Effect of amyloid peptides on serum withdrawal-induced cell differentiation and cell viability (original) (raw)
INTRODUCTION
Alzheimer's disease (AD) is a complex disorder that impairs cognitive and memory functions in the elder population. Aggregated amyloid peptide (Aβ) is the core component of brain senile plaques, a defining feature of Alzheimer's disease. Aβ is composed of 39-43 amino acid residues produced from a large precursor, amyloid precursor protein (APP), and plays a pivotal role in the dysfunction and death of neurons in AD. Among different subtypes of Aβ, Aβ1-40 is the most predominant form accounting for more than 90% of the total Aβ, whereas amyloid Aβ1-42 is the most toxic form.
APP is a type I integral membrane protein which is processed to generate several intracellular and secreted fragments. Among 10 identified isoforms of APP, APP695, which is composed of 695 amino acid residues, was mainly expressed in human brain. Genetic evidence 1 and the characterization of neurotoxicity of Aβ lead to numerous studies on the cell biology of APP and formation of Aβ 2. However, the functions of APP and Aβ still remain poorly understood until now. Although it is reported that APP promotes neural differentiation and neurite outgrowth 3, there was no information about whether and how APP or Aβ functions in regulating neurite growth. To investigate the role of APP/Aβ in neurite outgrowth and cell viability, mouse neuroblastoma N2a cells, which are capable of differentiating into neuron-like cells 4, were stably transfected with wild type or Swedish mutated APP695 and used in the study.
MATERIALS AND METHODS
Preparation of β-amyloid peptide
β-amyloid peptides Aβ1-40 and Aβ1-42 were purchased from Bachem (PA, USA) and prepared as previously described 5. The peptides were dissolved in double-distilled deionized water at a concentration of 1 mM. The stock solutions were 'aged' by incubation at room temperature for 24 h, followed by one freeze-thaw cycle. Aliquots were stored at −70 °C.
Cell culture
Untransfected mouse neuroblastoma N2a cell line (N2a/Wt), N2a cell lines transfected with empty vector pCB6 (N2a/vector), wild-type APP (N2a/APP695) and Swedish mutant form of APP (N2a/APPswe) were kindly gifted by Dr. Hua Xi XU (Rockefeller University, NY, USA) 6. The construction of plasmids was described previously 7 and plasmid pAPPswe harbors the Swedish FAD-specific amino acid substitutions (K595N and M596L). N2a cell lines were grown in 50 % Dulbecco's modified Eagle's medium (DMEM), 50 % OPTI-MEM plus 5 % fetal bovine serum (GibcoBRL, Grand Island, NY, USA) in the presence (stably transfected cells) or absence (N2a/Wt) of 200 mg/L G418 (GibcoBRL, Grand Island, NY, USA).
Cell differentiation
Cell differentiation was induced by serum withdrawal for 12 h. To detect the effect of exogenous Aβ on cell differentiation, N2a/Wt was co-cultured with 5 nM (almost the highest Aβ level detected in the medium of transfected cells) or 50 nM Aβ1-40 or Aβ1-42 (about 10-fold of the highest Aβ level detected in the medium of transfected cells) for 12 h together with serum withdrawal simultaneously. The Aβ concentrations used here were also higher than the physiological concentration of Aβ in blood and cerebrospinal fluid, in which Aβ normally presents in the range of 10-500 pM 8.
Double staining immunofluorescence
Cells were seeded at a concentration of 5×104 cells/well in 24-well plates. After differentiated by serum withdrawal for 12 h, cells were fixed for 10 min at room temperature with 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) and permeabilized for 5 min at room temperature with 0.3% Triton X-100 in PBS. Cells were further incubated with SMI33 (Sternberger Monoclonal Inc., Lutherville, MD, USA), a monoclonal antibody for neurofilament, for 2 h at 37ºC, and then incubated for 1 h at 37 °C with fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Santa Cruz Biotechnology, CA, USA). After washing, cells were probed with monoclonal antibody 6E10 (CalBiochem, San Diego, CA, USA), which recognize residues 1-17 of Aβ, for 2 h at 37 °C, and then incubated for 1 h at 37 °C with Texas Red-conjugated secondary antibodies (Santa Cruz Biotechnology, CA, USA). The cells were visualized by florescence microscopy (Olympus, Japan).
Western blot
Cells were lysed with RIPA buffer containing 50 mM Tris-HCl, pH 7.2, 150 mM sodium chloride, 1% NP-40, 12 mM sodium deoxycholate, 3 mM sodium dodecyl sulfate, 4 mM sodium azide, 0.57 mM phenylmethysulfonyl fluoride and 10 mg/L protease inhibitors (leupeptin, aprotinin and pepstatin). Protein concentrations were determined with BCA Protein Assay Reagent (Pierce, Rockford, USA). Proteins were separated by 10 % SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membrane (Amersham Pharmacia Biotech, Piscataway, NJ, USA). To determine the expression of APP, the blots were probed with the monoclonal antibody anti-APP (GibcoBRL, Grand Island, NY, USA) recognizing the N-terminus of APP, then incubated with ALP-labeled antibody to mouse IgG (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and developed with 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium (BCIP/NBT). α-tubulin was also detected as a control by monoclonal antibody DM1A (Sigma, St Louis, MO, USA).
ELISA
Sandwich enzyme linked immunosorbent assay (ELISA) was performed to measure the level of Aβ in culture medium and in cell lysates. In brief, Aβ40 and Aβ42 were captured with G2-10 (Aβeta, Germany), a monoclonal antibody specific for Aβ40, and G2-11 (Aβeta, Germany), a monoclonal antibody specific for Aβ42, respectively. The presence of Aβ was then detected specifically by antibody biotin-WO2 (Aβeta, Germany), and further developed with HRP-NeutrAvidin (Aβeta, Germany). HRP activity was assayed by color development using 3, 3′, 5, 5′-tetramethylbenzidine (TMB) microwell peroxidase system (Kirkegaard Perry Laboratories, Maryland, USA). The results were expressed as refered to dilutions of standard synthetic control peptides (Sigma, St Louis, MO, USA).
Cell viability assay
Cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetra- zolium bromide (MTT) assay. Cells were plated at a concentration of 5×103 cells/well in 96-well plates with 100 μl medium and grown for 24 h. Cells were rinsed with serum-free media and cultured in the presence or absence of 50 nM Aβ1-40 or Aβ1-42 for 48 h, then incubated with 0.5 mg/ml MTT in PBS for 4 h at 37 °C. After an additional 12 h of incubation with 100 μl of dimethyl sulfoxide at 37 °C, absorbance values at 570 nm were determined with a microtitre plate reader (TECAN, Austria). Experiments were repeated independently for 4∼5 times.
Statistics
The results were presented as means ± SEM. Statistical significance was determined by ANOVA followed by post-hoc test. P values less than 0.05 were considered as significance.
RESULTS
Both APP and Aβ were elevated in APP transfected cell lines
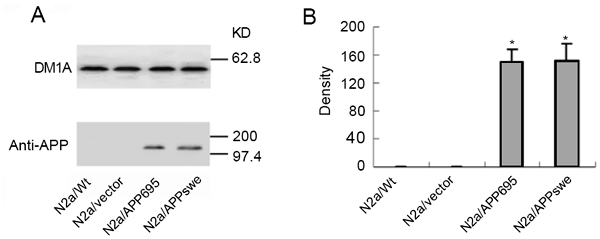
We determined the expression and metabolism of APP. As shown in Fig. 1, APP was present in N2a/APP695 and N2a/APPswe but deprived in N2a/Wt and N2a/vector cells. In addition, the expression levels of APP in N2a/APP695 and N2a/APPswe were comparable. Aβ1-40 and Aβ1-42 in culture medium and in cell lysates were assayed using ELISA. We found that Aβ1-40 both in culture medium and in cell lysates were significantly higher in APP transfected cells, especially in N2a/APPswe (Tab. 1). However, Aβ1-42 could only be detected in medium but was absent in cell lysate of the transfected cells, as well as in either fractions of N2a/Wt and N2a/vector (Tab. 1). The generation of Aβ1-40 and Aβ1-42 in N2a/APPswe were 1∼2 folds higher than those of N2a/APP695 though similar levels of APP were detected in these two cell lines.
Figure 1
The expression of APP in N2a/wt, N2a/vector, N2a/APP695 and N2a/APPswe. (A) APP expression was detected by western blot. DM1A was used to confirm the equal loading of each lane. (B) Statistical analysis of APP expression detected in (A). *presents P < 0.01, compared to N2a/Wt.
Table 1 Different levels of Aβ1-40 and Aβ1-42 detected in medium and cell lysate (n = 3).
Transfection of APP inhibits cell differentiation induced by serum withdrawal
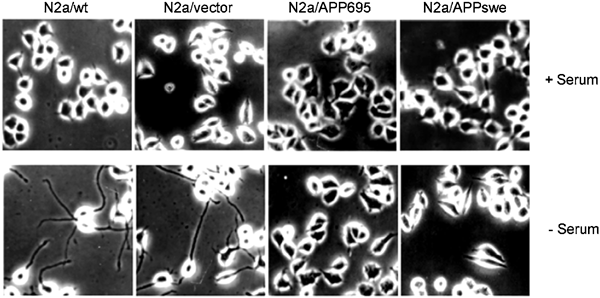
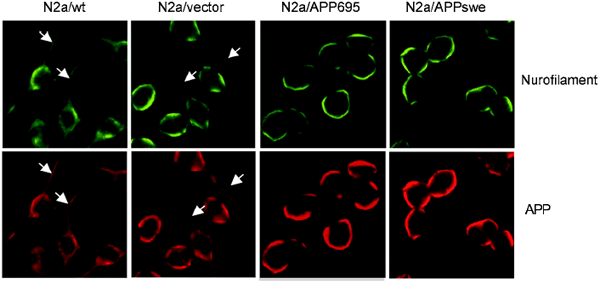
No cell processes or only very short ones were shown both in untransfected and transfected cells cultured in 5 % fetal bovine serum supplemented medium (Fig. 2, higher pannel). After serum withdrawal for 12 h, the majority of the N2a/Wt and N2a/vector extended long and branched neurites, whereas N2a/APP695 and N2a/APPswe displayed similar morphology to that of undifferentiated cells (Fig. 2, lower pannel). The result suggested that APP or Aβ might play a role in inhibiting cell differentiation in N2a/APP695 and N2a/APPswe cells. Immunostaining of APP, Aβ and neurofilament, a specific marker for neuron, was employed to further demonstrate the distribution of APP and its relationship with the outgrowth of cells. It was found that the localization of APP/Aβ and neurofilament in all of the undifferentiated N2a cells was mostly restricted in the cell body (data not shown), suggesting that transfection of APP gene did not change the localization of APP/Aβ. After differentiation induced by serum withdrawal, APP, Aβ and neurofilament in N2a/Wt and N2a/vector were found to be co-localized in both of the cell body and processes (Fig. 3); whereas those in N2a/APP695 and N2a/APPswe were still restricted within the cell body (Fig. 3), further confirming that the overexpression of APP or Aβ could inhibit cell differentiation in N2a/APP695 and N2a/APPswe cells.
Figure 2
Phase contrast microscopy showing inhibition of serum withdrawal induced differentiation. N2a/wt, N2a/vector, N2a/APP695 and N2a/APPswe were grown in medium containing 5% fetal bovine serum, then the cells were induced to differentiate by serum withdrawal for 12 h.
Figure 3
Immunostaining of neurofilament and APP in differentiated N2a/wt, N2a/vector, N2a/APP695 and N2a/APPswe cells.
Inhibition of serum withdrawal-induced cell differentiation could not be reproduced by exogenous addition of Aβ
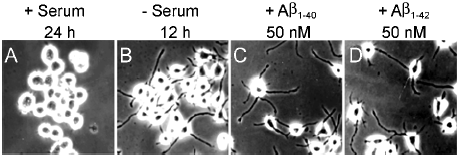
To further elucidate the involvement of Aβ overproduction in the above-mentioned inhibition of cell differentiation, 5 nM (data not shown), 50 nM Aβ1-40 or Aβ1-42 (about 10-times of the expression level generated by the transfected cells) were added into the medium of N2a/Wt at the time of serum withdrawal, respectively. It was found that N2a/Wt still extended long neurites and displayed similar morphology to that of untreated control cells following exposure to Aβ1-40 or Aβ1-42 (Fig. 4), indicating that neither Aβ1−40 nor Aβ1-42, when added exogenously, affected neurite outgrowth at the concentration used.
Figure 4
Effect of exogenous Aβ on the phenotype of differentiated N2a/wt detected by phase contrast micoscopy. (A) N2a/wt cells grown in medium containing 5% fetal bovine serum for 24 h. (B) Cells were induced to differentiate by serum withdrawal for 12 h. (C) 50 nM Aβ1-40 and (D) Aβ1-42 were added to the cultures, respectively.
Both endogenous production and exogenous addition of Aβ do not affect cell viability
To evaluate the toxicity of endogenously overproduced or exogenously added Aβ, cell viability was measured by MTT assay. No significant difference of the OD570 values in N2a/APP695, N2a/APPswe, N2a/vector and N2a/Wt (data not shown) were observed, although Aβ levels in these cell lines varied significantly (see Tab. 1), suggesting that the endogenous overproduction of Aβ did not affect the cell viability. Moreover, the OD570 value in N2a/Wt (1.12 ± 0.18) was not altered even after the cells were incubated with either Aβ1-40 (1.09 ± 0.29) or Aβ1-42 (1.10 ± 0.22) at 50 nM for 48 h.
DISCUSSION
Formation of senile plaques is one of the two major neuropathological features in AD brain. And Aβ, derived from the cleavage of APP, is the major insoluble component of the senile plaques. The presence of dystrophic neurites in the vicinity of senile plaques proposed the possible role of Aβ on neurite growth. The high density of APP in growth cones 9 and increasing expression during neurite outgrowth and synaptogenesis 10 suggest that APP might play a role in neurite outgrowth. However, the involvement of Aβ in this process is not well understood. In the present study, APP transfected cell lines N2a/APP695 and N2a/APPswe were resistant to serum-withdrawal-induced differentiation, while N2a/vector showed the same morphological feature as N2a/Wt, supporting the involvement of overexpression of APP in inhibiting cell differentiation. It was also observed that the transfection of APP gene did not alter the distribution of APP/Aβ, together with the co-localization of APP/Aβ and neurofilament, demonstrating the inhibitory effect of elevated APP/Aβ on serum deprivation-induced differentiation.
Given the fact that both APP and Aβ in N2a/APP695 and N2a/APPswe cells were significantly higher than that of the N2a/Wt and N2a/vector cells, N2a/Wt cells were co-cultured with exogenous Aβ to elucidate whether the overexpression of APP or Aβ was responsible for the inhibition of cell differentiation. Using the same concentration (5 nM) of Aβ generated in transfected cells or using a 10-times higher (50 nM) of the Aβ concentration, we did not observe any obvious inhibitory effect of the exogenous Aβ on neurite outgrowth. The above findings suggest that the overexpression of Aβ had no effect on inhibiting the serum withdrawal-induced cell differentiation. Another possibility lies in that the exogenous Aβ peptide may work in different way with the endogenous one. For example, we have observed that the administration of exogenous Aβ, up to 50 nM, which is a 100 to 5000-times of the normal endogenous Aβ concentration in blood or cerebrospinal fluid 8, showed no toxic effect on cell viability as detected by MTT assay. In addition, it should be noted that, in the previous studies, the concentration of Aβ used was usually hundreds or thousands times of the physiological concentration (in the level of micromole) 11,12,13,14, or different Aβ fragments were employed 11,13, or the cells were directly grew on the slices of AD tissue 15. The inconsistency between the present study and the previous ones might be partially due to the difference of Aβ concentration.
In addition to Aβ, APP was also elevated in the system, suggesting the effect of APP on inhibiting neurite outgrowth in N2a cell. Generally, APP is a marker for cell differentiation, however, previous studies also demonstrated that the overexpression of APP could inhibite neurite outgrowth in N2a cell 16. Its membrane location and its structural similarities with cysteine-rich growth factors suggest that APP might be functioning as a cell surface receptor or growth factor 17, and such proteins, such as G0 protein 18, Fe65 19, and low density lipoprotein receptor-related protein 20, have been reported as its ligands. However, APP is cleaved by different secretases to produce various fragments, whose functions remain largely unknown, except for Aβ. Therefore, no conclusion should be made to the effect of APP on inhibiting cell differentiation untill further studies on these fragments have been done. Furthermore, N2a is a neuroblastoma cell line which has limitation in studying neural differentiation.
Although APP was not detectable by Western blot in N2a/wt and N2a/vector, the expression and processing of endogenous APP in the cell lines were proved by the presence of Aβ in the culture media (see Tab. 1). In addition, the majority of Aβ produced from APP at physiological condition is Aβ1-40, and only about 10% is Aβ1-42/43. It was demonstrated in N2a cells that the production of Aβ1-40 was much higher than that of Aβ1-42, which was beyond the sensitivity of the method to be detected in N2a/Wt and N2a/vector. In accordance with previous studies that Swedish mutant APP could enhance the production of Aβ 1, more Aβ1-40 and Aβ1-42 were detected in N2a/APPswe cells. However, the expression of APP in was found to be comparable to that in N2a/APP695 cells.
Taken together, we have found in the present study that extracellular Aβ could not affect serum withdrawl-induced cell differentiation and cell viability. However, intracellular overproduction and accumulation of Aβ may be harmful to the cells. Therefore, further study on the role of intracellular Aβ is needed.
References
- Rocchi A, Pellegrini S, Siciliano G, Murri L . Causative and susceptibility genes for Alzheimer's disease: a review. Brain Res Bull 2003; 61:1–24.
Article CAS Google Scholar - Turner PR, O'Connor K, Tate WP, Abraham WC . Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol 2003; 70:1–32.
Article CAS Google Scholar - De Strooper B, Annaert W . Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci 2000; 113:1857–70.
Google Scholar - Munoz JP, Alvarez A, Maccioni RB . Increase in the expression of the neuronal cyclin-dependent protein kinase cdk-5 during differentiation of N2A neuroblastoma cells. Neuroreport 2000; 11:2733–8.
Article CAS Google Scholar - Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA . Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem 2002; 80:91–100.
Article CAS Google Scholar - Lo AC, Haass C, Wagner SL, Teplow DB, Sisodia SS . Metabolism of the “Swedish” amyloid precursor protein variant in Madin-Darby canine kidney cells. J Biol Chem 1994; 269:30966–73.
CAS PubMed Google Scholar - Gouras GK, Xu H, Gross RS, et al. Testosterone reduces neuronal secretion of Alzheimer's beta-amyloid peptides. Proc Natl Acad Sci U S A 2000; 97:1202–5.
Article CAS Google Scholar - Seubert P, Vigo-Pelfrey C, Esch F, et al. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature 1992; 359:325–7.
Article CAS Google Scholar - Masliah E, Mallory M, Saitoh T, Amyloid precursor protein is localized in growthing neuritis of neonatal rat brain. Brain Res 1992; 593:323–8.
Article CAS Google Scholar - Kirazov E, Kirazov L, Bigl V, Schliebs R . Ontogenetic changes in protein level of amyloid precursor protein (APP) in growth cones and synaptosomes from rat brain and prenatal expression pattern of APP mRNA isoforms in developing rat embryo. Int J Dev Neurosci 2001; 19:287–96.
Article CAS Google Scholar - Postuma RB, He W, Nunan J, et al. Substrate-bound β-amyloid peptides inhibit cell adhesion and neurite outgrowth in primary neuronal cultures. J Neurochem 2000; 74:1122–30.
Article CAS Google Scholar - Wei W, Wang X, Kusiak JW . Signaling events in amyloid beta-peptide-induced neuronal death and insulin-like growth factor I protection. J Biol Chem, 2002; 277:17649–56.
Article CAS Google Scholar - Dahlgren KN, Manelli AM, Stine WB Jr, et al. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem 2002; 277:32046–53.
Article CAS Google Scholar - Favit A, Grimaldi M, Alkon DL . Prevention of beta-amyloid neurotoxicity by blockade of the ubiquitin-proteasome proteolytic pathway. J Neurochem 2000; 75:1258–63.
Article CAS Google Scholar - Tolar M, Scott SA, Crutcher KA . Sympathetic neurite outgrowth is greater on plaque-poor vs. plaque-rich regions of Alzheimer's disease cryostat sections. Brain Res 1998; 787:49–58.
Article CAS Google Scholar - Lee JP, Chang KA, Kim HS, et al. APP carboxyl-terminal fragment without or with abeta domain equally induces cytotoxicity in differentiated PC12 cells and cortical neurons. J Neurosci Res 2000; 60:565–70.
Article CAS Google Scholar - Rossjohn J, Cappai R, Feil SC, et al. Crystal structure of the N-terminal,growth factor-like domain of Alzheimer amyloid precursor protein. Nature Struct Bio 1999; 6:327–31.
Article CAS Google Scholar - Nishimoto I, Okamoto T, Matsuura Y, et al. Alzheimer amyloid protein precursor complexes with brain GTP-binding protein G0. Nature, 1993; 362:75–9.
Article CAS Google Scholar - Zhao Q, Lee FS . The transcriptional activity of the APP intracellular domain-Fe65 complex is inhibited by activation of the NF-κB pathway. Biochemistry; 2003, 42:3627–34.
Article CAS Google Scholar - Rebeck GW, Moir RD, Mui S, et al. Association of membrane-bound amyloid precursor protein APP with the apolipoprotein E receptor LRP. Brain Res Mol Brain Res 2001, 87:238–45.
Article CAS Google Scholar
Acknowledgements
We thank Dr. Cheng Xin Gong from New York State Institute for Basic research for critical reading of the manuscript. This work was supported by grants from the National Natural Science Foundation of China (No. 30100057, 30170221, 30328007, 39925012), and Science and Technology Committee of China (No. G1999054007).
Author information
Author notes
- Yi Peng WANG and Ze Fen WANG: These authors contributed equally to this work.
Authors and Affiliations
- 1Department of Pathophysiology, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, China
Yi Peng WANG, Ze Fen WANG, Ying Chun ZHANG, Qing TIAN & Jian Zhi WANG
Authors
- Yi Peng WANG
You can also search for this author inPubMed Google Scholar - Ze Fen WANG
You can also search for this author inPubMed Google Scholar - Ying Chun ZHANG
You can also search for this author inPubMed Google Scholar - Qing TIAN
You can also search for this author inPubMed Google Scholar - Jian Zhi WANG
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toJian Zhi WANG.
Rights and permissions
About this article
Cite this article
WANG, Y., WANG, Z., ZHANG, Y. et al. Effect of amyloid peptides on serum withdrawal-induced cell differentiation and cell viability.Cell Res 14, 467–472 (2004). https://doi.org/10.1038/sj.cr.7290249
- Received: 05 January 2003
- Revised: 07 September 2004
- Accepted: 15 September 2004
- Issue Date: 01 December 2004
- DOI: https://doi.org/10.1038/sj.cr.7290249