, Lenoir MC, Maignan J, Charpentier B, Pilgrim WR, Reichert U, Shroot B . 1992 Identification of synthetic retinoids with selectivity for human nuclear retinoic acid receptor gamma Biochem. Biophys. Res. Commun. 186: 977–983
Article CAS Google Scholar - Sukhatme VP, Cao XM, Chang LC, Tsai-Morris CH, Stamenkovich D, Ferreira PC, Cohen DR, Edwards SA, Shows TB, Curran T, et al . 1988 A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization Cell 53: 37–43
Article CAS Google Scholar - Changelian PS, Feng P, King TC, Milbrandt J . 1989 Structure of the NGFI-A gene and detection of upstream sequences responsible for its transcriptional induction by nerve growth factor Proc. Natl. Acad. Sci. USA 86: 377–381
Article CAS Google Scholar - Christy BA, Lau LF, Nathans D . 1988 A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with “zinc finger” sequences Proc. Natl. Acad. Sci. USA 85: 7857–7861
Article CAS Google Scholar - Lemaire P, Vesque C, Schmitt J, Stunnenberg H, Frank R, Charnay P . 1990 The serum-inducible mouse gene Krox-24 encodes a sequence-specific transcriptional activator Mol. Cell. Biol. 10: 3456–3467
Article CAS Google Scholar - Varnum BC, Lim RW, Kujubu DA, Luner SJ, Kaufman SE, Greenberger JS, Gasson JC, Herschman HR . 1989 Granulocyte-macrophage colony-stimulating factor and tetradecanoyl phorbol acetate induce a distinct, restricted subset of primary-response TIS genes in both proliferating and terminally differentiated myeloid cells Mol. Cell. Biol. 9: 3580–3583
Article CAS Google Scholar - Liu C, Rangnekar VM, Adamson E, Mercola D . 1998 Suppression of growth and transformation and induction of apoptosis by EGR-1 Cancer Gene Ther. 5: 3–28
CAS PubMed Google Scholar - Li XS, Rishi AK, Shao ZM, Dawson MI, Jong L, Shroot B, Reichert U, Ordonez J, Fontana JA . 1996 Posttranscriptional regulation of p21WAF1/CIP1 expression in human breast carcinoma cells Cancer Res. 56: 5055–5062
CAS PubMed Google Scholar - Hollander MC, Alamo I, Jackman J, Wang MG, McBride OW, Fornace Jr AJ . 1993 Analysis of the mammalian gadd45 gene and its response to DNA damage J. Biol. Chem. 268: 24385–24393
CAS PubMed Google Scholar - Vairapandi M, Balliet AG, Fornace Jr AJ, Hoffman B, Liebermann DA . 1996 The differentiation primary response gene MyD118, related to GADD45, encodes for a nuclear protein which interacts with PCNA and p21WAF1/CIP1 Oncogene 12: 2579–2594
CAS PubMed Google Scholar - Harada H, Taniguchi T, Tanaka N . 1998 The role of interferon regulatory factors in the interferon system and cell growth control Biochimie 80: 641–650
Article CAS Google Scholar - Tanaka N, Ishihara M, Kitagawa M, Harada H, Kimura T, Matsuyama T, Lamphier MS, Aizawa S, Mak TW, Taniguchi T . 1994 Cellular commitment to oncogene-induced transformation or apoptosis is dependent on the transcription factor IRF-1 Cell 77: 829–839
Article CAS Google Scholar - Liu C, Calogero A, Ragona G, Adamson E, Mercola D . 1996 EGR-1, the reluctant suppression factor: EGR-1 is known to function in the regulation of growth, differentiation, and also has significant tumor suppressor activity and a mechanism involving the induction of TGF-beta1 is postulated to account for this suppressor activity Crit. Rev. Oncog. 7: 101–125
Article CAS Google Scholar - Sun SY, Yue P, Wu GS, El-Deiry WS, Shroot B, Hong WK, Lotan R . 1999 Implication of p53 in growth arrest and apoptosis induced by the synthetic retinoid CD437 in human lung cancer cells Cancer Res. 59: 2829–2833
CAS PubMed Google Scholar - Rolli M, Kotlyarov A, Sakamoto KM, Gaestel M, Neininger A . 1999 Stress-induced stimulation of early growth response gene-1 by p38/stress-activated protein kinase 2 is mediated by a cAMP-responsive promoter element in a MAPKAP kinase 2-independent manner J. Biol. Chem. 274: 19559–19564
Article CAS Google Scholar - de Belle I, Huang RP, Fan Y, Liu C, Mercola D, Adamson ED . 1999 p53 and Egr-1 additively suppress transformed growth in HT1080 cells but Egr-1 counteracts p53-dependent apoptosis Oncogene 18: 3633–3642
Article CAS Google Scholar - Yan SF, Lu J, Zou YS, Soh-Won J, Cohen DM, Buttrick PM, Cooper DR, Steinberg SF, Mackman N, Pinsky DJ, Stern DM . 1999 Hypoxia-associated induction of early growth response-1 gene expression J. Biol. Chem. 274: 15030–15040
Article CAS Google Scholar - Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J . 1998 Growth hormone stimulates phosphorylation and activation of elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2 J. Biol. Chem. 273: 31327–31336
Article CAS Google Scholar - Lim CP, Jain N, Cao X . 1998 Stress-induced immediate-early gene, egr-1, involves activation of p38/JNK1 Oncogene 16: 2915–2926
Article CAS Google Scholar - Zhang Y, Huang Y, Rishi AK, Sheikh MS, Shroot B, Reichert U, Dawson M, Poirer G, Fontana JA . 1999 Activation of the p38 and JNK/SAPK mitogen-activated protein kinase pathways during apoptosis is mediated by a novel retinoid Exp. Cell Res. 247: 233–240
Article CAS Google Scholar - Matsuura H, Sakaue M, Subbaramaih K, Kamitani H, Eling TE, Dannenberg AJ, Tanabe T, Inoue H, Arata J, Jetten AM . 1999 Regulation of cyclooxygenase-2 by interferon gamma and transforming growth factor alpha in normal human epidermal keratinocytes and squamous carcinoma cells. Role of mitogen-activated protein kinases J. Biol. Chem. 274: 29138–29148
Article CAS Google Scholar - Kaneda N, Oshima M, Chung SY, Guroff G . 1992 Sequence of a rat TIS11 cDNA, an immediate early gene induced by growth factors and phorbol esters Gene 118: 289–291
Article CAS Google Scholar - Gashler A, Sukhatme VP . 1995 Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors Prog. Nucleic Acid Res. Mol. Biol. 50: 191–224
Article CAS Google Scholar - Lai WS, Carballo E, Strum JR, Kennington EA, Phillips RS, Blackshear PJ . 1999 Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA Mol. Cell. Biol. 19: 4311–4323
Article CAS Google Scholar - Fornace Jr AJ . 1992 Mammalian genes induced by radiation; activation of genes associated with growth control Annu. Rev. Genet. 26: 507–526
Article CAS Google Scholar - Sun SY, Yue P, Shroot B, Hong WK, Lotan R . 1999 Implication of c-Myc in apoptosis induced by the retinoid CD437 in human lung carcinoma cells Oncogene 18: 3894–3901
Article CAS Google Scholar - Muthukkumar S, Nair P, Sells SF, Maddiwar NG, Jacob RJ, Rangnekar VM . 1995 Role of EGR-1 in thapsigargin-inducible apoptosis in the melanoma cell line A375-C6 Mol. Cell. Biol. 15: 6262–6272
Article CAS Google Scholar - Nair P, Muthukkumar S, Sells SF, Han SS, Sukhatme VP, Rangnekar VM . 1997 Early growth response-1-dependent apoptosis is mediated by p53 J. Biol. Chem. 272: 20131–20138
Article CAS Google Scholar - Huang RP, Liu C, Fan Y, Mercola D, Adamson ED . 1995 Egr-1 negatively regulates human tumor cell growth via the DNA-binding domain Cancer Res. 55: 5054–5062
CAS PubMed Google Scholar - Levin WJ, Press MF, Gaynor RB, Sukhatme VP, Boone TC, Reissmann PT, Figlin RA, Holmes EC, Souza LM, Slamon DJ . 1995 Expression patterns of immediate early transcription factors in human non-small cell lung cancer. The Lung Cancer Study Group Oncogene 11: 1261–1269
CAS Google Scholar - Nagarajan L, Zhao L, Lu X, Warrington JA, Wasmuth JJ, Siciliano M, Deisseroth AB, Liang JC . 1994 5q-chromosome. Evidence for complex interstitial breaks in a case of refractory anemia with excess blasts Cancer Genet. Cytogenet. 74: 8–12
Article CAS Google Scholar - Piedrafita FJ, Pfahl M . 1997 Retinoid-induced apoptosis and Sp1 cleavage occur independently of transcription and require caspase activation Mol. Cell. Biol. 17: 6348–6358
Article CAS Google Scholar - Ahmed MM, Sells SF, Venkatasubbarao K, Fruitwala SM, Muthukkumar S, Harp C, Mohiuddin M, Rangnekar VM . 1997 Ionizing radiation-inducible apoptosis in the absence of p53 linked to transcription factor EGR-1 J. Biol. Chem. 272: 33056–33061
Article CAS Google Scholar - Chiu JJ, Wung BS, Hsieh HJ, Lo LW, Wang DL . 1999 Nitric oxide regulates shear stress-induced early growth response-1. Expression via the extracellular signal-regulated kinase pathway in endothelial cells Circ. Res. 85: 238–246
Article CAS Google Scholar - Delescluse C, Cavey MT, Martin B, Bernard BA, Reichert U, Maignan J, Darmon M, Shroot B . 1991 Selective high affinity retinoic acid receptor alpha or beta-gamma ligands Mol. Pharmacol. 40: 556–562
CAS PubMed Google Scholar - Marchetti P, Zamzami N, Joseph B, Schraen-Maschke S, Mereau-Richard C, Costantini P, Metivier D, Susin SA, Kroemer G, Formstecher P . 1999 The novel retinoid 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid can trigger apoptosis through a mitochondrial pathway independent of the nucleus Cancer Res. 59: 6257–6266
CAS PubMed Google Scholar - Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, Xie Z, Fontana JA, Reed JC, Zhang X . 2000 Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3 Science 289: 1159–1164
Article CAS Google Scholar - Fanjul A, Dawson MI, Hobbs PD, Jong L, Cameron JF, Harlev E, Graupner G, Lu XP, Pfahl M . 1994 A new class of retinoids with selective inhibition of AP-1 inhibits proliferation Nature 372: 107–111
Article CAS Google Scholar - Lehmann JM, Jong L, Fanjul A, Cameron JF, Lu XP, Haefner P, Dawson MI, Pfahl M . 1992 Retinoids selective for retinoid X receptor response pathways Science 258: 1944–1946
Article CAS Google Scholar - Zhang J, Alter N, Reed JC, Borner C, Obeid LM, Hannun YA . 1996 Bcl-2 interrupts the ceramide-mediated pathway of cell death Proc. Natl. Acad. Sci. USA 93: 5325–5328
Article CAS Google Scholar
Acknowledgements
This research was supported in part by the Japanese Society for the Promotion of Science.
Author information
Author notes
- M Sakaue and H Adachi: M Sakaue and H Adachi contributed equally to this research
Authors and Affiliations
- Cell Biology Section, Laboratory of Pulmonary Pathobiology, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, North Carolina, 27709, NC, USA
M Sakaue, H Adachi & A M Jetten - Molecular Medicine Research Institute, Mountain View, CA 94043, California, USA
M Dawson
Authors
- M Sakaue
You can also search for this author inPubMed Google Scholar - H Adachi
You can also search for this author inPubMed Google Scholar - M Dawson
You can also search for this author inPubMed Google Scholar - A M Jetten
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toA M Jetten.
Additional information
Edited by C Thiele
Rights and permissions
About this article
Cite this article
Sakaue, M., Adachi, H., Dawson, M. et al. Induction of Egr-1 expression by the retinoid AHPN in human lung carcinoma cells is dependent on activated ERK1/2.Cell Death Differ 8, 411–424 (2001). https://doi.org/10.1038/sj.cdd.4400818
- Received: 19 June 2000
- Revised: 08 November 2000
- Accepted: 28 November 2000
- Published: 01 May 2001
- Issue Date: 01 April 2001
- DOI: https://doi.org/10.1038/sj.cdd.4400818