Imatinib dose increase up to 1200 mg daily can induce new durable complete cytogenetic remissions in relapsed Ph+ chronic myeloid leukemia patients (original) (raw)
- Spotlight Correspondence
- Published: 25 August 2005
- V Magistroni1,
- F Andreoni1,
- A Franceschino2,
- L Tornaghi3,
- M Varella-Garcia4,
- S Bungaro5,
- F Colnaghi5,
- G Corneo3,
- E M Pogliani3 &
- …
- C Gambacorti-Passerini1,3,6
Leukemia volume 19, pages 1985–1987 (2005)Cite this article
- 825 Accesses
- 8 Citations
- Metrics details
TO THE EDITOR
Resistance to imatinib can arise through point mutations in the ABL kinase domain or BCR-ABL gene amplification (reviewed in Gambacorti-Passerini et al1), by activation of the efflux pumps2 or by other unknown mechanisms. Resistance to imatinib frequently develops in patients with advanced disease. In this setting, resistance develops within few months of treatment and imatinib dose increase has a rather limited impact.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
Figure 1
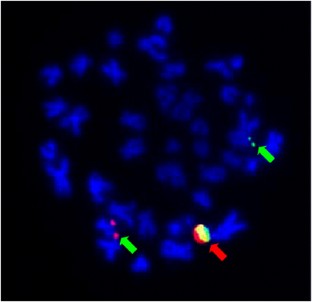
References
- Gambacorti-Passerini CB, Gunby RH, Piazza R, Galietta A, Rostagno R, Scapozza L . Molecular mechanisms of resistance to imatinib in Philadelphia-chromosome-positive leukaemias. Lancet Oncol 2003; 4: 75–85.
Article Google Scholar - Mahon FX, Belloc F, Lagarde V, Chollet C, Moreau-Gaudry F, Reiffers J et al. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood 2003; 101: 2368–2373.
Article CAS Google Scholar - Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med 2002; 346: 645–652.
Article CAS Google Scholar - Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med 2003; 349: 1423–1432.
Article CAS Google Scholar - Kantarjian H, Talpaz M, O’Brien S, Garcia-Manero G, Verstovsek S, Giles F et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood 2004; 103: 2873–2878.
Article CAS Google Scholar - Kantarjian HM, Talpaz M, O’Brien S, Giles F, Garcia-Manero G, Faderl S et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood 2003; 101: 473–475.
Article CAS Google Scholar - Zonder JA, Pemberton P, Brandt H, Mohamed AN, Schiffer CA . The effect of dose increase of imatinib mesylate in patients with chronic or accelerated phase chronic myelogenous leukemia with inadequate hematologic or cytogenetic response to initial treatment. Clin Cancer Res 2003; 9: 2092–2097.
CAS PubMed Google Scholar - Marin D, Goldman JM, Olavarria E, Apperley JF . Transient benefit only from increasing the imatinib dose in CML patients who do not achieve complete cytogenetic remissions on conventional doses. Blood 2003; 102: 2702–2703; author reply 2703–2704.
Article CAS Google Scholar - Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001; 344: 1031–1037.
Article CAS Google Scholar - Piazza RG, Magistroni V, Gasser M, Andreoni F, Galietta A, Scapozza L et al. Evidence for D276G and L364I Bcr-Abl mutations in Ph+ leukaemic cells obtained from patients resistant to imatinib. Leukemia 2005; 19: 132–134.
Article CAS Google Scholar - Warmuth M, Simon N, Mitina O, Mathes R, Fabbro D, Manley PW et al. Dual-specific Src and Abl kinase inhibitors, PP1 and CGP76030, inhibit growth and survival of cells expressing imatinib mesylate-resistant Bcr-Abl kinases. Blood 2003; 101: 664–672.
Article CAS Google Scholar
Acknowledgements
We thank Edoardo Marchesi and Silvia Portincasa for editorial and technical support. Grants: This work was funded by AIRC, EU (LSHB-CT-2004-503467), Ric. Fin. 2003, MIUR-COFIN and PRIN programs (2003, 2004), CNR, CIHR, NCIC, CFI.
Author information
Authors and Affiliations
- Department of Experimental Oncology, National Cancer Institute, Milan, Italy
R G Piazza, V Magistroni, F Andreoni & C Gambacorti-Passerini - University of Milano Bicocca, S Gerardo Hospital, Monza, Italy
R G Piazza & A Franceschino - Section of Hematology, S Gerardo Hospital, Monza, Italy
L Tornaghi, G Corneo, E M Pogliani & C Gambacorti-Passerini - Cancer Center, University of Colorado Health Sciences Center, Denver, CO, USA
M Varella-Garcia - M Tettamanti Research Center, Pediatric Clinic, University of Milano Bicocca, Monza, Milan, Italy
S Bungaro & F Colnaghi - Department of Oncology, McGill University, Montreal, Canada
C Gambacorti-Passerini
Authors
- R G Piazza
- V Magistroni
- F Andreoni
- A Franceschino
- L Tornaghi
- M Varella-Garcia
- S Bungaro
- F Colnaghi
- G Corneo
- E M Pogliani
- C Gambacorti-Passerini
Corresponding author
Correspondence toC Gambacorti-Passerini.
Rights and permissions
About this article
Cite this article
Piazza, R., Magistroni, V., Andreoni, F. et al. Imatinib dose increase up to 1200 mg daily can induce new durable complete cytogenetic remissions in relapsed Ph+ chronic myeloid leukemia patients.Leukemia 19, 1985–1987 (2005). https://doi.org/10.1038/sj.leu.2403928
- Received: 24 March 2005
- Accepted: 26 July 2005
- Published: 25 August 2005
- Issue date: 01 November 2005
- DOI: https://doi.org/10.1038/sj.leu.2403928