Evidence that many of the DISC1 isoforms in C57BL/6J mice are also expressed in 129S6/SvEv mice (original) (raw)
- Letter to the Editor
- Published: 26 September 2007
- J Chen3,
- S Taya4,
- W Li5,6,7,8,
- J K Millar9,
- Y Xu1,10,
- S J Clapcote11,
- C Hookway1,2,
- M Morita1,2,
- A Kamiya1,2,
- T Tomoda12,
- B K Lipska3,
- J C Roder11,
- M Pletnikov1,10,
- D Porteous9,
- A J Silva5,6,7,8,
- T D Cannon6,7,8,13,
- K Kaibuchi4,
- N J Brandon14,
- D R Weinberger3 &
- …
- A Sawa1,2,15
Molecular Psychiatry volume 12, pages 897–899 (2007)Cite this article
- 1246 Accesses
- 47 Citations
- 3 Altmetric
- Metrics details
Recently, Koike et al.1 identified a 25-bp deletion in a coding exon of the Disrupted-In-Schizophrenia (DISC1) gene in the 129S6/SvEv strain, which was also confirmed at the genomic level for all extant 129 mouse inbred substrains.2 This mutation could interfere with the production of the full-length DISC1 protein. When the 129S6/SvEv-derived DISC1 gene was transferred to C57BL/6J genetic background, the resultant mice displayed a subtle behavioral abnormality in working memory, without any other major deficits in behavior and brain anatomy.1 Several lines of evidence from animal models with RNA interference to DISC1, as well as studies in patient-derived lymphoblasts, suggest that loss of DISC1 function may be involved in the abnormalities underlying the pathophysiology of major mental conditions.3 The minor behavioral abnormality resulting from the 25-bp deletion is in contrast to the results of three independent groups who have generated partial loss-of-function models by expressing dominant-negative DISC1 constructs and obtained substantial changes in behavior, including deficits in prepulse inhibition, latent inhibition and working memory.4 Moreover, the absence of obvious anatomical changes in the mutant mice1 conflicts with cellular models based on the knockdown of DISC1 or expression of dominant-negative DISC1, which disrupts developmental processes critical for normal cortical architecture.5 Thus, if the 25-bp deletion completely abolishes the full-length DISC1 protein that is crucial for proper neurodevelopment, why are there such small phenotypic changes in mice with this mutation?
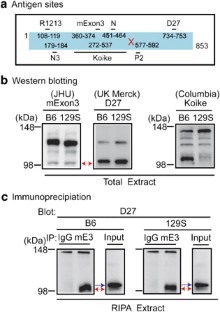
Thus, whereas we were able to confirm a differential immunoreactivity between 129S6/SvEv and C57BL/6J strains with the Koike et al. antibody, we did not find substantial detectable differences in DISC1 proteins between 129S6/SvEv and C57BL/6J strains by western blotting with most of currently available antibodies against DISC1. DISC1 has multiple isoforms at both mRNA and protein levels.7, 8 In addition to 13 coding exons for the full-length DISC1, a bioinformatic approach suggested that possible exons exist inside the genomic region of the DISC1 gene, such as those reported in the NCBI database (accession no.: XP_001002896). The antibodies used in this study, although their specificities have been confirmed in each laboratory (also shown in Supplementary Figures 2 and 3), depict different patterns of DISC1 bands in the western blotting, which may reflect the complexity of the molecular entity of DISC1. Immunoprecipitation with the mExon3 antibody followed by detection of the precipitates with the D27 antibody indicates that these two independent antibodies detect the same DISC1 molecule at 100 kDa, but an additional DISC1 band is detected by D27 at 105 kDa (Figure 1c). Therefore, this suggests that the antibody generated by Koike et al.1 might specifically detect a unique isoform of DISC1 at 100 kDa that is lost by the 25-bp deletion. Selective detection of this unique isoform in the immunoprecipitates with immunoglobulin G (IgG) from brain extracts of C57BL/6J, but not from those of 129S6/SvEv, suggests that this isoform has a specific binding affinity to IgG (Supplementary Figure 4). To resolve such complexity, including the possibility of more than one isoform of similar size, we propose to utilize two-dimensional gel electrophoresis combined with mass spectrometry for further studies. Of note, Arguello and Gogos also disclosed in their preliminary study that they obtained persistent isoforms of DISC1 in C57BL/6J mice that were transferred with the 25-bp deletion.9 Similar molecular complexity also occurs in another susceptibility gene for schizophrenia, neuregulin-1.10 The etiology of major mental disorders comprises a combination of changes in genetic factors together with environmental factors. Subtle disturbance of an isoform(s) of susceptibility gene products may well account for the etiology of these disorders. In this sense, the mice reported by Koike et al.1 would be useful in studies of major mental illnesses.
This is a preview of subscription content, access via your institution
Relevant articles
Open Access articles citing this article.
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
Figure 1
Accession codes
Accessions
GenBank/EMBL/DDBJ
References
- Koike H, Arguello PA, Kvajo M, Karayiorgou M, Gogos JA . Proc Natl Acad Sci USA 2006; 103: 3693–3697.
Article CAS Google Scholar - Clapcote SJ, Roder JC . Genetics 2006; 173: 2407–2410.
Article CAS Google Scholar - Sawa A, Snyder SH . Science 2005; 310: 1128–1129.
Article CAS Google Scholar - O'Tuathaigh CM, Babovic D, O'Meara G, Clifford JJ, Croke DT, Waddington JL . Neurosci Biobehav Rev 2007; 31: 60–78.
Article CAS Google Scholar - Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y et al. Nat Cell Biol 2005; 7: 1167–1178.
Article Google Scholar - Schurov IL, Handford EJ, Brandon NJ, Whiting PJ . Mol Psychiatry 2004; 9: 1100–1110.
Article CAS Google Scholar - Ishizuka K, Paek M, Kamiya A, Sawa A . Biol Psychiatry 2006; 59: 1189–1197.
Article CAS Google Scholar - Taylor MS, Devon RS, Millar JK, Porteous DJ . Genomics 2003; 81: 67–77.
Article CAS Google Scholar - Arguello PA, Gogos JA . Neuron 2006; 52: 179–196.
Article CAS Google Scholar - Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R et al. Proc Natl Acad Sci USA 2006; 103: 6747–6752.
Article CAS Google Scholar
Author information
Authors and Affiliations
- Departments of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, USA
K Ishizuka, Y Xu, C Hookway, M Morita, A Kamiya, M Pletnikov & A Sawa - Program in Molecular Psychiatry, Johns Hopkins University School of Medicine, Baltimore, MD, USA
K Ishizuka, C Hookway, M Morita, A Kamiya & A Sawa - Genes, Cognition and Psychosis Program, Clinical Brain Disorders Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, USA
J Chen, B K Lipska & D R Weinberger - Department of Cell Pharmacology, Graduate School of Medicine, Nagoya University, Nagoya, Aichi, Japan
S Taya & K Kaibuchi - Department of Neurobiology, University of California, Los Angeles, CA, USA
W Li & A J Silva - Department of Psychology, University of California, Los Angeles, CA, USA
W Li, A J Silva & T D Cannon - Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, CA, USA
W Li, A J Silva & T D Cannon - Brain Research Institute, University of California, Los Angeles, CA, USA
W Li, A J Silva & T D Cannon - Medical Genetics Section, Molecular Medicine Centre, University of Edinburgh, Edinburgh, UK
J K Millar & D Porteous - Division of Neurobiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Y Xu & M Pletnikov - Mount Sinai Hospital Research Institute, Toronto, ON, Canada
S J Clapcote & J C Roder - Division of Neurosciences, Beckman Research Institute of City of Hope, Los Angeles, CA, USA
T Tomoda - Department of Human Genetics, University of California, Los Angeles, CA, USA
T D Cannon - Schizophrenia and Bipolar Department, Wyeth Discovery Neuroscience, Princeton, NJ, USA
N J Brandon - Department of Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA
A Sawa
Authors
- K Ishizuka
- J Chen
- S Taya
- W Li
- J K Millar
- Y Xu
- S J Clapcote
- C Hookway
- M Morita
- A Kamiya
- T Tomoda
- B K Lipska
- J C Roder
- M Pletnikov
- D Porteous
- A J Silva
- T D Cannon
- K Kaibuchi
- N J Brandon
- D R Weinberger
- A Sawa
Corresponding author
Correspondence toA Sawa.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary information
Rights and permissions
About this article
Cite this article
Ishizuka, K., Chen, J., Taya, S. et al. Evidence that many of the DISC1 isoforms in C57BL/6J mice are also expressed in 129S6/SvEv mice.Mol Psychiatry 12, 897–899 (2007). https://doi.org/10.1038/sj.mp.4002024
- Published: 26 September 2007
- Issue Date: October 2007
- DOI: https://doi.org/10.1038/sj.mp.4002024