Lower extremity skeletal muscle function in persons with incomplete spinal cord injury (original) (raw)
Introduction
Approximately 200 000 persons with spinal cord injury (SCI) live in the United States alone; with roughly 11 000 new injuries occurring each year.[1](/articles/3101892#ref-CR1 " Ref-www.spinalcord.uab.edu
, accessed Jan 2005.") In addition, the relative number of new injuries is rising and expected to have increased by approximately 20% by 2010, compared to the 1994 prevalence. The reported costs associated with the care and treatment of persons after SCI is estimated to range between <span class="katex"><span class="katex-mathml"><math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow><mn>15000</mn><mi>a</mi><mi>n</mi><mi>d</mi></mrow><annotation encoding="application/x-tex">15 000 and </annotation></semantics></math></span><span class="katex-html" aria-hidden="true"><span class="base"><span class="strut" style="height:0.6944em;"></span><span class="mord">15000</span><span class="mord mathnormal">an</span><span class="mord mathnormal">d</span></span></span></span>125 000 annually, with an approximate lifetime total of <span class="katex"><span class="katex-mathml"><math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow><mn>435000</mn><mtext>–</mtext></mrow><annotation encoding="application/x-tex">435 000–</annotation></semantics></math></span><span class="katex-html" aria-hidden="true"><span class="base"><span class="strut" style="height:0.6444em;"></span><span class="mord">435000–</span></span></span></span>1 590 000.[1](/articles/3101892#ref-CR1 "
Ref-www.spinalcord.uab.edu
, accessed Jan 2005.") Interestingly, a significant proportion of these costs can be related to the loss of skeletal muscle mass and associated secondary health-related complications,[2](/articles/3101892#ref-CR2 "Shields RK . Muscular, skeletal, and neural adaptations following spinal cord injury. J Orthop Sports Phys Ther 2002; 32: 65–74.") (ie non-insulin dependent diabetes mellitus, cardiovascular disease, osteoporosis). A decrease in skeletal muscle function has been described as one of the most significant problems impacting the health care and quality of life of persons after SCI.[2](/articles/3101892#ref-CR2 "Shields RK . Muscular, skeletal, and neural adaptations following spinal cord injury. J Orthop Sports Phys Ther 2002; 32: 65–74."), [3](/articles/3101892#ref-CR3 "Stewart BG, Tarnopolsky MA, Hicks AL, McCartney N, Mahoney DJ, Staron RS et al. Treadmill training-induced adaptations in muscle phenotype in persons with incomplete spinal cord injury. Muscle Nerve 2004; 30: 61–68.") Consequently, the potential to decrease costs and improve quality of life by maintaining or partially restoring skeletal muscle size and function seems high.Due, in part, to advancements in the quality of emergency care, the relative number of injuries classified as incomplete has risen dramatically over the past 20 years.[4](/articles/3101892#ref-CR4 " www.ninds.nih.gov/disorders/sci
,accessed April 2005.") In fact, the majority of new injuries occurring annually are now classified as incomplete.[1](/articles/3101892#ref-CR1 "
Ref-www.spinalcord.uab.edu
, accessed Jan 2005.") Despite the rise in the proportion of persons with incomplete-SCI; the preponderance of scientific literature describing the effects of SCI on skeletal muscle involves persons with complete injuries.[5](/articles/3101892#ref-CR5 "Modlesky CM, Slade JM, Bickel CS, Meyer RA, Dudley GA . Deteriorated geometric structure and strength of the midfemur in men with complete spinal cord injury. Bone 2005; 36: 331–339."), [6](/articles/3101892#ref-CR6 "Castro MJ, Apple Jr DF, Rogers S, Dudley GA . Influence of complete spinal cord injury on skeletal muscle mechanics within the first 6 months of injury. Eur J Appl Physiol 2000; 81: 128–131."), [7](/articles/3101892#ref-CR7 "Mangold S, Keller T, Curt A, Dietz V . Transcutaneous functional electrical stimulation for grasping in subjects with cervical spinal cord injury. Spinal Cord 2005; 43: 1–13."), [8](/articles/3101892#ref-CR8 "Skold C, Harms-Ringdahl K, Seiger A . Movement-provoked muscle torque and EMG activity in longstanding motor complete spinal cord injured individuals. J Rehabil Med 2002; 34: 86–90.") To date, very little data exits describing muscle function in persons with chronic incomplete-SCI. Interestingly, the innate plasticity associated with incomplete-SCI furnishes these persons with the potential to progress functionally to a greater extent than the complete SCI population.[9](/articles/3101892#ref-CR9 "Waters RL, Adkins RH, Yakura JS, Sie I . Motor and sensory recovery following incomplete tetraplegia. Arch Phys Med Rehabil 1994; 75: 306–311."), [10](/articles/3101892#ref-CR10 "Muslumanoglu L, Aki S, Ozturk Y, Soy D, Filiz M, Karan A et al. Motor, sensory and functional recovery in patients with spinal cord lesions. Spinal Cord 1997; 35: 386–389.") In addition, novel intervention therapies have shown promise in promoting spinal plasticity and motor function after SCI. However, improvements in functional capacity in persons with I-SCI with rehabilitation vary greatly and the incidence of disability still remains high.[11](/articles/3101892#ref-CR11 "Herbison GJ, Isaac Z, Cohen ME, Ditunno JF . Strength post-spinal cord injury: myometer versus manual muscle test. Spinal Cord 1996; 34: 543–548."), [12](/articles/3101892#ref-CR12 "Edelle C . Field-fote. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil 2001; 82: 818–824.")In order to provide a foundation for the development of rehabilitation strategies targeting neuromuscular deficits in persons with incomplete-SCI, a need exists to characterize and objectively quantify existing impairments. Therefore, the purpose of this study was to quantify lower extremity muscle function in persons with chronic incomplete-SCI compared to age-, gender-, height- and body weight matched healthy controls. Specifically, we measured isometric peak torque and performed measures of explosive or instantaneous muscle strength in the knee extensor and ankle plantar flexor muscle groups and quantified voluntary activations deficits using a combination of voluntary contractile measurements and superimposed electrical stimulation.
Methods
Subjects
In total, 10 persons with chronic, upper motor neuron lesions and motor incomplete-SCI participated. Characteristics of the persons with incomplete-SCI are provided in Table 1. Average age, height and body mass±standard deviation (SD) at the time of the study enrollment were 45.4±14.8 years, 155.9±9.4 cm, and 79.9±12.2 kg. Eight of the subjects were classified ASIA D and two as ASIA C. Four subjects were able to ambulate over ground, while six used a wheelchair as their primary mode of locomotion. The incomplete-SCI subjects were matched on the basis of age, gender, height and weight with 10 recreationally active controls (45.1±14.9 years, 159.1±9.0 cm, and 78.0±11.7 kg). Prior to participating in the study, written informed consent was obtained from all subjects, as approved by the Institutional Review Board at the University of Florida, Gainesville.
Table 1 Characteristics of incomplete SCI subjects
Experimental setup
Voluntary and electrically elicited contractile measurements were performed in the self-reported more-involved and less-involved limbs for the knee extensor and plantar flexor muscle groups, using a Biodex System 3 Dynamometer (Biodex Medical Systems, Inc., 20 Ramsay Road, Shirley, NY 11967, USA). Knee extensor testing was performed with subjects seated in an upright position with hips flexed to ∼85° and knees flexed to ∼90°. The axis of rotation of the dynamometer was aligned with the axis of the knee joint and the lever arm secured against the anterior aspect of the leg, proximal to the lateral malleolus. Testing of the plantar flexor muscle group was performed with the hips flexed at 90–100°, the knee flexed at ∼10° and the ankle at ∼0° plantar flexion, as previously described.13, 14 The anatomical axis of the ankle was aligned with the axis of the dynamometer, while the foot was secured to the footplate with straps placed at the forefoot and ankle. Proximal stabilization was achieved with straps across the chest, hips and thigh. Electrical stimulation was performed using a Grass S8800 stimulator with a Grass Model SIU8T stimulus isolation unit (Grass Instruments, West Warwick, Rhode Island, USA). Electrically induced contractions were delivered through two 3.0″ by 5.0″ self-adhesive neuromuscular stimulation electrodes placed over the proximal and distal portions of the muscle group being tested.
The stimulator and the dynamometer were interfaced with a personal computer through a commercially available hardware system (MP150 system) (BIOPAC systems Inc., Goleta, CA, USA). The data were sampled at 400 Hz and analyzed with commercially available software (AcqKnowledge 3.7.1).
Voluntary contractile measurements
Prior to testing, subjects performed three warm-up contractions to get familiarized with the testing procedures. Subjects then performed three maximal voluntary isometric contractions (∼5 s each with 1 min rest intervals) while being given verbal encouragement. Peak torque was defined as the highest value obtained during the three maximal isometric contractions. In the event that the peak torque values differed by more than 10%, additional contractions were performed.
In addition to peak torque we also determined the average rate of torque development (ARTD) and the torque200, as indices of explosive muscle strength. The ARTD was defined as the average increase in torque generated in unit time, and was calculated in the time interval corresponding to 20–80% of peak amplitude, starting from muscle perturbation. This time interval was selected to reduce the effect of errors in calculating peak amplitude. Hence, ARTD was calculated through numerical differentiation as

where, N is the total number of time slots for numerical differentiation, δf i is the change in torque in the time slot i and δt is the unit time duration for a slot. Torque200 was defined as the absolute torque reached at 200 ms during a maximal voluntary contraction (Nm).
Electrically elicited contractile measurements
Peak twitch torque, time to peak twitch and twitch half-relaxation time were determined by delivering a supra-maximal electrical stimulus (600 _μ_s pulse duration) to the muscles at rest. Supra-maximal intensity was determined by increasing the current voltage until twitch torque production plateaued. Time to peak twitch and twitch half-relaxation time were calculated from the peak twitch contractions.
Voluntary activation deficits
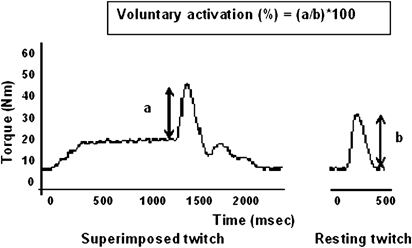
Voluntary activation deficits were determined using the twitch interpolation method.15 Briefly, a single biphasic and supra-maximal pulse was delivered at rest and during maximal voluntary isometric contraction. The voluntary activation deficit was calculated based on the ratio between the torques produced by the superimposition of a supra-maximal twitch on a peak isometric contraction (a) and the torque produced by the same stimulus in the potentiated, resting muscle (b).

Statistical analyses
Independent sample _T_-tests were used to determine if differences existed between the groups. Comparisons were made between the self-reported dominant side of the controls and both the self-reported more involved side and less involved side of the incomplete-SCI group. For all analyses, significance was established when P<0.05. Data are presented as means±standard error of mean. All statistical analyses were performed using SPSS for Windows, Version 11.0.1.
Results
Voluntary contractile measurements
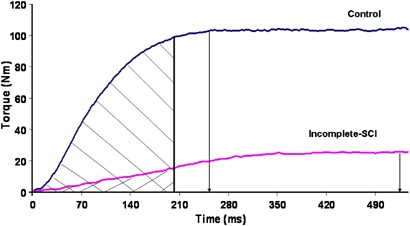
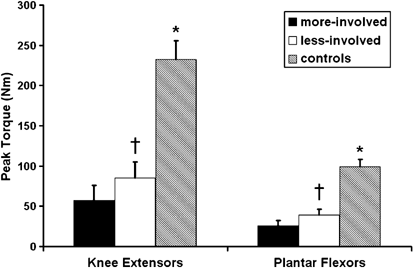
Individuals after incomplete-SCI demonstrated significant deficits in their ability to generate peak isometric torque relative to noninjured controls in both the knee extensor and plantar flexor muscle groups (P<0.05). A representative ankle plantar flexor torque trace acquired during a peak isometric contraction of both incomplete-SCI and control subject is provided in Figure 1. The peak torque deficit measured in both muscle groups was of similar magnitude (Figure 2). Specifically, persons after incomplete-SCI were able to produce 36 and 24% of the knee extensor torque and 38 and 26% of the plantar flexor torque generated by noninjured controls in the less-involved and more-involved limbs, respectively (P<0.01). Significant bilateral asymmetries were noted in peak torque production between the self-reported more-involved versus the less-involved limb in both the knee extensor (57±18 versus 85±20 Nm) and plantar flexor muscle groups (26±6 versus 39±7 Nm; P<0.01).
Figure 1
Representative torque–time curve. Drop down arrows indicate time points at which peak torque is reached in a representative incomplete-SCI and control subject. Shaded areas indicate torque200 in both subjects
Figure 2
Peak torque (Nm) for the knee extensor and plantar flexor muscle groups, comparing the dominant side of the control with the more involved (more-involved) and less involved limb (less-involved) of the incomplete-SCI group. *Significant difference between control group and incomplete-SCI group. †Significant difference between the less-involved versus the more-involved (P<0.05)
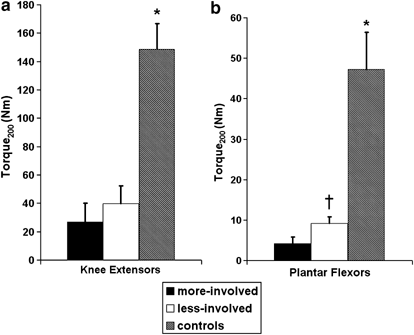
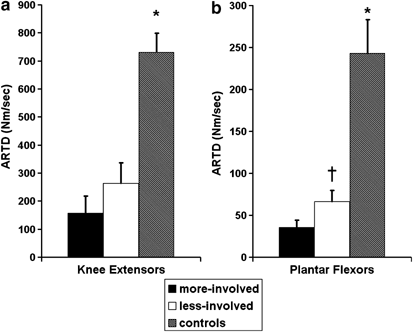
Both indices of explosive muscle strength, ARTD and torque200, were significantly lower in persons with incomplete-SCI relative to controls in both muscle groups tested (P<0.01; Figures 3 and 4). Bilateral asymmetries in torque200 and ARTD were specific to the ankle plantar flexor muscles. Of interest to note is that both indices of explosive muscle strength showed more pronounced deficits in the ankle plantar flexor muscles compared to the knee extensor muscles. In particular, large deficits were noted in the torque200 of the ankle plantar flexor muscles with an 11.7-fold difference between the torque200 measured in the self-reported more involved limb and a five-fold difference in the less-involved limb compared to control muscles (Figures 1 and 3). The torque200 was 4.2±1.6 Nm in the plantar flexor muscles of the more-involved limb, 9.2±1.6 Nm in the less-involved limb and 47.2±9.2 Nm in the noninjured controls, respectively. In contrast, a 5.5-fold difference and 3.7-fold difference was noted in the torque200 measured in the knee extensor muscles of the self-reported more involved (27.0±13.2 Nm) and less involved limb (39.9±12.3 Nm) of incomplete-SCI persons compared to noninjured controls (148.6±18.3 Nm). Torque200 and ARTD data are summarized in Figures 3 and 4.
Figure 3
(a, b) Torque200 (Nm) for the knee extensor and plantar flexor muscle groups, comparing the dominant side of the control with the more involved (more-involved) and less involved limb (less-involved) of the incomplete-SCI group. *Significant difference between control group and incomplete-SCI group. †Significant difference between the less-involved versus the more-involved (P<0.05)
Figure 4
(a, b) Average rate of torque development (ARTD) (Nm/s) for the knee extensor and plantar flexor muscle groups, comparing the dominant side of the control with the more involved (more-involved) and less involved limb (less-involved) of the incomplete-SCI group. *Significant difference between control group and incomplete-SCI group. †Significant difference between the less-involved versus the more-involved (P<0.05)
Electrically elicited contractile measurements
No significant differences were found either within or between-subject groups for measures of peak twitch torque, time to peak twitch or half-relaxation times in either the knee extensor or plantar flexor muscle groups (Table 2).
Table 2 Electrically elicited contractile measurements
Voluntary activation deficits
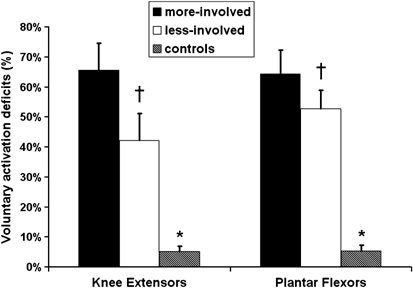
A significant injury related effect on the ability to voluntarily activate the plantar flexor and knee extensor muscle groups was noted. Activation deficits in the knee extensors were 42±8 and 66±9% in the less involved and more involved side, respectively, compared to only a 5±2% deficit in noninjured controls. The incomplete-SCI group also demonstrated a 53±6% voluntary activation deficit in the less involved side and a 64±8% deficit in the more involved side for the plantar flexor muscle group, compared to a 5±2% deficit in noninjured controls (Figure 5). Significant bilateral asymmetries existed for both muscle groups for voluntary activation deficits (P<0.05, Figure 5). A representative torque trace acquired during a peak isometric voluntary contraction with interpolated twitch is provided in Figure 6.
Figure 5
Voluntary activation deficits (%) for the knee extensor and plantar flexor muscle groups, comparing the dominant side of the control with the more involved (more-involved) and less involved limb (less-involved) of the incomplete-SCI group. *Significant difference between control group and incomplete-SCI group. †Significant difference between the less-involved versus the more-involved (P<0.05)
Figure 6
Torque trace acquired during MVIC with interpolated twitch to quantify muscle activation deficit. A single supramaximal intensity electrical stimulus was superimposed on a maximal voluntary isometric contraction (a), as well as on a resting, potentiated plantar flexor muscle (b)
Discussion
The development of novel intervention therapies to promote the recovery of skeletal muscle function after incomplete-SCI is one of the exciting paths of current rehabilitation research.16, 17, 18, 19, 20, 21, 22 However, the translation of these experimental therapies to the SCI population is enormously challenging given the extreme heterogeneity in presentation and response to treatment of this population. As such, a comprehensive examination of skeletal muscle function in this patient population might aid in the development of targeted therapies aimed at the recovery of muscle function after incomplete-SCI. Accordingly, the present study demonstrates that after chronic upper motor lesions and incomplete-SCI, both knee extensor and plantar flexor skeletal muscles (1) generate ∼70% less peak torque, (2) demonstrate significant bilateral asymmetry in peak torque, which matches the hierarchy for self-reported functional deficits, (3) experience voluntary activation deficits ranging between 42 and 66%, and (4) demonstrate large deficits in the rate of torque development and instantaneous muscle strength. While in this study, both muscle groups demonstrated significant impairments in ARTD and torque200, more pronounced deficits were noted in the ankle plantar flexor muscles and bilateral asymmetries in ARTD and torque200 were specific to the ankle plantar flexor muscles. Given the role of the ankle plantar flexor muscles in propulsion during gait, we put forward that the latter impairments should be targeted in rehabilitative interventions aiming to restore or promote locomotion in this population.
The deficits noted between persons after incomplete SCI and controls in their ability to generate peak torque in the plantar flexor and knee extensor muscle groups may appear somewhat intuitive. In addition, the bilateral asymmetries observed may be considered obvious by many after this type of injury. However, no quantitative measurements of muscle function have previously been reported in this population. Moreover, we contend that the methodologies described here are more suitable than traditional evaluative tests in assessing impairments of muscle function in persons with incomplete-SCI. Muscle strength assessments in persons with incomplete-SCI are typically performed using manual muscle tests during ASIA evaluations. The ASIA is used routinely to describe the level of injury and impairment and imply severity of injury.23, 24 However; this evaluative tool may not be adequate to direct targeted rehabilitation interventions in persons with incomplete-SCI. Manual muscle tests are subject to a ceiling effect, lack sensitivity to change and have a relatively poor inter-rater reliability, especially at scores greater than 3.25, 26
A myriad of physiological changes occur in persons after SCI. Many of these changes are due to the direct effects of the injury (ie neural circuitry disruption) while others are secondary in nature and attributable to a resultant decrease in neuromuscular activity. An inability to voluntarily activate skeletal muscles may be a product of both primary and secondary mechanisms. Twitch interpolation is a commonly used method to estimate the extent to which a person can voluntarily activate a given muscle or muscle group.27, 28, 29, 30 Our findings of small activation deficits (∼5%) in the quadriceps and ankle plantar flexor muscles of noninjured controls are consistent with those from other laboratories.30 The activation deficits measured in persons with incomplete-SCI (42–66%) are larger in magnitude compared to those measured in patients early after surgery or long-term immobilization.15, 31 Accordingly, persons with incomplete-SCI may benefit from rehabilitation strategies that target voluntary activation deficits to maximize skeletal muscle function, that is, functional electrical stimulation or bio-feedback.32 While these interventions may not directly impact the primary injury, they may be able to ameliorate the loss of muscle function secondary to disuse or lack of neuromuscular activity.
Perhaps the most functionally relevant characteristics of muscle torque production for persons with incomplete-SCI are the indices of explosive strength. ARTD is reflective of the average rate of contractile torque development during maximum voluntary contraction while torque200 is the absolute torque generated within the initial 200 ms of contraction and is indicative of the magnitude of instantaneous torque. Both ARTD and torque200 were significantly reduced in the ankle plantar flexor and quadriceps muscle groups of persons with incomplete-SCI. However, the deficit in instantaneous strength was more pronounced in the ankle plantar flexor muscles. It is our contention that the initial rate of torque development and the instantaneous strength may be most critical for performance of functional tasks (ie walking). For example, steady-state walking is characterized by repetitive, reciprocal contractions of the plantar flexor muscles (ie propulsion at push off) that must be accomplished in finite periods of time. A speed commonly deemed necessary for persons to safely ambulate in the community is 1.2 m/s.17 At this speed, the time it takes to complete one gait cycle (ie right heel strike to right heel strike) is ∼1.0 s. Given that the plantar flexor muscles are reported to be active for ∼40% of the gait cycle and approximately 1/2 of that time is spent generating concentric torque, roughly 200 ms is available for torque generation by this muscle group.33 Given this available time, plantar flexor muscles must generate torque of sufficient magnitude and at precise rates so as to propel the mass of the body forward, translating to movement or walking.34 We speculate that the large deficits in instantaneous torque in the ankle plantar flexors observed in this study (11.7 and five-fold difference in torque200) may potentially limit locomotor function in persons with incomplete-SCI. Thus, rehabilitative strategies must be employed that result in improved rates of torque production and enhanced instantaneous torque to meet the imposed demands of walking at community ambulating speeds.35 Although we chose to examine torque generation at 200 ms based on our calculations of muscular demands at a functionally minimal gait speed (1.0 m/s), consideration should also be given to the fact that as functional improvements are realized, the contractile demands (ie magnitude and rate of force production) will continue to increase and the available time to generate torque will decrease.
An interesting finding in the present study was the lack of difference in the electrically elicited contractile properties between persons after incomplete-SCI and noninjured controls. Previous studies have used these properties as a means to explain molecular and histochemical changes that occur in skeletal muscle.2 Studies using both animal and human models have provided evidence for faster contractile properties following SCI.2, 36, 37, 38 However, we observed no differences in the rate of rise or relaxation of electrically elicited contractions in muscles after incomplete-SCI relative to non-injured controls. This is somewhat surprising in that both the knee extensor and plantar flexor muscle groups have been characterized by faster contractile speeds following SCI.36, 37 These findings have been used to support the idea of a fiber type transformation following SCI (slow → fast). However, whether a fiber type transition occurs after incomplete-SCI and the timeline for any potential shift are yet unclear. Thus, further research and tissue sampling is warranted before we can make any suggestions towards the muscle fiber type transformation based on contractile properties in this population.
In conclusion, this study characterizes the impairments in lower extremity skeletal muscle function in persons after incomplete SCI relative to noninjured controls. The examination of knee extensor and plantar flexor muscle groups in this study is clinically meaningful given the antigravity responsibilities of each of these muscle groups and their purported roles in standing and locomotor function.39, 40 Reduced peak torque production, ARTD and torque200, as well as increased voluntary activation deficits were found to be characteristic of affected muscles below the level of incomplete SCI. In addition, a hierarchy of these impairments existed between limbs with significant bilateral asymmetries in the plantar flexor muscle group for all variables tested. This characteristic asymmetry suggests that recovery and response to rehabilitation may be specific to each side, with rate-limiting factors to functional performance potentially being limb rather than subject specific. We speculate that the large deficit in the rate of torque development and instantaneous torque in the ankle plantar flexors of persons with incomplete-SCI limits locomotor function.
References
- Ref-www.spinalcord.uab.edu, accessed Jan 2005.
- Shields RK . Muscular, skeletal, and neural adaptations following spinal cord injury. J Orthop Sports Phys Ther 2002; 32: 65–74.
Article PubMed PubMed Central Google Scholar - Stewart BG, Tarnopolsky MA, Hicks AL, McCartney N, Mahoney DJ, Staron RS et al. Treadmill training-induced adaptations in muscle phenotype in persons with incomplete spinal cord injury. Muscle Nerve 2004; 30: 61–68.
Article PubMed Google Scholar - www.ninds.nih.gov/disorders/sci,accessed April 2005.
- Modlesky CM, Slade JM, Bickel CS, Meyer RA, Dudley GA . Deteriorated geometric structure and strength of the midfemur in men with complete spinal cord injury. Bone 2005; 36: 331–339.
Article PubMed Google Scholar - Castro MJ, Apple Jr DF, Rogers S, Dudley GA . Influence of complete spinal cord injury on skeletal muscle mechanics within the first 6 months of injury. Eur J Appl Physiol 2000; 81: 128–131.
Article CAS PubMed Google Scholar - Mangold S, Keller T, Curt A, Dietz V . Transcutaneous functional electrical stimulation for grasping in subjects with cervical spinal cord injury. Spinal Cord 2005; 43: 1–13.
Article CAS PubMed Google Scholar - Skold C, Harms-Ringdahl K, Seiger A . Movement-provoked muscle torque and EMG activity in longstanding motor complete spinal cord injured individuals. J Rehabil Med 2002; 34: 86–90.
Article PubMed Google Scholar - Waters RL, Adkins RH, Yakura JS, Sie I . Motor and sensory recovery following incomplete tetraplegia. Arch Phys Med Rehabil 1994; 75: 306–311.
Article CAS PubMed Google Scholar - Muslumanoglu L, Aki S, Ozturk Y, Soy D, Filiz M, Karan A et al. Motor, sensory and functional recovery in patients with spinal cord lesions. Spinal Cord 1997; 35: 386–389.
Article CAS PubMed Google Scholar - Herbison GJ, Isaac Z, Cohen ME, Ditunno JF . Strength post-spinal cord injury: myometer versus manual muscle test. Spinal Cord 1996; 34: 543–548.
Article CAS PubMed Google Scholar - Edelle C . Field-fote. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil 2001; 82: 818–824.
Article Google Scholar - Shaffer MA, Okereke E, Esterhai Jr JL, Elliott MA, Walker GA, Yim SH et al. Effects of immobilization on plantar-flexion torque, fatigue resistance, and functional ability following an ankle fracture. Phys Ther 2000; 80: 769–780.
CAS PubMed Google Scholar - Pathare N, Walter GA, Stevens JE, Yang Z, Okereke E, Gibbs JD et al. Changes in inorganic phosphate and force production in human skeletal muscle following cast immobilization. J Appl Physiol 2005; 98: 307–314.
Article CAS PubMed Google Scholar - Allen GM, Middleton J, Katrak PH, Lord SR, Gandevia SC . Prediction of voluntary activation, strength and endurance of elbow flexors in post polio patients. Muscle Nerve 2004; 30: 172–181.
Article PubMed Google Scholar - Thoumie P, Mevellec E . Relation between walking speed and muscle strength is affected by somatosensory loss in multiple sclerosis. J Neurol Neurosurg Psychiatry 2002; 73: 313–315.
Article CAS PubMed PubMed Central Google Scholar - Kim CM, Eng JJ, Whttaker MW . Level walking and ambulatory capacity in persons with incomplete spinal cord injury: relationship with muscle strength. Spinal Cord 2004; 42: 156–162.
Article CAS PubMed PubMed Central Google Scholar - Gregory CM, Vandenborne K, Huang HF, Ottenweller JE, Dudley GA . Effects of testosterone replacement therapy on skeletal muscle after spinal cord injury. Spinal Cord 2003; 41: 23–28.
Article CAS PubMed Google Scholar - Chilibeck PD, Jeon J, Weiss C, Bell G, Burnham R . Histochemical changes in muscle of individuals with spinal cord injury following functional electrical stimulated exercise training. Spinal Cord 1999; 37: 264–268.
Article CAS PubMed Google Scholar - Sloan KE, Bremner LA, Byrne J, Day RE, Scull ER . Musculoskeletal effects of an electrical stimulation induced cycling programme in the spinal injured. Paraplegia 1994; 32: 407–415.
CAS PubMed Google Scholar - Crameri RM, Weston AR, Rutkowski S, Middleton JW, Davis GM, Sutton JR . Effects of electrical stimulation leg training during the acute phase of spinal cord injury: a pilot study. Eur J Appl Physiol 2000; 83: 409–415.
Article CAS PubMed Google Scholar - Donaldson N, Perkins TA, Fitzwater R, Wood DE, Middleton F . FES cycling may promote recovery of leg function after incomplete spinal cord injury. Spinal Cord 2000; 38: 680–682.
Article CAS PubMed Google Scholar - Maynard FM, Bracken Jr MB . International standards for neurological and functional classification of spinal cord injury. American spinal injury association. Spinal Cord 1997; 35: 266–274.
Article PubMed Google Scholar - Dobkin BH, Apple D, Barbeau H, Basso M, Behrman A, Deforge D et al. Methods for a randomized trial of weight-supported treadmill training versus conventional training for walking during inpatient rehabilitation after incomplete traumatic spinal cord injury. Neurorehabil Neural Repair 2003; 17: 153–167.
Article PubMed PubMed Central Google Scholar - Herbison GJ, Isaac Z, Cohen ME, Ditunno Jr JF . Strength post-spinal cord injury: myometer versus manual muscle test. Spinal Cord 1996; 34: 543–548.
Article CAS PubMed Google Scholar - Noreau L, Vachon J . Comparison of three methods to assess muscular strength in individuals with spinal cord injury. Spinal Cord 1998; 36: 716–723.
Article CAS PubMed Google Scholar - Shield A, Zhou S . Assessing voluntary muscle activation with the twitch interpolation technique. Sports Med 2004; 34: 253–267.
Article PubMed Google Scholar - Todd G, Gorman RB, Gandevia SC . Measurement and reproducibility of strength and voluntary activation of lower-limb muscles. Muscle Nerve 2004; 29: 834–842.
Article PubMed Google Scholar - Pap G, Machner A, Awiszus F . Strength and voluntary activation of the quadriceps femoris muscle at different severities of osteoarthritic knee joint damage. J Orthop Res 2004; 22: 96–103.
Article PubMed Google Scholar - Norregaard J, Bulow PM, Vestergaard-Poulsen P, Thomsen C, Danneskiold-Samoe B . Muscle strength, voluntary activation and cross-sectional muscle area in patients with fibromyalgia. Br J Rheumatol 1995; 34: 925–931.
Article CAS PubMed Google Scholar - Stevens JE, Mizner RL, Snyder-Mackler L . Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J Orthop Res 2003; 2195: 775–779.
Article Google Scholar - Binder-Macleod SA . Electromyographic biofeedback to improve voluntary motor control. In: Snyder-Mackler L, Robinson AJ (eds). Clinical Electrotherapy: Electrotherapy and Electrophysiological Testing 2nd edn. Williams and Wilkins Co: Baltimore, MD 1995, pp 433–449.
Google Scholar - Neptune RR, Kautz SA, Zajac FE . Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech 2001; 34: 1387–1398.
Article CAS PubMed Google Scholar - Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Poulsen D . Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol 2002; 93: 1318–1326.
Article PubMed Google Scholar - Bemben MG, Tuttle TD, Bemben DA, Knehans AW . Effects of creatine supplementation on isometric force-time curve characteristics. Med Sci Sports Exerc 2001; 33: 1876–1881.
Article CAS PubMed Google Scholar - Gerrits HL, Haan A, Hopman MTE, Woude LHV, Sargeant AJ . Influence of muscle temperature on the contractile properties of the quadriceps muscle in humans with spinal cord injury. Clin Sci 2000; 98: 31–38.
Article CAS Google Scholar - Gerrits HL, Haan A, Hopman MTE, Woude LHV, Jones DA, Sargeant AJ . Contractile properties of the quadriceps muscle in individuals with spinal cord injury. Muscle Nerve 1999; 22: 1249–1256.
Article CAS PubMed Google Scholar - Shields RK . Fatigability, relaxation properties, and electromyographic responses of the human paralyzed soleus muscle. J Neurophysiol 1995; 73: 2195–2206.
Article CAS PubMed Google Scholar - Ostchega Y, Dillon CF, Lindle R, Carroll M, Hurley BF . Isokinetic leg muscle strength in older americans and its relationship to a standardized walk test: data from the national health and nutrition examination survey 1999–2000. J Am Geriatr Soc 2004; 52: 977–982.
Article PubMed Google Scholar - Mueller MJ, Minor SD, Schaaf JA, Strube MJ, Sahrmann SA . Relationship of plantar-flexor peak torque and dorsiflexion range of motion to kinetic variables during walking. Phys Ther 1995; 75: 684–693.
Article CAS PubMed Google Scholar
Acknowledgements
We sincerely thank Neeti Pathare and Chetan Phadke for their assistance with data collection and Shivkumar Swaminathan for his help with the data analysis. We also thank all the study participants for their dedication towards this research. Grant support for this study was provided by NIH-KO1HD01348 and the Evelyn F and William L McKnight Brain Institute of the University of Florida. ‘We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research’.
Author information
Authors and Affiliations
- Department of Physical Therapy, University of Florida, Gainesville, FL, USA
A Jayaraman, C M Gregory, M Bowden, J E Stevens, P Shah, A L Behrman & K Vandenborne - Malcom Randall VAMC, Brain Rehabilitation Research Center, Gainesville, FL, USA
M Bowden, J E Stevens & A L Behrman
Authors
- A Jayaraman
You can also search for this author inPubMed Google Scholar - C M Gregory
You can also search for this author inPubMed Google Scholar - M Bowden
You can also search for this author inPubMed Google Scholar - J E Stevens
You can also search for this author inPubMed Google Scholar - P Shah
You can also search for this author inPubMed Google Scholar - A L Behrman
You can also search for this author inPubMed Google Scholar - K Vandenborne
You can also search for this author inPubMed Google Scholar
Additional information
These results were presented in part at the Society for Neuroscience Annual Conference, San Diego, CA, 2004
Rights and permissions
About this article
Cite this article
Jayaraman, A., Gregory, C., Bowden, M. et al. Lower extremity skeletal muscle function in persons with incomplete spinal cord injury.Spinal Cord 44, 680–687 (2006). https://doi.org/10.1038/sj.sc.3101892
- Published: 13 December 2005
- Issue Date: 01 November 2006
- DOI: https://doi.org/10.1038/sj.sc.3101892