GABRA2 Alleles Moderate the Subjective Effects of Alcohol, Which are Attenuated by Finasteride (original) (raw)
INTRODUCTION
Two genome-wide scans in humans provided evidence of linkage of alcohol dependence to a region of chromosome 4p that includes a cluster of four genes encoding GABAA receptor subunits (Reich et al, 1998; Long et al, 1998). Edenberg et al (2004), by fine mapping this region, found that numerous single-nucleotide polymorphisms (SNPs) in the gene encoding the GABAA _α_-2 subunit (GABRA2), but not in other members of the gene cluster, were associated with alcohol dependence. In addition, all 3-SNP haplotypes examined in the 3′ region of GABRA2 were significantly associated with alcohol dependence (Edenberg et al, 2004). Covault et al (2004) replicated this haplotypic association in a region of the gene that overlaps the region identified by Edenberg et al (2004). These findings, together with evidence that GABAA receptors mediate several behavioral effects of alcohol (Grobin et al, 1998; Davies, 2003), underscore the potential contribution of variation at GABRA2 to the risk for alcohol dependence.
As alcohol elevates plasma and brain concentrations of GABAergic neuroactive steroids, including 3_α_–5_α_-THP (allopregnanolone or ALLO), and 3_α_–5_α_-THDOC (allotetrahydrodeoxycorticosterone), it has been hypothesized that these compounds may contribute to specific behavioral actions of ethanol by modulating GABAA receptor function (Morrow et al, 1999; Barbaccia et al, 1999; VanDoren et al, 2000). Two recent reports in humans suggest that acute alcohol exposure stimulates neuroactive steroid production (Torres and Ortega, 2003, 2004). Compared to adolescents seen for nonalcohol-related reasons, plasma levels of ALLO and progesterone were elevated in both male and female adolescents presenting to the emergency department with acute alcohol intoxication. The effects were seen among females during both the luteal and follicular phases of the ovarian cycle. Studies in chronic drinkers have shown 40–50% lower plasma concentrations of ALLO and THDOC in the first week of abstinence, with normalization during the subsequent 3 weeks (Romeo et al, 1996, 2000). These studies suggest a direct link between increased neuroactive steroid levels and acute alcohol intoxication in humans, with chronic intoxication producing the opposite effect, findings that parallel those described in the animal literature (Barbaccia et al, 1999; VanDoren et al, 2000; Khisti et al, 2003; Finn et al, 2004).
The present study examined the moderating effects of GABRA2 alleles and high-dose finasteride on subjective and physiological effects of a standardized dose of alcohol in healthy social drinkers. Finasteride, a 5-α steroid reductase (5AR) inhibitor that limits the conversion of testosterone to dihydrotestosterone, is FDA approved for the treatment of benign prostatic hypertrophy. Finasteride also reduces the metabolism of progesterone to the 5_α_-reduced neuroactive steroids ALLO and THDOC. Based on these considerations, we hypothesized that GABRA2 alleles would moderate the subjective effects of alcohol measured during the ascending limb of the breath alcohol concentration (BrAC) curve and that finasteride would attenuate the subjective response to alcohol. We focused on the ascending limb of the BrAC curve, since it is commonly associated with the stimulating effects of alcohol (de Wit et al, 1987), which are in turn associated with alcohol preference (de Wit et al, 1987; Chutuape and de Wit, 1994) and heavy drinking (Holdstock et al, 2000).
METHODS
Subjects
In all, 27 subjects (15 men) were recruited from the greater Hartford, CT area by advertisement and were paid for their participation. After an initial telephone interview, a psychiatric history was obtained using the Structured Clinical Interview for DSM-IV (First et al, 1995). A 90-day timeline follow-back interview (Sobell and Sobell, 1992) was used to quantify recent alcohol and drug use. Subjects underwent a medical history and physical examination and routine laboratory tests (including complete blood count, liver and renal function tests, blood glucose and electrolyte concentration, and among female subjects, a serum pregnancy test). All subjects gave written informed consent to participate in the protocol, as approved by the University of Connecticut Health Center Institutional Review Board.
Subjects were included in the study if they reported moderate social drinking (ie a minimum of three drinks per week and at least three drinks on one occasion in the past month) and had a body mass index of 18.5–30 kg/m2. They were excluded if they ever met the criteria for DSM-IV (American Psychiatric Association, 1994) substance abuse or dependence or another major psychiatric disorder, had evidence of liver dysfunction, were using benzodiazepines or other psychotropic medications, were smokers, or were pregnant or nursing. Women were included only if they reported having regular menstrual cycles and were not using oral contraceptives or other hormones and had no history of endocrine or reproductive abnormalities.
Study Design
The study involved a balanced design, in which each subject served as his or her own control, with two experimental sessions, separated by 1 month. At 24 h prior to each laboratory session, and 2 h before the first drink, subjects were pretreated with high-dose finasteride or matching placebo, under double-blind conditions. The order of the sessions was randomized. Among women, the experimental sessions were held during the follicular phase of two consecutive menstrual cycles (based on a 28-day menstrual cycle). The sessions were scheduled 5–9 days following the onset of menstruation, with the menstrual cycle phase determined by counting the days from the onset of menstruation. To ensure comparability, the laboratory sessions were separated by 1 month for men also.
Subjective Effects
Subjective effects were measured using the following self-report questionnaires:
Alcohol Sensation Scale (SS; Maisto et al, 1980) consists of 26 items that are divided into six subscales measuring somatic sensations produced by alcohol, particularly on the ascending limb of the blood alcohol concentration curve: the Central-Stimulant Subscale measures sensations associated with effects on the brain (four items), the Dynamic-Peripheral Subscale measures sensations associated with excitation, including increased breathing and heart rate (three items), the Warmth-Glow Subscale measures blushing sensations (three items), the Anesthetic Subscale measures sensations associated with loss of feeling or decreased sensitivity to feeling (nine items), the Gastrointestinal Subscale measures sensations felt in the stomach (four items), and the Impaired-Function Subscale measures perceived changes in psychomotor performance (three items).
Biphasic Alcohol Effects Scale (BAES; Martin et al, 1993) is a 14-item unipolar adjective rating scale designed to measure both the stimulant and sedative effects of alcohol. Subjects rated the adjectives on a scale of 0 (not at all) to 10 (extremely). We used the items from the Stimulation Subscale (seven items), which has been experimentally associated with increasing BrAC (de Wit et al, 1987; Martin et al, 1993).
Drug Effects Questionnaire (DEQ; Holdstock and de Wit, 1998) consists of four items that measure current drug effects that were adapted to measure alcohol effects: ‘feel alcohol’, ‘feel high’, ‘like alcohol’, and ‘want more alcohol’. Subjects indicated on a 100-mm line the extent to which they endorsed each statement, with anchors from ‘not at all’ on the left end of the scale to ‘extremely’ on the right end of the scale.
We also measured BrAC, blood pressure, and heart rate (while seated).
Laboratory Procedures
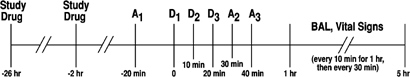
To maximize the antagonism of 5AR activity (Ohtawa et al, 1991), subjects received either finasteride 100 mg or placebo in identical capsules 24 h prior to each laboratory session (see Figure 1) and were instructed to avoid alcohol, nicotine, or other drugs (except caffeine) until after completion of the laboratory session. Subjects arrived at the laboratory at 0800, having been instructed to fast from solid foods for 8 h prior to the session. At 2 h prior to the first dose of alcohol (ie time (t)=−120 min), a second dose of finasteride 100 mg or placebo was administered, followed by a light breakfast. An alcoholic drink, which was divided into three equal portions, was served at _t_=0, 10, and 20 min, with subjects having 10 min to consume each drink. The dosage of alcohol was chosen to ensure a measurable subjective effect (Holdstock and de Wit, 1998; Swift et al, 1994), with an adjustment based on sex differences in alcohol pharmacokinetics (Watson, 1989): men received 0.8 g/kg and women received 0.7 g/kg.
Figure 1
Timeline of events. A1=1st Assessment Battery (SS, BAES, and DEQ); A2=2nd Assessment Battery; A3=3rd Assessment Battery; D1=1st Alcoholic Drink; D2=2nd Alcoholic Drink; D3=3rd Alcoholic Drink; BrAC: Breath Alcohol Concentration. AA (_n_=7), G-allele carriers (_n_=20).
Subjects completed the SS, BAES, and DEQ 20 min before ingesting the first alcoholic drink, and then along the ascending limb of the BrAC curve, that is, at 10 and 20 min after the third drink was consumed (ie 30 and 40 min after the onset of alcohol consumption). BrAC and physiological measures (heart rate and blood pressure) were recorded at consecutive 10-min intervals after consumption of the first drink.
Genotyping
DNA was purified from venous blood samples using the PureGene kit (GentraSystems, Minneapolis, MN). The rs279858 SNP, which is a synonymous A-to-G substitution (K132K) in exon 4 of the GABRA2 gene, was genotyped using the TaqMan 5′ nuclease assay with ABI Assay-on-Demand #2073557. This SNP lies in the middle of the haplotype block in GABRA2 that was observed to be associated to alcohol dependence by Edenberg et al (2004) in a family-based study and by Covault et al (2004) in a case–control study. Although the G-allele of this SNP was less common overall, it was over-represented among alcohol-dependent individuals (Covault et al, 2004), and may therefore be considered a potential ‘risk’ allele. Conversely, since the A-allele, although more common overall, was less frequently transmitted (Edenberg et al, 2004) among alcoholics or was over-represented among healthy controls (Covault et al, 2004), it may be considered a ‘protective’ allele.
Data Analysis
The data were examined using a full factorial, repeated measures analysis of variance model. Within-subject variables were Genotype (A-allele homozygotes (_n_=7) vs G-allele carriers (AG, _n_=18 or GG, _n_=2)), Study Drug (finasteride vs placebo), and Time (−20 min and 30 and 40 min after the first alcoholic drink). Significant interactions were decomposed to reveal the specific nature of the effects. Statistical significance was defined as p<0.05.
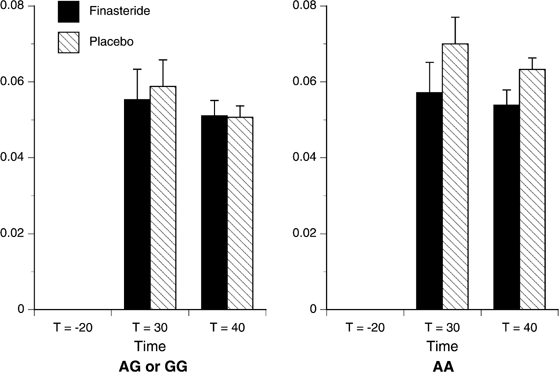
The dependent measures examined in the analyses were the six SS subscales scores, the BAES Stimulation Subscale score and the four DEQ items. BrAC did not differ by Study Drug or Genotype (Figure 2), nor was it significant as a time-varying covariate, so it was removed from the analysis, with no substantial change in the observed effects. Similarly, when included as a between-subjects factor, the order of laboratory sessions had no effect on the results, and so it too was removed from the analysis. When the assumption of sphericity was not met (as determined by Mauchley's W), we used the Greenhouse–Geisser adjusted F test for within-subjects effects and within-/between-subjects interaction effects.
Figure 2
Mean (SEM) BrAC by Study Drug and Genotype. Significant effect of Time (p<0.001), BrAC did not differ by Study Drug or Genotype. AA (_n_=7), G-allele carriers (_n_=20).
In view of the potential for population differences to confound the effects of genotype, all analyses were repeated including only non-Hispanic European-American subjects, the most common population subgroup.
RESULTS
Subjects
Of the 27 participants, 15 (56%) were male, 22 (82%) were European-American, and five (18%) were Hispanic (all of whom were heterozygous for the SNP examined). The average age of the subjects was 29.2 years (SD=6.7). The majority of subjects (74%) were college graduates. Most subjects were single (56%), and were either working (_n_=12) or attending graduate school (_n_=14). In total, 14 subjects received finasteride (10 G-allele carriers) and 13 subjects (10 G-allele carriers) received placebo prior to the first laboratory session. As shown in Table 1, the genotype groups did not differ on sociodemographic or clinical characteristics, including recent drinking history (all _p_-values >0.10).
Table 1 Demographic and Drinking Features by GABRA2 Genotypea
Laboratory Sessions
Physiological and adverse effects
As shown in Figure 2, there was a main effect of time on BrAC (F(2,46)=135.24, p<0.001), with an increase in BrAC from _t_=−20 to 30 min, followed by a decrease from _t_=30 to 40 min. There were also main effects of time on heart rate (F(2,46)=15.05, p<0.001), and both systolic (F(2,46)=9.53, p<0.001) and diastolic (F(2,46)=7.74, _p_=0.001) blood pressure. Alcohol produced increases in all three physiological measures from _t_=−20 to 30 min, followed by a decrease from _t_=30 to 40 min for the systolic and diastolic blood pressures, while remaining stable for heart rate. There were no statistically significant main or interactive effects of Genotype or Study Drug on any of these measures.
A total of 17 subjects (63%) reported 25 adverse events during or after the laboratory session in which finasteride was administered, and 15 subjects (56%) reported 20 adverse events during or after placebo administration. For both sessions, the adverse event reported most frequently was headache (finasteride=50.0%, placebo=47.6%), followed by nausea (finasteride=4.2%, placebo=9.5%). There was no Study Drug effect on either the proportion of subjects reporting adverse events (McNemar _χ_2 (1 df)=0.06, _p_=0.80) or the number of adverse events reported (t(23)=0.87, _p_=0.40).
Subjective Effects
Alcohol SS
All six of the SS subscale scores showed a significant effect of Time, with increased scores following alcohol administration. There were also Genotype and/or Study Drug effects on four of the subscale scores.
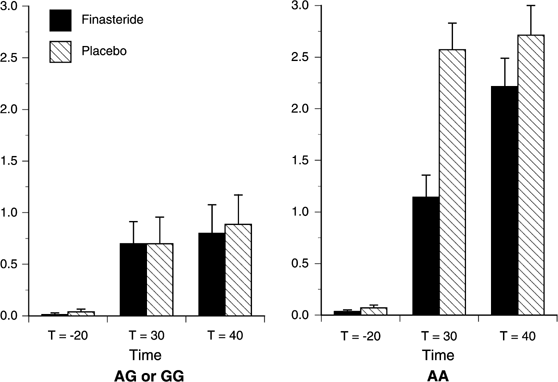
Central-Stimulant Subscale
All main and interactive effects on this subscale score were statistically significant: Time (F(2,50)=29.68, p<0.001), Genotype (F(1,25)=10.61, _p_=0.003), Study Drug (F(1,25)=8.25, _p_=0.008), Time × Genotype (F(2,50)=6.97, _p_=0.002), Time × Study Drug (F(2,50)=6.34, _p_=0.004), Genotype × Study Drug (F(1,25)=6.56, _p_=0.017), and Time × Genotype × Study Drug (F(2,50)=7.11, _p_=0.002). As shown in Figure 3, the increase in Central-Stimulant Subscale scores following alcohol administration was greater among the A-allele homozygotes than the G-allele carriers. However, it should be noted that G-allele carriers showed a significant response to alcohol on this measure (F(2,38)=8.32, _p_=0.004). Overall, pretreatment with finasteride reduced scores on this measure relative to the placebo session. There was no effect of Genotype prior to alcohol administration, but a significantly greater increase in scores among A-allele homozygotes at _t_=30 min (F(1,25)=7.28, _p_=0.012), but not at _t_=40 min (F(1,25)=1.63, _p_=0.213). Decomposition of the three-way interaction revealed only that finasteride significantly attenuated Central-Stimulant Subscale scores for the A-allele homozygotes compared to the G-allele carriers at _t_=30 min (F(1,25)=10.87, _p_=0.003), an effect that was no longer significant at _t_=40 min (F(1,25)=1.27, _p_=0.27).
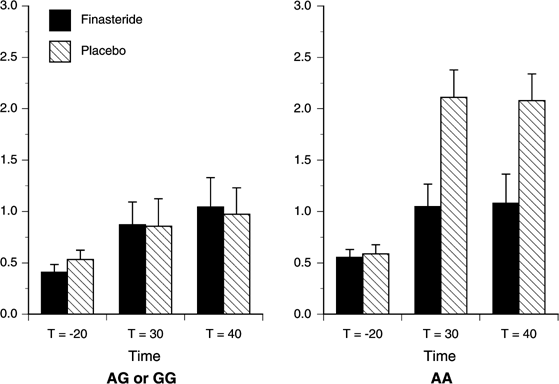
Figure 3
Estimated mean (SEM) for the Central-Stimulant Subscale score by Study Drug and Genotype. There were significant effects of Time (p<0.001), Genotype (_p_=0.003), Study Drug (_p_=0.008), Time × Genotype (_p_=0.002), Time × Study Drug (_p_=0.004), Genotype × Study Drug (_p_=0.017), and Time × Genotype × Study Drug (_p_=0.002). Finasteride significantly attenuated Central-Stimulant Subscale scores for the A-allele homozygotes compared to the G-allele carriers at _t_=30 min (_p_=0.003). AA (_n_=7), G-allele carriers (_n_=20).
Dynamic-Peripheral Subscale
There were significant effects of Time (F(2,50)=9.28, _p_=0.002), Study Drug (F(1,25)=4.41, _p_=0.046), and Time × Study Drug (F(2,50)=3.99, _p_=0.037) on this subscale score. As shown in Figure 4, finasteride attenuated the increase in scores on this subscale following alcohol administration. The effect was seen at _t_=30 min (F(1,25)=6.11, _p_=0.021), but was no longer significant at _t_=40 min (F(1,25)=3.28, _p_=0.082).
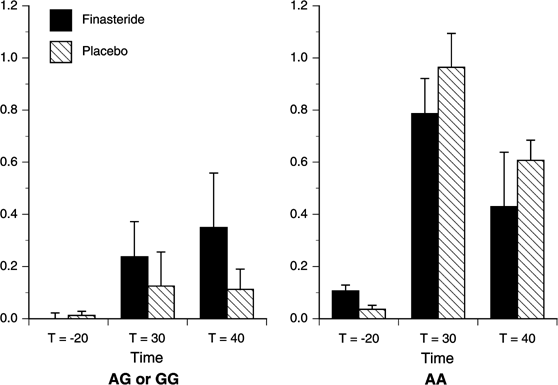
Figure 4
Estimated mean (SEM) for the Dynamic-Peripheral Subscale Score of the SS by Study Drug and Genotype. There were significant effects of Time (_p_=0.002), Study Drug (_p_=0.046), and Time × Study Drug (_p_=0.037). Finasteride significantly attenuated the increase in Dynamic-Peripheral Subscale scores at _t_=30 min (_p_=0.021). AA (_n_=7), G-allele carriers (_n_=20).
Anesthetic Subscale
There were significant effects of Time (F(2,50)=9.27, _p_=0.002), Study Drug (F(1,25)=4.57, _p_=0.043), Genotype × Study Drug (F(1,25)=4.27, _p_=0.049), and Time × Genotype × Study Drug (F(2,50)=4.78, _p_=0.024) on this subscale score. As shown in Figure 5, finasteride attenuated the increase in scores resulting from alcohol administration. The effects of finasteride were significant among A-allele homozygotes at _t_=30 min (F(1,25)=6.05, _p_=0.021), but were reduced to the level of a nonsignificant trend at _t_=40 min (F(1,25)=3.74, _p_=0.065).
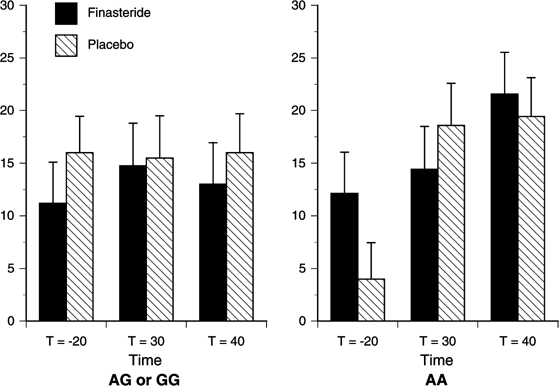
Figure 5
Estimated mean (SEM) for the Anesthetic Subscale Score of the SS by Study Drug and Genotype. There were significant effects of Time (_p_=0.002), Study Drug (_p_=0.043), Genotype × Study Drug (_p_=0.049), and Time × Genotype × Study Drug (_p_=0.024). Finasteride significantly attenuated the increase in Anesthetic Subscale scores for the A-allele homozygotes compared to the G-allele carriers at _t_=30 min (_p_=0.021). AA (_n_=7), G-allele carriers (_n_=20).
Gastrointestinal Subscale
There were significant effects of Time (F(2,50)=12.76, p<0.001), Genotype (F(1,25)=7.18, _p_=0.013), and Time × Genotype (F(2,50)=5.18, _p_=0.009) on this subscale score. As shown in Figure 6, the increase in scores following alcohol administration was greater among A-allele homozygotes than among G-allele carriers. At _t_=30 min A-allele homozygotes had higher scores on this measure (F(1,25)=10.94, _p_=0.003), an effect that was no longer significant at _t_=40 min (F(1,25)=1.83, _p_=0.19).
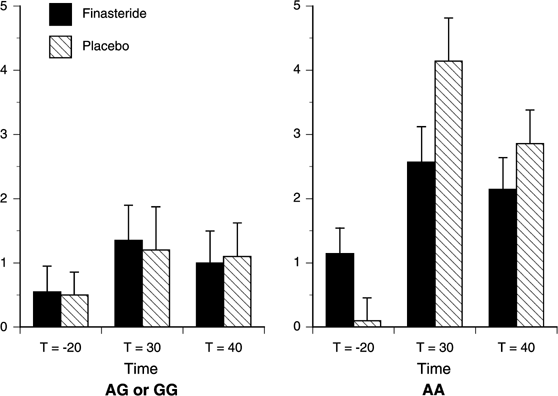
Figure 6
Estimated mean (SEM) for the Gastrointestinal Subscale Score of the SS by Study Drug and Genotype. There were significant effects of Time (p<0.001), Genotype (_p_=0.013), and Time × Genotype (_p_=0.009). Increased scores on the Gastrointestinal Subscale Score following alcohol administration were greater among A-allele homozygotes than among G-allele carriers at _t_=30 min (_p_=0.003). AA (_n_=7), G-allele carriers (_n_=20).
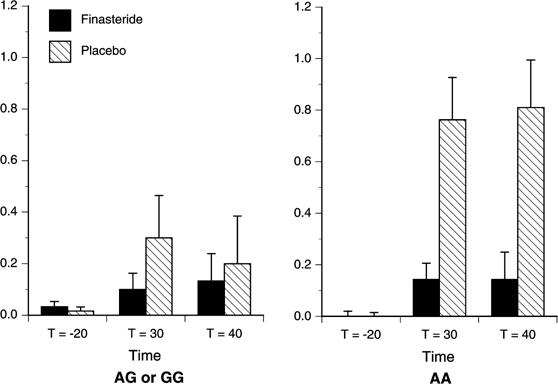
Stimulation Subscale of the BAES
There was a significant effect of Time (F(2,50)=4.97, _p_=0.018) and of Time × Genotype × Study Drug (F(2,50)=3.56, _p_=0.036) on this subscale score (Figure 7). Decomposition of the three-way interaction showed that, among G-allele carriers, scores remained stable across the three time points, during both the finasteride and placebo sessions. However, among subjects homozygous for the A-allele, the increase from baseline to _t_=40 min was significantly steeper during the placebo session than during the finasteride session (F(1,25)=6.86, _p_=0.015), an effect that was due only to the lower score on this measure at −20 min among A-allele carriers during the placebo session. However, analysis of covariance, in which the baseline placebo value was used as a covariate, revealed a significant main effect of genotype (F(1,24)=5.39, _p_=0.029), with A-allele homozygotes reporting greater stimulation following alcohol administration.
Figure 7
Estimated mean (SEM) for the Stimulation Subscale Score of the BAES as a function of Study Drug and Genotype. There were significant effects of Time (_p_=0.018) and Time × Genotype × Study Drug (_p_=0.036). Among subjects homozygous for the A-allele, the increase from baseline to _t_=40 min was significantly steeper during the placebo session than during the finasteride session (_p_=0.015). AA (_n_=7), G-allele carriers (_n_=20).
Drug Effects questionnaire
There was only a main effect of Time for the DEQ items ‘feel alcohol effect’ (F(2,50)=68.95, p<0.001), ‘feel alcohol high’ (F(2,50)=28.56, p<0.001), and ‘like alcohol’ (F(2,50)=11.64, _p_=0.001). For the item ‘want more alcohol’, there was a significant effect of Time (F(2,50)=6.76, _p_=0.010), Time × Study Drug (F(2,50)=4.03, _p_=0.025), and Time × Genotype × Study Drug (F(2,50)=4.33, _p_=0.020). As shown in Figure 8, although the genotype groups were comparable on this measure prior to alcohol administration, there was a trend for higher scores among A-allele homozygotes at _t_=30 min (F(1,25)=3.56, _p_=0.071). Decomposition of the three-way interaction showed that at _t_=30 min, A-allele homozygotes reported a greater desire to drink than did G-allele carriers, but only during the placebo session (F(1,25)=4.32, _p_=0.047).
Figure 8
Estimated mean (SEM) for the ‘want more alcohol’ item of the DEQ by Study Drug and Genotype. There were significant effects of Time (_p_=0.010), Time × Study Drug (_p_=0.025), and Time × Genotype × Study Drug (_p_=0.020). At _t_=30 min, there was a trend for higher scores among A-allele homozygotes (_p_=0.071) than G-allele carriers and a greater increase among A-allele homozygotes than among G-allele carriers, but only following placebo treatment (_p_=0.047). AA (_n_=7), G-allele carriers (_n_=20).
When analyses were repeated including only European-Americans (ie excluding the five Hispanic subjects), the overall pattern of effects was comparable to that seen in the larger sample, but reflected the reduced statistical power attributable to the smaller sample size.
DISCUSSION
The most consistent finding in this study was an effect of genotype on many of the subjective effects of alcohol. There was also evidence that finasteride antagonized some alcohol effects, particularly among A-allele homozygotes. These findings are interesting in view of the animal and human literature implicating both GABA and neuroactive steroids in alcohol consumption and dependence.
There is consistent evidence that GABAergic systems mediate alcohol self-administration in animal models, probably by stimulating reward circuitry in the mesolimbic system (Chester and Cunningham, 2002). GABAA receptor agonists increase both the speed of acquisition of alcohol drinking behavior (Petry, 1997; Smith et al, 1992) and the volume of alcohol consumed (Boyle et al, 1993; Pohorecky and Brick, 1988). Consistent with these findings, GABAergic inverse agonists diminish the reinforcing effects of alcohol (Rassnick et al, 1993; Samson et al, 1987) and its intake (Balakleevsky et al, 1990; McBride et al, 1998).
Several GABAA receptor subunits have been implicated in alcohol's action, so that the specific subunit composition of the receptor may be an important determinant of alcohol's CNS effects. The _α_-2 subunit of the GABA receptor mediates the anxiolytic effects of benzodiazepines (Harris et al, 1998; Rudolph et al, 1999; Low et al, 2000; Tobler et al, 2001), and enhances the hypnotic, but not the sedative, effects of combined exposure to alcohol and benzodiazepines (Tauber et al, 2003). In the present study, individuals homozygous for the A-allele at GABRA2 reported greater stimulant and gastrointestinal subjective effects of alcohol (as measured on the Alcohol SS) compared with subjects having one or more G-alleles.
A recent genetic study has shown the G-allele to be over-represented among alcohol-dependent individuals (Covault et al, 2004). Alcoholism risk has been associated with a low level of response to alcohol, as measured by subjective feelings of intoxication following an alcohol challenge (Schuckit, 1984, 1994; Bauer and Hesselbrock, 1993; Schuckit et al, 1996). A family history of alcoholism has also been associated with a diminished response to alcohol in nonalcoholics (Moss et al, 1989; O'Malley and Maisto, 1985; Pollock et al, 1986; Savoie et al, 1988; Schuckit, 1984; Schuckit et al, 2000; cf. Newlin and Thompson, 1990; see Pollock, 1992 for a meta-analysis). Studies of monozygotic twins show greater similarity in sensitivity to an alcohol challenge than do dizygotic twins (Martin et al, 1985; Martin, 1988; Heath and Martin, 1992; Viken et al, 2003), providing evidence that alcohol sensitivity is an inherited trait. In view of this literature, one interpretation of the findings reported here is that the risk of alcoholism associated in a population study with the G-allele (Covault et al, 2004) may, in part, be mediated by the decreased subjective response to alcohol of individuals carrying this allele.
The findings reported here indicate that A-allele individuals experience the stimulating effects of a moderate dose of alcohol soon after their BrAC rises. It is possible that, because of these subjective effects, such individuals may be less likely to continue drinking. In contrast, individuals with one or two copies of the G-allele may need to drink more alcohol to achieve a comparable level of alcohol-induced stimulation and may be more likely to drink if given access to alcohol. This interpretation appears to be contradicted by the finding that the A-allele homozygotes reported a greater desire for alcohol as measured by the DEQ item ‘want more alcohol’. It is unclear whether self-reported desire for alcohol leads to subsequent drinking, so studies are needed that directly examine whether a priming dose of alcohol leads to greater subsequent alcohol consumption among A-allele homozygotes than among G-allele carriers. Furthermore, studies are needed to determine the effects of GABRA2 alleles on drinking behavior as it occurs in natural settings.
In this study, we also examined the effects of finasteride, a 5AR inhibitor, on the subjective response to a moderate dose of alcohol in social drinkers. As hypothesized, finasteride reduced a number of the self-reported stimulant effects of alcohol, as measured by two subscales of the Alcohol SS (ie the Central-Stimulant and Dynamic-Peripheral Subscales), as well as the Stimulation Subscale of the BAES. These findings are consistent with the results of studies in rodents, which show that the pregnane neuroactive steroids, especially ALLO, mediate some of the effects of acute alcohol administration (Morrow et al, 1999, 2001). In rats, the time course of the alcohol-induced increase in brain ALLO concentration paralleled the appearance of specific behavioral and electrophysiological effects of alcohol (Finn et al, 2004; VanDoren et al, 2000), which were reversed by finasteride (VanDoren et al, 2000; Khisti et al, 2003).
Finasteride also reduced the numbing effects of alcohol, as measured by the SS Anesthetic Subscale. The mechanisms implicated in the numbing effects of alcohol are not as well understood as the general anesthetic effects. A recent study suggests that neuroactive steroids contribute to the hypnotic effects of ethanol in rats (Khisti et al, 2003). Moreover, several studies (Mihic et al, 1997; Koltchine et al, 1999; Mascia et al, 2000; Ueno et al, 2001) have suggested that general anesthesia can be produced, at least in part, by enhancing neuronal inhibition mediated by the GABAA _α_-2 subunit.
The strengths of this study include the choice of an SNP within GABRA2 to predict the response to alcohol based on findings from two recent association studies that yielded highly convergent results of an allelic association to alcohol dependence. These findings were based on the positional and functional implication of GABRA2 as a candidate contributing to alcoholism risk. The hypothesis that finasteride would attenuate the effects of alcohol were based on a well-developed animal literature and a developing human literature demonstrating that neuroactive steroids mediate a number of the effects of alcohol. In animal studies, finasteride has been shown to dampen those effects. Other strengths of the study include the fact that the subjects were well characterized and careful attention was paid to avoid potential confounders. In addition, the double-blind, placebo-controlled crossover design involved laboratory sessions separated by a period of time adequate to reduce the risk of a carryover effect.
The findings reported here must nonetheless be viewed in the context of the study's limitations. Although of interest in relation to alcoholism risk, the GABRA2 polymorphism that was examined in this study as a moderator of alcohol's effects has not been shown to be functional; the variation in this gene that underlies the association with alcohol dependence remains to be determined. Although finasteride is a potent antagonist of type 2 5AR, it has modest antagonist activity at type 1 5AR (Tian et al, 1994; Tian, 1996), the predominant isoform in the mature human brain (Poletti et al, 1998; Stoffel-Wagner et al, 1998). To compensate for the lower antagonist activity of finasteride at the human brain type 1 5AR, we administered a high dose of the drug (ie a total of 200 mg over 24 h, which is 40 times the standard daily dose). Human studies using lower doses of finasteride, which inhibit only peripheral 5AR, would help to define the relative contribution of central or peripheral effects of finasteride on the subjective effects of alcohol in humans. Further, the lack of a placebo alcohol condition limited our capacity to ascertain whether the impact of finasteride on subjective responses was pharmacological or psychological (eg reflecting expectancies), or to determine the subjective effects produced by finasteride alone.
Since the literature on the effects of neuroactive steroids in humans is limited to ALLO (Torres and Ortega, 2003, 2004), we have focused primarily on that compound as a potential mediator of the observed effects of finasteride. However, we did not measure peripheral neuroactive steroid concentrations, so it is possible that the observed effects of finasteride were mediated by changes in other brain steroids. For example, changes in testosterone concentration may have mediated the observed subjective effects, consistent with a recent finding that testosterone concentrations are elevated in both men and women following acute alcohol intake (Sarkola and Eriksson, 2003; Sarkola et al, 2000). Although alcohol may also have increased levels of 5_β_-reduced steroids, such as pregnanolone, finasteride blocks the conversion of progesterone and deoxycorticosterone to the 5_α_-reduced metabolites, with no effect on the concentration of 5_β_-reduced metabolites. This makes it unlikely that changes in concentration of the 5_β_-reduced steroids provide an adequate explanation for the observed subjective effects.
Although the primary outcome measures used in the study were based on participants’ self-report, the instruments that were chosen are used widely to evaluate alcohol-induced subjective effects. In this study, A-allele homozygotes were more sensitive to the subjective effects of alcohol. However, it is possible that the presence of the G-allele changed the threshold at which subjects experienced alcohol's effects, which may also have limited the potential effects of finasteride in the G-allele group. A study design in which the dose of alcohol is varied could be used to test this hypothesis by determining the concentration at which G-allele carriers experience substantial subjective effects. Subsequent studies of the impact of a 5AR inhibitor on a behavioral measure of alcohol's effects in humans should also include a physiological measure that is sensitive to alcohol effects, such as static ataxia, or by using an operant paradigm to measure alcohol self-administration.
The comparatively small number of subjects in this study, in particular the fact that only seven individuals were homozygous for the A-allele, raises the possibility that the findings occurred by chance. In addition, because only two individuals were homozygous for the G-allele, we were able to test only for a dominant effect of the G-allele by comparing carriers (ie AG or GG genotypes) with A-allele homozygotes. A larger sample would make it possible to examine an additive effect of the alleles by using all three genotype groups to examine both the response to alcohol and its modification by finasteride. In addition, the functional effects of the allelic variation at GABRA2 are not understood (Edenberg et al, 2004; Covault et al, 2004), so that additional research is required to elucidate the potential mechanism by which the gene influences the response to alcohol.
Despite these limitations, the results of this study provided support for the hypothesis that GABRA2 alleles moderate the stimulating effects of alcohol, and that these effects can be attenuated by finasteride. The effects of finasteride observed here are consistent with findings from an extensive animal literature and a growing human literature that implicate neuroactive steroids as mediators of some of the effects of alcohol. Further investigations of neuroactive steroid interactions with GABAA receptor variants and their potential role in determining risk of alcohol dependence are warranted.
References
- American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th edn. American Psychiatric Press: Washington, DC.
- Balakleevsky A, Colombo G, Fadda F, Gessa GL (1990). Ro19-4603, a benzodiazepine inverse agonist, attenuates voluntary ethanol consumption in rats selectively bred for high ethanol preference. Alcohol Alcohol 25: 449–452.
CAS PubMed Google Scholar - Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R et al (1999). Ethanol markedly increases ‘GABAergic’ neurosteroids in alcohol-preferring rats. Eur J Pharmacol 384: R1–R2.
Article CAS Google Scholar - Bauer LO, Hesselbrock VM (1993). EEG, autonomic and subjective correlates of the risk for alcoholism. J Stud Alcohol 54: 577–589.
Article CAS Google Scholar - Boyle AE, Segal R, Smith BR, Amit Z (1993). Bidirectional effects of GABAergic agonists and antagonists on maintenance of voluntary ethanol intake in rats. Pharmacol Biochem Behav 46: 179–182.
Article CAS Google Scholar - Chester JA, Cunningham CL (2002). GABA(A) receptor modulation of the rewarding and aversive effects of ethanol. Alcohol 26: 131–143.
Article CAS Google Scholar - Chutuape MA, de Wit H (1994). Relationship between subjective effects and drug preferences: ethanol and diazepam. Drug Alcohol Depend 34: 243–251.
Article CAS Google Scholar - Covault C, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR (2004). Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet 129B: 104–109.
Article Google Scholar - Davies M (2003). The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci 28: 263–274.
PubMed PubMed Central Google Scholar - de Wit H, Uhlenhuth EH, Johanson CE (1987). Individual differences in behavioral and subjective responses to alcohol. Alcohol Clin Exp Res 11: 52–59.
Article CAS Google Scholar - Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO et al (2004). Variations in GABRA2, encoding the alpha 2 subunit of the GABAA receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet 74: 705–714.
Article CAS Google Scholar - Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ (2004). Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience 123: 813–819.
Article CAS Google Scholar - First MB, Spitzer RL, Gibbon M, Williams JBW (1995). Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID—I/P, Version 2.0). Biometrics Research Department, New York State Psychiatric Institute: New York.
Google Scholar - Grobin AC, Matthews DB, Devaud LL, Morrow AL (1998). The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 139: 2–19.
Article CAS Google Scholar - Harris RA, Mihic SJ, Valenzuela CF (1998). Alcohol and benzodiazepines: recent mechanistic studies. Drug Alcohol Depend 51: 155–164.
Article CAS Google Scholar - Heath AC, Martin NG (1992). Genetic differences in psychomotor performance decrement after alcohol: a multivariate analysis. J Stud Alcohol 53: 262–271.
Article CAS Google Scholar - Holdstock L, de Wit H (1998). Individual differences in the biphasic effects of ethanol. Alcohol Clin Exp Res 22: 1903–1911.
Article CAS Google Scholar - Holdstock L, King AC, de Wit H (2000). Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res 24: 789–794.
Article CAS Google Scholar - Khisti RT, VanDoren MJ, O'Buckley T, Morrow AL (2003). Neuroactive steroid 3 alpha-hydroxy-5 alpha-pregnan-20-one modulates ethanol-induced loss of righting reflex in rats. Brain Res 980: 255–265.
Article CAS Google Scholar - Koltchine VV, Finn SE, Jenkins A, Nikolaeva N, Lin A, Harrison NL (1999). Agonist gating and isoflurane potentiation in the human gamma-aminobutyric acid type A receptor determined by the volume of a second transmembrane domain residue. Mol Pharmacol 56: 1087–1093.
Article CAS Google Scholar - Long JC, Knowler WC, Hanson RL, Robin RW, Urbanek M, Moore E et al (1998). Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an Am Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet 81: 216–221.
Article CAS Google Scholar - Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA et al (2000). Molecular and neuronal substrate for the selective attenuation of anxiety. Science 290: 131–134.
Article CAS Google Scholar - Maisto SA, Connors GJ, Tucker JA, McCollam JB (1980). Validation of the sensation scale, a measure of subjective physiological responses to alcohol. Behav Res Ther 18: 37–43.
Article CAS Google Scholar - Martin CS, Earleywire M, Musty RE, Perrine MW, Swift RM (1993). Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res 17: 140–146.
Article CAS Google Scholar - Martin NG (1988). Twin studies of alcohol consumption, metabolism and sensitivity. Aust Drug Alcohol Rev 7: 9–12.
Article Google Scholar - Martin NG, Oakeshott JG, Gibson JB, Starmer GA, Perl J, Wilks AV (1985). A twin study of psychomotor and physiological responses to an acute dose of alcohol. Behav Genet 15: 305–347.
Article CAS Google Scholar - Mascia MP, Trudell JR, Harris RA (2000). Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci USA 97: 9305–9310.
Article CAS Google Scholar - McBride WJ, Murphy JM, Lumeng Land Li TK (1998). Effects of Ro15-4513, fluoxetine and desipramine on intake of ethanol water and food in alcohol preferring and non-preferring lines of rats. Pharmacol Biochem Behav 30: 1045–1050.
Article Google Scholar - Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE et al (1997). Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 389: 385–389.
Article CAS Google Scholar - Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Jan PH et al (1999). Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of action? Alcohol Clin Exp Res 23: 1933–1940.
Article CAS Google Scholar - Morrow AL, VanDoren MJ, Penland SN, Matthews DB (2001). The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev 37: 98–109.
Article CAS Google Scholar - Moss HB, Yao JK, Maddock JM (1989). Responses by sons of alcoholic fathers to alcoholic and placebo drinks: perceived mood, intoxication, and plasma prolactin. Alcohol Clin Exp Res 13: 252–257.
Article CAS Google Scholar - Newlin DB, Thompson JB (1990). Alcohol challenge with sons of alcoholics: a critical review analysis. Psychol Bull 108: 383–402.
Article CAS Google Scholar - Ohtawa M, Morikawa H, Shimazaki J (1991). Pharmacokinetic and biochemical efficacy after a single and multiple oral administration of _N_-(2methyl-2propyl)-3-oxo-4-aza-5-_α_-androst-1-ene-17-carboxamide, a new type of specific competitive inhibitor of testosterone 5-_α_-reductase, in volunteers. Eur J Drug Metab Pharmacokinet 16: 15–21.
Article CAS Google Scholar - O'Malley SS, Maisto SA (1985). Effects of family drinking history and expectancies on responses to alcohol in men. J Stud Alcohol 46: 289–297.
Article CAS Google Scholar - Petry NM (1997). Benzodiazepine–GABA modulation of concurrent ethanol and sucrose reinforcement in the rat. Exp Clin Psychopharmacol 5: 183–194.
Article CAS Google Scholar - Pohorecky LA, Brick J (1988). Pharmacology of ethanol. Pharmacol Ther 36: 335–427.
Article CAS Google Scholar - Poletti A, Negri-Cesi P, Rabuffetti M, Colciago A, Celotti F, Martini L (1998). Transient expression of the 5-reductase type 2 isozyme in the rat brain in late fetal and early postnatal life. Endocrinology 139: 2171–2178.
Article CAS Google Scholar - Pollock VE (1992). Meta-analysis of subjective sensitivity to alcohol in sons of alcoholics. Am J Psychiatry 149: 1534–1538.
Article CAS Google Scholar - Pollock VE, Teasdale TW, Gabrielli WF, Knop J (1986). Subjective and objective measures of response to alcohol among young men at risk for alcoholism. J Stud Alcohol 47: 297–304.
Article CAS Google Scholar - Rassnick S, D'Amico E, Riley E, Koob GF (1993). GABA antagonist and benzodiazepine partial inverse agonist reduce motivated responding for ethanol. Alcohol Clin Exp Res 17: 124–130.
Article CAS Google Scholar - Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P et al (1998). Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet 8: 207–215.
Article Google Scholar - Romeo E, Brancati A, De Lorenzo A, Fucci P, Furnari C, Pompili E et al (1996). Marked decrease of plasma neuroactive steroids during alcohol withdrawal. Clin Neuropharmacol 19: 366–369.
Article CAS Google Scholar - Romeo E, Pompili E, di Michele F, Pace M, Rupprecht R, Bernardi G et al (2000). Effects of fluoxetine, indomethacine and placebo on 3 alpha, 5 alpha tetrahydroprogesterone (THP) plasma levels in uncomplicated alcohol withdrawal. World J Biol Psychiatry 1: 101–104.
Article CAS Google Scholar - Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM et al (1999). Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature 401: 796–800.
Article CAS Google Scholar - Samson HH, Tolliver GA, Pfeffer AU, Sadeghi KG, Mills FG (1987). Oral ethanol reinforcement in the rat: effect of the partial inverse benzodiazepine agonist Ro15-4513. Pharmacol Biochem Behav 27: 517–519.
Article CAS Google Scholar - Sarkola T, Eriksson CJ (2003). Testosterone increases in men after a low dose of alcohol. Alcohol Clin Exp Res 27: 682–685.
Article CAS Google Scholar - Sarkola T, Fukunaga T, Makisalo H, Peter Eriksson CJ (2000). Acute effect of alcohol on androgens in premenopausal women. Alcohol Alcohol 35: 84–90.
Article CAS Google Scholar - Savoie TM, Emory EK, Moody-Thomas S (1988). Acute alcohol intoxication in socially drinking female and male offspring of alcoholic fathers. J Stud Alcohol 49: 430–435.
Article CAS Google Scholar - Schuckit MA (1984). Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry 41: 879–884.
Article CAS Google Scholar - Schuckit MA (1994). Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry 151: 184–189.
Article CAS Google Scholar - Schuckit MA, Smith TL, Kalmijn J, Tsuang J, Hesselbrock V, Bucholz K (2000). Response to alcohol in daughters of alcoholics: a pilot study and a comparison with sons of alcoholics. Alcohol Alcohol 35: 242–248.
Article CAS Google Scholar - Schuckit MA, Tsuang JW, Anthenelli RM, Tipp JE, Nurnberger Jr JI (1996). Alcohol challenges in young men from alcoholic pedigrees and control families: a report from the COGA project. J Stud Alcohol 57: 368–377.
Article CAS Google Scholar - Smith BR, Robidoux J, Amit Z (1992). GABAergic involvement in the acquisition of voluntary ethanol intake in laboratory rats. Alcohol Alcohol 27: 227–231.
CAS PubMed Google Scholar - Sobell LC, Sobell MD (1992). Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J (eds). Measuring Alcohol Consumption. Human Press: Clifton, NJ. pp 41–65.
Chapter Google Scholar - Stoffel-Wagner B, Watzka M, Steckelbroeck S, Wickert L, Schramm J, Romalo G et al (1998). Expression of 5-reductase in the human temporal lobe of children and adults. J Clin Endocrinol Metab 83: 3636–3642.
CAS PubMed Google Scholar - Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H (1994). Naltrexone-induced alterations in human ethanol intoxication. Am J Psychiatry 151: 1463–1467.
Article CAS Google Scholar - Tauber M, Calame-Droz E, Prut L, Rudolph U, Crestani F (2003). Alpha2-gamma-aminobutyric acid (GABA)A receptors are the molecular substrates mediating precipitation of narcosis but not of sedation by the combined use of diazepam and alcohol in vivo. Eur J Neurosci 18: 2599–2604.
Article Google Scholar - Tian G (1996). In vivo time-dependent inhibition of human steroid 5 alpha-reductase by finasteride. J Pharm Sci 85: 106–111.
Article CAS Google Scholar - Tian G, Stuart JD, Moss ML, Domanico PL, Bramson HN, Patel IR et al (1994). 17 beta-(_N_-tert-butylcarbamoyl)-4-aza-5 alpha-androstan-1-en-3-one is an active site-directed slow time-dependent inhibitor of human steroid 5 alpha-reductase 1. Biochemistry 33: 2291–2296.
Article CAS Google Scholar - Tobler I, Kopp C, Deboer T, Rudolph U (2001). Diazepam-induced changes in sleep: role of the alpha 1 GABA(A) receptor subtype. Proc Natl Acad Sci USA 98: 6464–6469.
Article CAS Google Scholar - Torres JM, Ortega E (2003). Alcohol intoxication increases allopregnanolone levels in female adolescent humans. Neuropsychopharmacology 28: 1207–1209.
Article CAS Google Scholar - Torres JM, Ortega E (2004). Alcohol intoxication increases allopregnanolone levels in male adolescent humans. Psychopharmacology (Berl) 172: 352–355.
Article CAS Google Scholar - Ueno S, Harris RA, Messing RO, Sanchez-Perez AM, Hodge CW, McMahon T et al (2001). Alcohol actions on GABA(A) receptors: from protein structure to mouse behavior. Alcohol Clin Exp Res 25: 76S–81S.
Article CAS Google Scholar - VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL (2000). Neuroactive steroid 3-alpha-hydroxy-5-alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci 20: 1982–1989.
Article CAS Google Scholar - Viken RJ, Rose RJ, Morzorati SL, Christian JC, Li TK (2003). Subjective intoxication in response to alcohol challenge: heritability and covariation with personality, breath alcohol level, and drinking history. Alcohol Clin Exp Res 27: 795–803.
Article Google Scholar - Watson PE (1989). Total body water and blood alcohol levels: updating the fundamentals. In: Crow KE, Batt RD (eds). Human Metabolism of Alcohol. CRC Press: Boca Raton, FL. pp 41–56.
Google Scholar
Acknowledgements
This study was supported by NIH Grants AA13736, AA07290, AA03510, and RR06192 (University of Connecticut General Clinical Research Center (GCRC)). Contributions by the staff of the Clinical Research and Evaluation Unit of the Alcohol Research Center and the GCRC are gratefully acknowledged. We also thank Lawrence Raisz and Joel Gelernter for their helpful review and comments on the manuscript.
Author information
Authors and Affiliations
- Department of Psychiatry, Alcohol Research Center, University of Connecticut School of Medicine, Farmington, CT, USA
Amira Pierucci-Lagha, Jonathan Covault, Richard Feinn, Maggie Nellissery, Carlos Hernandez-Avila & Henry R Kranzler - Department of Medicine, University of Connecticut School of Medicine, Farmington, CT, USA
Cheryl Oncken - Departments of Psychiatry and Pharmacology, Bowles Center for Alcohol Studies, University of North Carolina, Chapel Hill, NC, USA
A Leslie Morrow
Authors
- Amira Pierucci-Lagha
You can also search for this author inPubMed Google Scholar - Jonathan Covault
You can also search for this author inPubMed Google Scholar - Richard Feinn
You can also search for this author inPubMed Google Scholar - Maggie Nellissery
You can also search for this author inPubMed Google Scholar - Carlos Hernandez-Avila
You can also search for this author inPubMed Google Scholar - Cheryl Oncken
You can also search for this author inPubMed Google Scholar - A Leslie Morrow
You can also search for this author inPubMed Google Scholar - Henry R Kranzler
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toHenry R Kranzler.
Rights and permissions
About this article
Cite this article
Pierucci-Lagha, A., Covault, J., Feinn, R. et al. GABRA2 Alleles Moderate the Subjective Effects of Alcohol, Which are Attenuated by Finasteride.Neuropsychopharmacol 30, 1193–1203 (2005). https://doi.org/10.1038/sj.npp.1300688
- Received: 06 May 2004
- Revised: 10 December 2004
- Accepted: 15 December 2004
- Published: 09 February 2005
- Issue Date: 01 June 2005
- DOI: https://doi.org/10.1038/sj.npp.1300688