Elevation of Glucocorticoids is Necessary but not Sufficient for the Escalation of Cocaine Self-Administration by Chronic Electric Footshock Stress in Rats (original) (raw)
INTRODUCTION
The loss of control over cocaine self-administration (SA) and the resulting escalation of drug use are defining features of cocaine addiction. These aspects of cocaine addiction have been studied using rodent models in which progressively escalating patterns of intravenous drug intake emerge when the period of time that cocaine is available for SA each day is prolonged (see eg Ahmed and Koob, 1998, 1999; Mantsch et al, 2004). Such models have provided valuable insight into potential neurobiological mediators of the transition between controlled drug use and addiction (Koob et al, 2004). Further characterization of factors that promote escalating SA patterns in rodents should facilitate the identification of determinants of human cocaine addiction and the neurobiological substrates through which they act.
A role for stress in cocaine addiction has been established (see Sinha (2001) for review). The incidence of a number of stress-related disorders, including post-traumatic stress disorder (PTSD; Najavits et al, 1998; Back et al, 2000) and panic disorder (Anthony et al, 1989), is higher in cocaine-dependent individuals. In fact, it has been reported that up to 43% of cocaine-dependent individuals meet the DSM III-R criteria for lifetime PTSD (Back et al, 2000). In one study, 95.5% of subjects with concurrent cocaine dependence and PTSD reported a functional relationship between cocaine use and PTSD symptoms, with 86.4% indicating that a worsening of PTSD symptoms was commonly associated with an increase in cocaine use (Back et al, 2006). These data suggest that, at least in a subpopulation of cocaine users, stressful life events may act as precipitating factors for cocaine abuse and/or addiction. This interpretation is supported by findings that the presentation of personalized stress imagery to abstinent cocaine-dependent individuals in a laboratory setting directly elicits drug craving (Sinha et al, 1999, 2000).
Pre-clinical findings from studies using rodent models further support a role for stress in cocaine abuse (see Goeders (2002) for review). A number of investigators have demonstrated that stressors facilitate the acquisition of cocaine SA (see eg Goeders and Guerin, 1994; Haney et al, 1995; Miczek and Mutschler, 1996; Ramsey and Van Ree, 1993; Campbell and Carroll, 2001; Kosten et al, 2000; Schenk et al, 1987) and reinstate extinguished cocaine-seeking behavior (Erb et al, 1996; Ahmed and Koob, 1997; Mantsch and Goeders, 1999a; Shalev et al, 2003). By contrast, the ability of stress to produce escalating patterns of cocaine SA has not been extensively examined. Covington and Miczek (2001) have reported that a history of social-defeat stress prolongs responding by rats during periods of unlimited access, thereby increasing the cumulative amount of cocaine consumption over an extended period of time, suggesting that prior stress may indeed promote a loss of control over cocaine SA. However, apart from an earlier finding that food restriction elevates post-acquisition cocaine SA in rats (Carroll, 1985), the impact of chronic stress administered concurrently across an extended period of ongoing cocaine SA has not been reported.
Glucocorticoids, secreted as a result of activation of the hypothalamic-pituitary-adrenal (HPA) axis, are important mediators of physiological and behavioral responses that permit organisms to adapt to and/or cope with stressors. These adaptational responses appear to include effects on motivational neurocircuitry that promote illicit drug use (see Marinelli and Piazza (2002) for review). For example, the stressor-induced facilitation of the acquisition of cocaine SA by rats is positively correlated with systemic corticosterone (CORT) levels (Goeders and Guerin, 1996a) and can be reproduced by CORT injections at doses that produce blood concentrations within the stressor-induced range (Mantsch et al, 1998), whereas rats that have been adrenalectomized do not acquire cocaine SA at any dose (Goeders and Guerin, 1996b). It has also been reported that basal levels of glucocorticoids are critical for the stressor-induced reinstatement of cocaine-seeking behavior (Mantsch and Goeders, 1999a; Erb et al, 1998) and that CORT infusions can mimic the reinstating effects of stressors (Deroche et al, 1997). Altogether, these findings suggest that glucocorticoids may serve as important substrates through which stressors influence cocaine abuse.
The goals of the present study were (1) to investigate the ability of chronic stress to escalate cocaine SA, and (2) to examine the potential role of glucocorticoids in the escalation of cocaine SA by chronic stress. We chose to use uncontrollable EFS as the chronic stressor because it has been reported that EFS facilitates the acquisition of SA (Goeders and Guerin, 1994) and reinstates extinguished cocaine-seeking behavior (Erb et al, 1996; Ahmed and Koob, 1997; Mantsch and Goeders, 1999a). The specificity of the effects of EFS on cocaine SA was assessed by also examining EFS-induced alterations in food-reinforced lever pressing. The potential role of glucocorticoids in the effects of EFS on SA was determined in adrenalectomized rats with diurnal CORT replacement and by administration of CORT under conditions that produce blood concentrations within the stressor-induced range.
MATERIALS AND METHODS
Subjects
A total of 104 male Sprague–Dawley rats (Charles River Laboratories, Inc., Wilmington, MA), approximately 90 days old (325 g) were used for the study. Rats were housed individually in a temperature- and humidity-controlled AAALAC-accredited animal facility and had access to food at all times except when in the experimental chambers. With the exception of the rats used for Experiments 3a and 3c, all rats were housed under a standard light cycle (lights on at 0600 hours and lights off at 1800 hours) and were tested during the light phase. All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
Catheterization Surgery
For the cocaine SA experiments, rats were implanted with chronic indwelling catheters under ketamine HCl (100 mg/kg, i.p., Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (2 mg/kg, i.p., Lloyd Laboratories, Shenandoah, IA) anesthesia. A silicon tubing catheter (Silastic®; Dow Corning Co., Midland, MI; 0.64 mm i.d.; 1.19 mm o.d.) was inserted into the right posterior facial vein and down into the jugular vein so that it terminated at the right atrium. The catheter was sutured to the vein and continued subcutaneously to the animal's back where it exited 2 cm posterior to the scapulae via a back-mounted 22-gauge guide cannula (Plastics One Inc., Roanoke, VA) attached using dental acrylic to a piece of polypropylene monofilament surgical mesh (Atrium Medical, Co., Hudson, NH) to permit connection of a polyurethane delivery line (0.51 mm i.d. × 1.52 mm o.d.) encased in a stainless steel connector assembly spring leash (Plastics One Inc., Roanoke, VA). The delivery line was connected to a 30-ml syringe in a motor-driven pump (Razel, Stamford, CT) via a leak-proof fluid swivel (Instech Lab. Inc., Plymouth Meeting, PA) suspended above the chamber to allow drug solution delivery. The swivel and leash assembly was counterbalanced to permit relatively unrestrained movement. Rats were allowed to recover for at least 3 days before SA testing during which time they were provided acetaminophen (480 mg/l) in their drinking water. After implantation, rats were injected with a sterile cefazolin antibiotic solution (15 mg, i.v.; West-Ward Pharmaceutical Co., Eatontown, NJ) each day. Catheters were filled daily with a heparin solution (83 IU/ml; Elkins-Sinn, Inc., Cherry Hill, NJ) and capped whenever the leash/delivery line assembly was disconnected.
SA Apparatus
Sixteen plastic and stainless steel operant conditioning chambers encased in sound attenuating cubicles (MED-Associates Inc., St Albans, VT) were used for SA. The chambers were equipped with three retractable levers with stimulus lights located above each lever, food pellet dispensers, and water bottles. Two levers and their respective stimulus lights were mounted on the front wall of the chamber, with the food pellet dispenser located between them. The third lever and light were located on the back wall. Cubicles were equipped with exhaust fans that provided ventilation and white noise to mask extraneous sound.
SA Training
Following recovery from surgery, rats were trained to self-administer cocaine (0.5 mg/kg/inf, i.v., National Institute on Drug Abuse, Bethesda, MD) by pressing a lever under a FR4 schedule during daily sessions consisting of four 30-min components separated by 5-min drug-free periods. During the SA components, the cocaine (ie right) lever was extended into the chamber and the corresponding stimulus light was illuminated. Depression of this lever resulted in an i.v. infusion of drug solution (200 _μ_l delivered over 5.0 s) followed by a 25-s time-out period during which the stimulus light was extinguished but the lever remained extended. Responding on a second, inactive (ie back) lever was recorded but had no programmed consequences. During the drug-free periods, the stimulus light was extinguished and levers were retracted. Once stable SA under the FR1 schedule was observed, response requirements were gradually increased such that before testing, each rat was self-administering under a FR4 schedule. Testing began once stable response patterns were observed (total responding <10% variation from the mean over three consecutive sessions).
ADX and Diurnal CORT Replacement
To investigate the involvement of CORT in SA in the presence or absence of stress, some rats were bilaterally adrenalectomized via the dorsal approach under ketamine and xylazine anesthesia. Diurnal CORT replacement consisted of subcutaneous implantation of a 25% CORT (Sigma Chemicals, St Louis, MO) pellet in the nape of the neck to produce blood concentrations similar to those observed at the nadir of the circadian cycle along with inclusion of 0.025% CORT in the drinking water to emulate the circadian peak observed during the active (dark) phase, within which most drinking typically occurs (Jacobson et al, 1988). Twenty-five percent CORT pellets were made by melting a 1:3 CORT:cholesterol ratio mixture over a flame and pouring it into a pellet mold, creating a 1.5 × 0.5 × 0.5 cm (l × w × h) pellet for implantation (Meyer et al, 1979). CORT was dissolved in ethanol before introduction into the drinking water resulting in a final ethanol concentration of 1 mg/l (0.01%). In order to replace depleted sodium secondary to the loss of aldosterone as a result of ADX, drinking water for all rats consisted of a 0.9% NaCl solution. CORT pellets were replaced every 7 days under brief anesthesia induced by sodium methohexital (1.5 mg, i.v., Monarch Pharmaceuticals, Bristol, TN) injected via the implanted catheter. Control rats underwent sham ADX procedures and were implanted with 100% cholesterol pellets. Sham operations were identical to ADX surgeries, except that, once exposed, the adrenal glands were not removed during the procedure.
Experiment 1: Effects of Repeated Daily EFS on Cocaine SA
To investigate the ability of daily stress to escalate cocaine SA, rats were tested for SA over a 14-day period in the presence or absence of daily electric footshock stress (EFS). These rats also received sham ADX surgeries and subcutaneous cholesterol pellet implantation before SA so that they could serve as controls for the ADX studies described below. Shocked rats received EFS (sequences of three 0.6 mA, 100 ms shocks administered at 1-s intervals) delivered through the scrambled grid floors of the SA chambers under a variable time 45-s schedule during the 5-min periods preceding each 30-min SA component within the daily SA sessions. Rats received an average of 28.5 shock sequences each day and 7.25 shock sequences per 5-min period. During the 5-min shock periods, the house light in the SA chamber was illuminated but all response levers were retracted and stimulus lights were extinguished. Additional groups of rats were exposed to daily EFS under parameters identical to those described above except that EFS was delivered 4 h after the daily SA test sessions ended for 14 days either inside or outside of the SA context in order to determine if EFS effects on SA were context-dependent and/or relied on a close temporal relationship between EFS and SA. These rats were returned to their home cages during the 4-h period between SA testing and the EFS sessions. Rats that were shocked outside of the SA environment received EFS inside ventilated 41 × 30.8 × 34.4 cm (l × w × h) plastic chambers equipped with electrifiable stainless steel grid floors.
Experiment 2: Effects of Repeated Daily EFS on Food-Reinforced Lever Pressing
To determine if the effects of EFS were selective for cocaine-reinforced responding, we also examined the effects of repeated daily EFS on food-reinforced lever pressing. Rats were trained to press a response lever under a FR4 schedule of food reinforcement during daily sessions consisting of four 15-min components separated by 5-min time-out periods. During the sessions, pressing the front, left response lever four times resulted in delivery of a sucrose-sweetened food pellet (45 mg, P.J. Noyes, Lancaster, NH). Once stable response patterns were observed (total responding <10% variation from the mean over three consecutive sessions), the effects of repeated daily EFS on responding were determined in half of the rats by administering EFS, as described above, across 14 days of testing. The remaining rats were tested for 14 days in the absence of EFS.
Experiment 3: Role of Elevated Glucocorticoids in the Escalation of Cocaine SA by EFS
In order to investigate the potential role of elevated glucocorticoids in the escalation of cocaine SA by EFS, rats underwent surgical ADX with diurnal CORT replacement or sham treatment before testing for cocaine SA in the presence or absence of repeated EFS.
Experiment 3a: Effects of ADX and diurnal CORT replacement on plasma CORT under basal, EFS, and SA conditions
The effects of ADX and CORT replacement on circadian and EFS-induced increases in plasma CORT were examined in 16 rats housed under a reverse light–dark cycle (lights on at 1800 hours and off at 0600 hours). Ten days after surgical ADX with diurnal CORT replacement or sham surgery with cholesterol pellet implantation and following 1 week of habituation to the blood sampling procedure, blood for CORT determination was collected from the tail vein of conscious rats. Rats were wrapped in a hand towel, and 1–2 mm was cut from the tip of the tail. Blood (approximately 100 μl) was collected into tubes containing heparin and placed on ice. Blood was centrifuged to allow separation of plasma, which was collected and frozen at −80°C until measurement of CORT in duplicate using a commercial radioimmunoassay kit (ICN Biomedical, Irvine, CA). Three blood samples were acquired from each rat on consecutive days and in the following sequence: one sample at 0700 hours, just after the start of the dark phase; one sample at 1900 hours, 1 h into the light phase in the absence of EFS; and one sample at 1900 hours, 15 min after a 5-min EFS session identical to those used in the SA study. The effects of cocaine SA on plasma CORT were examined in rats from which blood was acquired via the implanted catheters immediately following SA sessions in the absence of EFS. For statistical analysis, post-SA CORT levels were compared to basal CORT levels measured at 1900 hours, near the nadir of the circadian cycle.
Experiment 3b: Effects of ADX and diurnal CORT replacement on the EFS-induced escalation of cocaine SA
To determine if stressor-induced CORT secretion was necessary for the stressor-induced escalation of cocaine SA, ADX rats with diurnal CORT replacement and sham controls were tested for SA in the presence or absence of EFS. For shocked rats, EFS was administered during the 5-min periods preceding each 30-min SA component within the daily SA sessions, as described above. SA by ADX rats with CORT replacement was compared to pre-surgical SA levels and to SA by sham-treated rats.
Experiment 3c: Effect of repeated daily EFS on EFS-induced increases in plasma CORT
A role for EFS-induced CORT secretion in the effects of repeated EFS on SA would suggest that the CORT response to EFS is sustained with repeated administration. However, it has been reported that repeated exposure to the same stressor results in an attenuation of the glucocorticoid response to that stressor (see Armario et al (2004) for review). To determine if basal or EFS-induced CORT secretion was altered over the course of 14 days of EFS exposure, plasma CORT was measured under basal conditions and following EFS in drug-naive rats with no prior history of EFS and in drug-naive rats exposed to the 14-day EFS regimen used in our SA experiment. Tail blood was acquired under basal conditions and following EFS from 16 drug-naive rats that received 14 days of EFS under conditions identical to those used in the SA experiments described above. As was the case in Experiment 3a, blood samples were acquired at 1900 hours, 1 h into the light phase under basal conditions and 15 min after a 5-min EFS session.
Experiment 4: Effects of IP CORT Administration on Cocaine SA
To determine if daily elevation of CORT alone was sufficient to escalate cocaine SA or if elevated CORT could restore the EFS-induced escalation of cocaine SA in ADX/CORT-replaced rats, ADX rats with diurnal CORT replacement received i.p. CORT injections (3.0 mg/kg, i.p. suspended in extra virgin olive oil) 30 min before daily SA testing in the presence or absence of EFS.
Experiment 4a: Effects of IP CORT administration on plasma CORT levels
The effect of a 3.0 mg/kg i.p. CORT injection on plasma CORT levels was initially determined in ADX rats with no CORT replacement. The time course of increases in plasma CORT levels following i.p. CORT injection was examined using RIA in tail blood acquired at various times following CORT administration.
Experiment 4b: Effects of repeated daily IP CORT administration in the presence or absence of EFS on cocaine SA in ADX rats
To determine if CORT could reproduce or restore the effects of EFS on cocaine SA, ADX rats with diurnal CORT replacement received daily i.p. CORT injections 30 min before SA testing for 14 days in the presence or absence of EFS. SA by these rats was compared to pre-surgical SA levels and to SA by ADX rats with diurnal CORT replacement who did not receive daily i.p. CORT injections. Escalated cocaine SA as a result of the i.p. CORT injections in the absence of EFS would suggest that elevated CORT alone is sufficient to produce escalation. By contrast, a restoration of EFS-induced escalation by CORT administration without an effect of CORT alone would indicate that elevated CORT is necessary for EFS-induced escalation but alone is insufficient to produce escalation in the absence of stress.
Statistical Analyses
Effects of EFS and/or altered CORT levels on cocaine SA, food-reinforced responding, and plasma CORT concentrations were examined using 2- or 3-way repeated measures ANOVA followed, when appropriate, by post-hoc analysis using the Fisher's PLSD test. Statistical significance was set at P<0.05.
RESULTS
Experiment 1: Effects of Repeated Daily EFS on Cocaine SA
Figure 1 shows the effects of repeated EFS on cocaine SA. A 2-way ANOVA examining SA under basal conditions and across the 14 days of SA testing revealed a significant overall effect of SA day (F14,350=2.28; P<0.01) and a significant interaction between EFS condition and SA day (F42,350=2.01; P<0.0001). Separate 1-way ANOVAs showed that significant time-dependent increases in SA emerged when rats were repeatedly exposed to EFS in the SA environment immediately before testing (F14,84=3.623; P<0.001), but not when rats did not receive EFS or when EFS was delivered temporally removed from (ie 4 h after) the SA session either inside or outside of the SA environment. Post-hoc analysis using the Fisher's LSD test showed that responding by these rats was significantly increased compared to baseline on SA days 3, 7, 10, 11, 12, and 14 (P<0.05). No significant differences in inactive lever responding were found (data not shown).
Figure 1
Effects of repeated daily electric footshock (EFS) on cocaine SA. Rats were tested for cocaine SA over a 14-day period in the presence (EFS) or absence (No EFS; _n_=9) of daily EFS. EFS escalated cocaine SA when delivered within the SA chambers at the time of SA testing (EFS/SA CONTEXT/SA; _n_=7), but not when delivered within the SA chambers 4 h after completion of the daily SA sessions (EFS/SA CONTEXT/POST-SA; _n_=6) or in a different chamber within which SA did not occur, 4 h after SA (EFS/NON-SA CONTEXT/POST-SA; _n_=7). Data represent the mean number of self-administered infusions per 2-h session (±SE) under basal conditions (BAS) and across 14 days of SA testing in each group. *P<0.05, significant vs BAS.
Effects of Repeated EFS on Body Weight
A 2-way ANOVA showed a significant main effect of SA test day (F14,196=6.612; P<0.05) on body weight, but no significant effect of EFS condition or interaction between EFS condition and SA test day. Rats gained weight across the 14-day SA test period regardless of whether or not they received daily EFS.
Experiment 2: Effects of Repeated Daily EFS on Food-Reinforced Lever Pressing
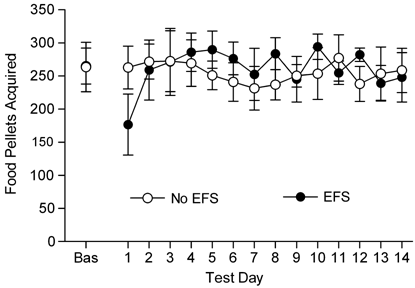
In contrast to cocaine SA, food-reinforced responding across a 14-day period was not significantly altered as a result of repeated EFS (see Figure 2). Although responding was reduced in rats receiving EFS on the first day of testing, a 2-way ANOVA examining responding under basal conditions and across the 14 days of testing failed to demonstrate significant overall effects of EFS condition or test day on food-reinforced responding or a significant EFS/test day interaction. Although the mean numbers of food pellets acquired under the FR4 schedule during the daily sessions were always well below the maximum possible number, it is possible that escalating effects of EFS may have been observed if rats were trained to respond under conditions that maintained substantially lower levels of food intake and behavior.
Figure 2
Effects of repeated daily electric footshock (EFS) on food-reinforced lever pressing. EFS had no significant effects on food-reinforced responding. Data represent the mean number of food pellets acquired per 1-h session (±SE) under basal conditions (BAS) and across 14 days of testing in rats that received EFS at the time of testing (EFS; _n_=8) and in rats tested for food-reinforced responding in the absence of EFS (No EFS; _n_=8).
Experiment 3: Role of Elevated Glucocorticoids in the Escalation of Cocaine SA by EFS
Experiment 3a: Effects of ADX and diurnal CORT replacement on plasma CORT under basal, EFS, and SA conditions
Figure 3 shows plasma CORT concentrations at the peak and nadir of the circadian rhythm and plasma CORT following EFS or cocaine SA at the circadian nadir in ADX rats receiving diurnal CORT replacement and sham-treated rats. A 2-way time-of-day (peak vs nadir; repeated measure) × CORT condition (ADX/CORT vs sham) ANOVA showed, as expected, a significant main effect of time-of-day (peak vs nadir) on CORT (F1,10=21.588; P<0.05). No significant effect of CORT condition or CORT × time-of-day interaction was observed, suggesting that the ADX along with diurnal CORT replacement effectively reproduced the circadian pattern of plasma CORT levels. A separate 2-way EFS condition (repeated measure) × CORT condition ANOVA examining nadir CORT levels under basal conditions and following EFS in ADX/CORT-replaced rats and sham controls showed significant main effects of EFS (F1,10=43.411; P<0.001) and ADX/CORT replacement (F1,10=48.038; P<0.001), and a significant stress × CORT condition interaction (F1,10=72.78; P<0.001). EFS significantly increased plasma CORT in sham-treated rats compared to basal nadir values, but had no effect on CORT in ADX rats with CORT replacement. A 2-way SA condition × CORT condition ANOVA examining nadir CORT levels under basal conditions and following SA in ADX/CORT-replaced rats and sham controls failed to show significant main effects of SA or ADX/CORT replacement condition on plasma CORT but did show a significant SA × CORT condition interaction (F1,10=13.038; P<0.01). Although the CORT response was substantially less than that evoked by EFS, SA increased CORT compared to basal nadir values in sham-treated but not ADX/CORT-replaced rats. Altogether these data indicate that the ADX/CORT replacement regimen mimicked the diurnal rhythm of CORT but eliminated the CORT responses to EFS and SA.
Figure 3
Circadian and EFS- and SA-induced increases in plasma corticosterone (CORT) in adrenalectomized rats with diurnal CORT replacement and sham-operated/cholesterol pellet-implanted controls. Surgical adrenalectomy (ADX) along with diurnal CORT replacement reproduced the circadian rhythm for plasma CORT but eliminated the plasma CORT responses to EFS or SA. Data represent the mean plasma CORT concentration (ng/ml±SE) at the peak and nadir of the circadian cycle, 15 min following a 5-min EFS exposure (post-EFS) or immediately following a 2-h SA session (post-SA) in ADX rats with diurnal CORT replacement (ADX/C) and Sham-treated controls (Sham). EFS and SA CORT determinations were made at the circadian nadir. *P<0.05, significant vs ADX/C.
Experiment 3b: Effects of ADX and diurnal CORT replacement on the EFS-induced escalation of cocaine SA
Figure 4 shows the effects of ADX and diurnal CORT replacement on cocaine SA in rats tested in the presence or absence of repeated EFS. A 3-way ANOVA examining effects of ADX, EFS, and SA under basal conditions and across the 14 days of SA testing showed a significant main effect of SA day (F14,392=1.953; P<0.05), but not EFS or ADX conditions and significant interactions between EFS and ADX conditions (F1,28=4.022; _P_=0.05) and between SA day and EFS and ADX conditions (F14,392=3.931; P<0.001). Separate 1-way ANOVAs showed that significant time-dependent increases in SA emerged only in sham-operated rats that were repeatedly exposed to EFS (F14,84=3.623; P<0.001) and not in ADX/CORT-replaced or sham-treated rats that did not receive daily EFS or in ADX/CORT-replaced rats that received daily EFS. In fact, in ADX rats, post-hoc testing showed that EFS produced significant reductions in SA on days 1, 5, 6, 7, and 11 of testing (Fisher's LSD; P<0.05). ADX along with diurnal CORT replacement failed to alter cocaine SA in the absence of EFS.
Figure 4
Effects of adrenalectomy (ADX) and diurnal corticosterone (CORT) replacement on the EFS-induced escalation of cocaine SA. Surgical ADX along with diurnal CORT replacement (ADX/C) prevented the escalation of cocaine SA by repeated daily EFS but had no significant effects on SA in the absence of EFS. In fact, EFS-induced decreases in SA were observed in ADX/CORT-replaced rats. Data represent the mean number of self-administered infusions per 2-h session (±SE) under basal conditions (BAS) and across 14 days of SA testing in sham-treated rats (SHAM) and ADX rats with diurnal CORT replacement (ADX/C) tested with (EFS) or without (No EFS) repeated daily EFS (SHAM+No EFS, _n_=9; SHAM+EFS, _n_=7; ADX/C+No EFS, _n_=9; ADX/C+EFS, _n_=7). *P<0.05, significant increase vs BAS; #P<0.05, significant decrease vs BAS.
Experiment 3c: Effect of repeated daily EFS on EFS-induced increases in plasma CORT
To examine the possibility that basal or EFS-induced CORT secretion was altered over the course of 14 days of EFS exposure, plasma CORT was measured at the circadian nadir under basal conditions and following EFS in drug-naive rats with no prior history of EFS and in drug-naive rats exposed to the 14-day EFS regimen used in our SA experiment. A 2-way EFS condition (EFS vs baseline nadir) × EFS history (no prior EFS vs 13 days prior EFS) ANOVA failed to show significant effects of EFS history or a significant EFS condition × EFS history interaction. Basal CORT levels were 50.56 (±10.68) ng/ml in rats with no EFS history and 92.9 (±35.62) ng/ml in rats that received 14 days of EFS. CORT levels following EFS were 293.91 (±9.37) ng/ml in rats exposed to EFS for the first time and 265.17 (±30.77) ng/ml in rats with a 14-day history of EFS. Although these findings suggest that the CORT response to EFS was likely not altered with repeated testing in our rats, we did not examine alterations in EFS-induced increases in plasma CORT in rats that received daily EFS and were tested for cocaine SA.
Experiment 4: Effects of IP CORT Administration on Cocaine SA
Experiment 4a: Effects of IP CORT administration on plasma CORT levels
Intraperitoneal CORT administration (3.0 mg/kg, i.p. in extra virgin olive oil) to ADX rats without circadian CORT replacement produced a transient increase in plasma CORT. At the time of administration, CORT levels were undetectable. One hour after administration the plasma CORT concentration increased to 292.7 ng/ml (±33.6). After 2, 4, and 8 h, the plasma CORT concentrations were 348.4 ng/ml (±26.7), 368.6 ng/ml (±18.0), and 125.5 ng/ml (±20.7), respectively. Twenty-four hours after administration, plasma CORT was undetectable in all but one rat (15.8±14.1).
Experiment 4b: Effects of repeated daily IP CORT administration in the presence or absence of EFS on cocaine SA in ADX Rats
Figure 5 shows the effects of repeated i.p. CORT administration on cocaine SA in ADX rats with diurnal CORT replacement in the presence or absence of EFS. A 3-way ANOVA examining effects of EFS, CORT administration, and SA under basal conditions and across the 14 days of SA testing in ADX/CORT-replaced rats showed no significant main effects but a significant EFS, CORT administration, and test-day interaction (F14,364=2.129; _P_=0.01). Separate 1-way ANOVAs were performed to examine time-dependent alterations in SA in each group. As described in Experiment 3b, EFS-induced escalation was not observed in ADX/CORT-replaced rats in the absence of i.p. CORT injections. In fact, post-hoc testing showed that EFS produced significant reductions in SA on days 1, 5, 6, 7, and 11 of testing in ADX/CORT-replaced rats (P<0.05). However, significant EFS-induced increases in SA were observed when ADX rats with diurnal CORT replacement were injected with CORT before EFS each day (F14,84=3.446; P<0.01), suggesting that CORT administration restored the effects of EFS. Post-hoc analysis using the Fisher's LSD test showed that responding by these rats was significantly increased compared to baseline on SA days 3, 7, 10, 11, 12, and 14 (P<0.05). By contrast, when CORT was administered without EFS significant increases in SA did not emerge.
Figure 5
Effects of i.p. corticosterone (CORT) administration in the presence or absence of EFS on cocaine SA in adrenalectomized rats with diurnal CORT replacement. Daily i.p. CORT injections administered to ADX rats with diurnal CORT replacement (ADX/C) failed to reproduce the effects of repeated daily EFS on cocaine SA. However, CORT administration before EFS restored the EFS-induced escalation of SA. Data represent the mean number of self-administered infusions per 2-h session (±SE) under basal conditions (BAS) and across 14 days of SA testing in ADX rats with diurnal CORT replacement (ADX/C) that received neither CORT injections nor EFS (ADX/C ONLY; _n_=9), rats that received no CORT injections but received EFS (ADX/C+EFS; _n_=7), rats that received CORT injections but no EFS (ADX/C+IP CORT; _n_=7), or rats that received both CORT injections and EFS (ADX/C+IP CORT AND EFS; _n_=8). *P<0.05, significant increase vs BAS; #P<0.05, significant decrease vs BAS.
DISCUSSION
This study demonstrates that repeated daily exposure to a stressor, uncontrollable EFS, escalates drug intake by cocaine self-administering rats. By contrast, EFS administered under similar conditions did not increase responding on a second, inactive lever and failed to augment food-reinforced responding, suggesting that the observed escalation of cocaine intake was not the consequence of nonspecific stimulatory effects of EFS on behavior. Escalating patterns of drug SA have been proposed to reflect the loss of control over drug use that is fundamental to human cocaine addiction. Previously, it has been reported that such patterns of drug consumption emerge when daily access to cocaine for SA is prolonged and are associated with a persistently heightened motivation to engage in cocaine-seeking behavior (Ahmed and Koob, 1998, 1999; Deroche-Gamonet et al, 2004; Mantsch et al, 2004), suggesting that the experience of using cocaine can predispose individuals towards addiction. Here we report that chronic stress during a period of ongoing cocaine SA also escalates drug intake, implying that environmental factors may also facilitate the onset of addiction by reproducing and/or promoting pathogenic cocaine-induced neuroplasticity.
A number of studies have established that exposure to a variety of stressors, including uncontrollable EFS, facilitates the acquisition of cocaine SA by rats (see eg Goeders and Guerin, 1994; Haney et al, 1995; Miczek and Mutschler, 1996; Ramsey and Van Ree, 1993; Campbell and Carroll, 2001; Kosten et al, 2000; Schenk et al, 1987) and reinstates extinguished cocaine-seeking behavior (Erb et al, 1996; Ahmed and Koob, 1997; Mantsch and Goeders, 1999a; Shalev et al, 2003). Our finding that daily EFS delivered across a period of ongoing SA escalates cocaine intake after acquisition has occurred extends these findings and is consistent with reports that food deprivation elevates post-acquisition cocaine SA (Carroll, 1985) and that repeated social-defeat stress extends the duration of SA and increases the cumulative amount of cocaine self-administered by rats during unlimited-access ‘binges’ (Covington and Miczek, 2001). Altogether, these findings indicate that stress can influence a number of aspects of the addiction process and therefore should serve as a therapeutic target for the management of cocaine addiction.
In the present study, EFS-induced escalation of cocaine SA was observed when EFS was administered within the SA chambers immediately before daily SA testing, but not when it was delivered under identical parameters either inside or outside of the SA environment but temporally removed from (ie 4 h after) the daily SA sessions. The requirement for EFS delivery at the time of SA for the escalating effects of EFS can be interpreted in several ways. First, it is possible that concurrent exposure to EFS and cocaine is required to produce neuroplasticity leading to escalated SA, in which case EFS administered in the absence of self-administered cocaine would not be expected to escalate SA. Secondly, it is possible that EFS produces transient effects on cocaine SA that only emerge with repeated testing as significant increases in SA did not appear immediately, but took several sessions to develop and continued to intensify across SA testing. A progressive augmentation of stressor-induced cocaine SA could be attributable to a recruitment of new inputs relaying stressor-related signals to the motivational neurocircuitry responsible for cocaine-seeking behavior or could be the result of a strengthening of existing inputs.
It is also possible that the increased SA observed in the present study represented a conditioned response, such that rats learned to self-administer more cocaine in the presence of EFS. Like EFS, food deprivation at the time of cocaine SA has been reported to transiently elevate cocaine SA once it has been acquired (Carroll and Meisch, 1984) and to reinstate cocaine-seeking behavior following a period of extinction (Carroll, 1985; Shalev et al, 2003). Carroll (1985) reported that increases in cocaine-seeking behavior by food deprivation were only observed in rats with a history of deprivation at the time of SA and dissipated when rats were repeatedly food deprived in the absence of cocaine, suggesting that the increase in SA observed with food deprivation was attributable to classical conditioning of the interoceptive state produced by the stressor with the reinforcing effects of cocaine. This interpretation is consistent with our own finding that EFS escalated SA when presented at the time of SA testing but not when EFS administration was temporally removed from the SA sessions. The ability of an EFS-induced interoceptive state to set the stage for SA would suggest that (1) EFS-induced increases in SA should subside when rats are tested for SA in the absence of EFS; (2) rats self-administering in the presence of EFS should be more susceptible to EFS-induced reinstatement; and (3) repeated presentation of EFS in the absence of SA should attenuate EFS-induced effects on cocaine-seeking behavior. These issues and the potential role of classical conditioning in EFS-induced escalation will be investigated in future studies.
In order to examine the potential role of CORT in the effects of repeated EFS on cocaine SA, we tested surgically adrenalectomized rats with diurnal CORT replacement for EFS-induced escalation. These rats displayed a normal circadian rhythm of plasma CORT concentrations but failed to show evoked increases in CORT in response to either EFS or cocaine SA. In contrast to rats that received sham treatment, repeated EFS failed to escalate cocaine SA in ADX/CORT-replaced rats, suggesting that elevated CORT at the time of EFS, either as a consequence of cocaine SA or EFS, was necessary for EFS-induced escalation. Despite preventing EFS-induced escalation, ADX and diurnal CORT replacement failed to alter cocaine SA in the absence of EFS, a finding that is consistent with previous reports that cocaine-induced CORT secretion is not required for the reinforcing or reinstating effects of cocaine in rats (Deroche et al, 1997; Erb et al, 1998; Mantsch and Goeders, 1999b), non-human primates (Broadbear et al, 1999a, 1999b; Lee et al, 2003) and human cocaine addicts (Ward et al, 1998; Kosten et al, 2002). Interestingly, complete elimination of circulating CORT via ADX in the absence of circadian CORT replacement has been reported to attenuate ongoing cocaine SA by rats (Goeders and Guerin, 1996b; Deroche et al, 1997). However, as was the case in our study, it has been reported that SA is restored to control levels once circadian CORT fluctuations are re-established (Deroche et al, 1997).
When ADX rats with diurnal CORT replacement were tested for SA in the presence of EFS, not only was EFS-induced escalation prevented, but significant EFS-induced reductions in cocaine SA were observed. Thus, EFS had opposite effects on SA in the presence and absence of a CORT response, suggesting that elevated CORT levels are not only required for stressor-induced escalation but are also necessary for sustained levels of responding in the presence of stress. Although it is unclear how this occurs, we speculate that CORT either inhibits or offsets one or more secondary response-restricting effects of EFS, such that in the absence of elevated CORT, stressor-induced reductions in SA emerge. The ability of CORT to counteract stressor-induced suppression of behavior may provide insight into its potential role as mediator of behavioral responses that permit organisms to cope with stress.
After establishing that elevated CORT was necessary for the escalation of cocaine SA by EFS, we next tried to reproduce the effects of repeated EFS by administering daily i.p. injections of CORT at a dose that produced transient increases in plasma CORT comparable to those evoked by EFS. In contrast to EFS, daily CORT administration failed to escalate SA. This finding was somewhat surprising, considering that administration of CORT at doses that produce blood concentrations within the stressor-induced range has been previously reported to mimic the effects of EFS on the acquisition of cocaine SA (Mantsch et al, 1998) and reinstatement of extinguished cocaine-seeking behavior by EFS (Deroche et al, 1997). Based on these observations, we hypothesized that CORT was not directly mediating the effects of EFS on SA, but rather was exerting a permissive role in the effects of EFS by enabling EFS-induced escalation. To test this hypothesis, we simulated EFS-induced CORT secretion by administering i.p. CORT immediately before delivery of EFS to ADX/CORT-replaced rats during SA testing. Despite having no effects on SA by itself, i.p. CORT injections restored the escalation of SA by EFS, suggesting that elevated CORT was necessary, but alone was not sufficient, for the effects of EFS on SA. A similar permissive role for CORT in the reinstatement of cocaine-seeking behavior by acute food deprivation stress has been reported (Shalev et al, 2003).
The lack of effect of repeated CORT administration on cocaine SA in the absence of EFS in the present study differs from our previously published report that CORT administration under a similar regimen facilitated the acquisition of cocaine SA (Mantsch et al, 1998). There were a number of differences between the two studies that may have accounted for the disparate findings. Most importantly, whereas the present study examined the effects of CORT on cocaine SA after the acquisition of SA had already occurred, the earlier study examined the effects of CORT on SA by drug-naive rats during the acquisition phase. Elevated CORT at the time of an emotionally arousing event has been reported to improve the consolidation of memory of that event (Buchanan and Lovallo, 2001; Roozendaal, 2002 for review). Thus it is possible that the effects of CORT on acquisition represent facilitated learning of the SA operant task rather than the production of addiction-related neuroplasticity. Additionally, in our previous study rats were maintained at 85% of their free-feeding weights in order to permit concurrent determination of food-reinforced behavior. As described above, food restriction is a stressor and has been reported to elevate cocaine SA (see eg Carroll, 1985). Thus, in contrast to the present study, the effects of CORT on the acquisition of SA were examined during a period of ongoing stress. Notably, in the present study, CORT escalated SA when delivered along with EFS. It would be of interest to determine whether or not a facilitated acquisition of cocaine SA by CORT can be produced in free-feeding rats.
Consistent with prior reports (Mantsch et al, 2000, 2003; Galici et al, 2000; Broadbear et al, 1999a, 1999b), cocaine SA in the absence of EFS elevated CORT in our rats. Although SA-induced increases in plasma CORT were less pronounced than those evoked by EFS, the observed elevation of CORT in the absence of increased SA provides further evidence that elevated CORT alone is insufficient to produce escalation and suggests that CORT is exerting its permissive effects on neurobiological systems that are directly responsive to stressors and not cocaine. Although it is not clear exactly which glucocorticoid-dependent stressor-responsive target is responsible for the escalating effects of EFS, a strong case can be made for corticotropin releasing factor (CRF), which has been identified as a mediator of EFS-induced but not cocaine-induced reinstatement of extinguished cocaine-seeking behavior (Erb et al, 1998; Shaham et al, 1998 and see Sarnyai et al, 2001 for review). Determination of the precise role of CRF in the glucocorticoid-dependent effects of repeated EFS on cocaine SA will require further investigation.
In summary, glucocorticoids are important mediators of the physiological and behavioral responses that permit organisms to adapt to and/or cope with stressors. Such responses are beneficial for short-term adaptation to a stressor, but with constant activation, can produce effects that are detrimental to an organism, a consequence of chronic stress referred to as allostatic load (McEwen, 1998). In the case of motivation neurocircuitry within the brain, which is likely critical for the behavioral adaptations to stressors, an apparent glucocorticoid-dependent consequence of chronic stress is a heightened responsiveness to drugs of abuse that may increase susceptibility to addiction. Further characterization of the relationship between stress, glucocorticoids, and cocaine-seeking behavior will likely lead to the development of more effective approaches for the management of cocaine addiction, particularly in individuals whose drug use is stress related.