Understanding the nature of “superhard graphite” (original) (raw)
- Article
- Open access
- Published: 28 June 2012
Scientific Reports volume 2, Article number: 471 (2012)Cite this article
- 5297 Accesses
- 85 Citations
- 17 Altmetric
- Metrics details
Subjects
Abstract
Numerous experiments showed that on cold compression graphite transforms into a new superhard and transparent allotrope. Several structures with different topologies have been proposed for this phase. While experimental data are compatible with most of these models, the only way to solve this puzzle is to find which structure is kinetically easiest to form. Using state-of-the-art molecular-dynamics transition path sampling simulations, we investigate kinetic pathways of the pressure-induced transformation of graphite to various superhard candidate structures. Unlike hitherto applied methods for elucidating nature of superhard graphite, transition path sampling realistically models nucleation events necessary for physically meaningful transformation kinetics. We demonstrate that nucleation mechanism and kinetics lead to _M_-carbon as the final product. _W_-carbon, initially competitor to _M_-carbon, is ruled out by phase growth. Bct-C4 structure is not expected to be produced by cold compression due to less probable nucleation and higher barrier of formation.
Similar content being viewed by others
Introduction
Computational materials design is a central grand challenge of modern science. Recent progress in the development of theoretical methodologies for structure prediction, especially evolutionary algorithms1, has led to important discoveries confirmed by subsequent experiments2,3. In addition to thermodynamically stable solid phases, metastable modifications are worth exploring both computationally and experimentally because of their sometimes superior physical properties. However, among the infinite number of all possible metastable phases for each compound, only a handful are synthesizable for kinetic reasons. A metastable phase is synthesizable if it has the highest kinetic likelihood of formation at given conditions.
The recently revived debate4,5,6,7,8,9 on the nature of the metastable product of cold compression of graphite10,11,12,13 represents a special case of interest. The transformation of graphite to the thermodynamically stable cubic diamond occurs at high pressures and temperatures (> 5 GPa and 1200–2800 K) and in the presence of catalysts. At ambient temperature, however, a new phase is formed above 15–19 GPa10,11,12,13 and the transition is accompanied by clear changes in the physical properties of carbon4,7.
Experiments have reported an increase in the electrical resistivity12,14,15,16,17,18 and optical transmittance12,15. Raman spectra measurements up to 14 GPa indicate that the frequencies of the 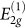 rigid-layer shear mode at 44 cm−1 and the
rigid-layer shear mode at 44 cm−1 and the 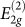 in-plane mode at 1579 cm−1 increase sublinearly under pressure with initial pressure coefficients of 4.8 and 4.7 cm−1/GPa, respectively14. Changes indicating the formation of a new phase have been also observed in the x-ray diffraction pattern13,16,17,18 and in the near _K_-edge spectroscopy13. More remarkable is the increase in hardness, as evidenced by the ability of the new phase to indent diamond anvils13.
in-plane mode at 1579 cm−1 increase sublinearly under pressure with initial pressure coefficients of 4.8 and 4.7 cm−1/GPa, respectively14. Changes indicating the formation of a new phase have been also observed in the x-ray diffraction pattern13,16,17,18 and in the near _K_-edge spectroscopy13. More remarkable is the increase in hardness, as evidenced by the ability of the new phase to indent diamond anvils13.
The difficulty to resolve the crystal structure of this mysterious superhard phase, which we call “superhard graphite”, from experiments has stimulated theoretical efforts4,5,6,7. Hexagonal and cubic diamond have been ruled out because of the absence of their characteristic Raman bands at 1330 cm−1 19. Amorphization was dismissed because of the persistence of Bragg peaks4,13.
Theoretically, candidate structures with distinct topologies featuring different patterns, combining odd (5 and 7) and even (4, 6 and 8) rings, have been proposed. A low-enthalpy monoclinic structure called _M_-carbon (space group _C_2/m, Z = 16) was discovered20 using evolutionary algorithm USPEX and was later identified with “superhard graphite”4. All carbon atoms in this structure are four-coordinate and form 5-membered and 7-membered rings when the structure is projected in (010). Later, a body-centered tetragonal structure called bct-C4 (space group I/4 mmm, Z = 8)5,6,21 was proposed as another candidate. The four-coordinate carbon atoms form 4-membered and 8-membered rings on the (001) projection. Shortly afterwards, an orthorhombic candidate structure called _W_-carbon (space group Pnma, Z = 16) was proposed7. This structure is very similar to that of _M_-carbon, featuring 5-membered and 7-membered rings when the structure is projected in (010). Very recently, an orthorhombic structure, called _o_C16-II (Cmmm, Z = 16), combining even-membered rings (4, 6 and 8) was predicted using particle-swarm optimization method8 and metadynamics simulations9. Using the latter method, it was demonstrated that a large variety of superhard structures can be expected (_e.g. o_C16-I, _m_C12 and _m_C32)9.
The simulated x-ray diffraction patterns of the predicted structures (_M_-carbon, _W_-carbon, bct-C4 and _o_C16-II) are in satisfactory agreement with experimental data4,5,7,9. Their respective bulk moduli (and hardness22) are 392.6 (82.7), 391.8 (83.1), 393.4 (82.0) and 408.4 (84.4) GPa9. Band gaps calculated using the GW method23 are 5.0024, 4.397 and 3.78 eV5, for _M_-carbon, _W_-carbon and bct-C4, respectively. All candidate structures show no imaginary phonon frequencies and can explain the experimental observations of hardness and transparency of cold compressed graphite.
Physical properties and energetics of these phases have been theoretically well studied4,5,6,7,25,26, but the existing evidence is insufficient to decide which one was obtained in experiments10,11,12,13. In order to clearly identify the product of cold compression of graphite, we need to explore graphite transformation kinetics and compute energy barriers for transition regimes characterized by phase coexistence.
Investigations of transition routes to superhard graphite6,7 using nudged elastic band method provided inconsistent results, especially inferring that graphite to cubic diamond has lower transition barrier than transformations to other three possible structures7. In addition, Ref.7 reports a different sequence of energy barriers (cubic diamond < _M_-carbon < bct-C4) from Ref.6 (bct-C4 < cubic diamond < _M_-carbon), despite the use of the same computational method. These conflicting results highlight the intrinsic complexity of a reliable elucidation of transition mechanisms and associated barriers.
In a complex system, like a three dimensional solid, the lowest energy state of a system is not always the most relevant27. The viability of any candidate structure as a metastable product is strictly connected to the corresponding transformation pathway and the associated transition barriers or dynamical bottlenecks1,28,29. Transition barriers can be computed and bottlenecks identified with (stationary) saddle points representing transition states on the potential energy surface30. In complex systems with very rugged energy landscapes, saddle points cease to be characteristic points of the free energy barrier31. The latter barrier encompasses a large set of configurations and the associated relevant degrees of freedom are very difficult to anticipate1,30,31. To overcome this problem, we can use transition path sampling, a method based on collecting ensembles of dynamical trajectories1,28,29,30,31.
Results
In this work, we elucidate the nature of the metastable product of cold compression of graphite and compute the associated energy barrier from true dynamical pathways. The mechanistic details of this pressure-induced phase transformation are determined at the atomic level with full account for relevant events of nucleation. To achieve a reliable and detailed mechanistic picture of the transition we employ a strategy that combines isothermal-isobaric (NpT) molecular dynamics with transition path sampling (TPS)32,33,34 (see Methods). This methodology has proven very effective in the simulation of activated processes1,30,35 with phase coexistence and growth28,29,36.
How TPS works
Transition path sampling is a generalization of Monte Carlo procedures in the space of dynamical trajectories connecting two states (graphite and metastable superhard phase in this case) separated by a high barrier in a rough energy landscape32,33,34. Therein, the relevance of a transition pathway is biased by path probability. The overall simulation approach is iterative and develops from an initial trajectory. By analogy with conventional MC simulations, the first step of TPS consists of equilibrating an initial pathway and gradually converging the trajectory regime to more probable regions. Accordingly, the first trajectory does not need to be a probable one or include mechanistic details of the real transition regime.
In a situation characterized by the possibility of many theoretically viable candidate structures, TPS is a very helpful simulation strategy because it allows starting from a regime of very low probability or even a model constructed from a mapping between the limiting phases of the transition of interest. The convergence is ensured by Monte Carlo sampling of the space of trajectories. In the case of cold compression of graphite, we started from a pathway connecting graphite to cubic diamond generated by propagation of configurations obtained from a modeling approach based on transforming periodic nodal surfaces calculated from a short Fourier summation for each structure37,38 (see Methods).
Finding the real transition route
The importance of nucleation events during the compression of graphite was pointed out for the transition to diamond at high pressures and temperatures39. In the following, we investigate the kinetics of graphite transformation at ambient temperature due to intrinsic differences between nucleation and growth patterns of predicted superhard structures.
The first set of TPS runs was started from trajectories connecting graphite to cubic diamond. Since diamond (cubic and hexagonal) was ruled out by experiments19 and due to high activation barrier separating diamond from graphite, starting with this pathway will not only optimize the search in the path ensemble but will help converging to a more probable end point structure. The appearance of a new different phase instead of diamond is allowed by setting a flexible order parameter that can accommodate all four-coordinate structures (see Methods).
The set of transition pathways harvested in the course of TPS iterations shows a quick departure of the trajectory regime from the collective motion inherited from the geometrical modeling. The graphite-diamond trajectories underwent three drastic shifts in the transition regime.
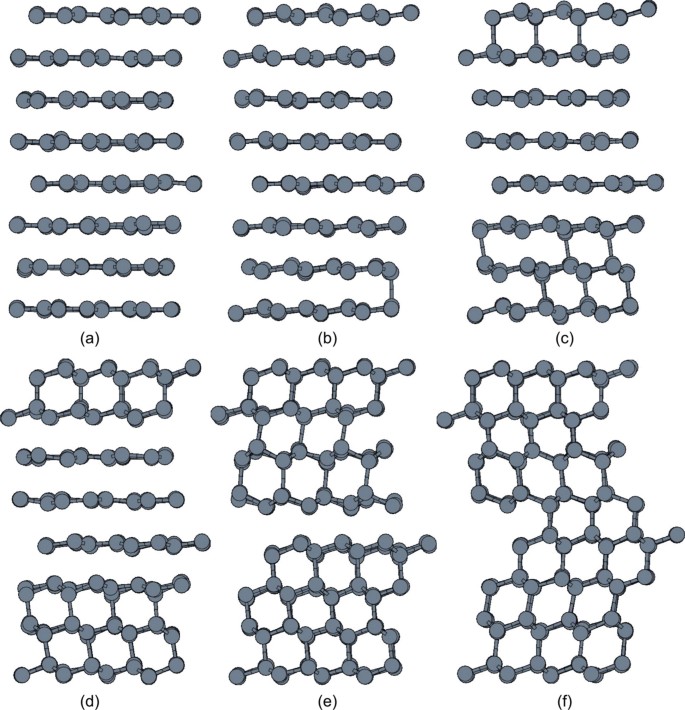
First, after 15–20 iterations, the end point of the initial trajectory, graphite to cubic diamond, is replaced by a polytype intermediate between hexagonal and cubic diamond (Fig. 1). The appearance of this polytype is due to opposite sliding directions of graphene layers giving rise to layers of hexagonal diamond. This evolution of the transition regime is similar to the results of a molecular dynamics investigation of the pressure-induced transformation path of graphite of diamond at high temperatures40.
Figure 1
Snapshots from a dynamical trajectory collected from transition path sampling connecting (a) graphite to (f) a polytype intermediate between cubic and hexagonal diamond.
The buckling of graphene layers in initiated by the formation of C–C bonds along [001]graphite (b)–(c). Domains of cubic diamond are formed with different orientation (d)–(e). The interface between the latter domains defines a region of hexagonal diamond (e)–(f).
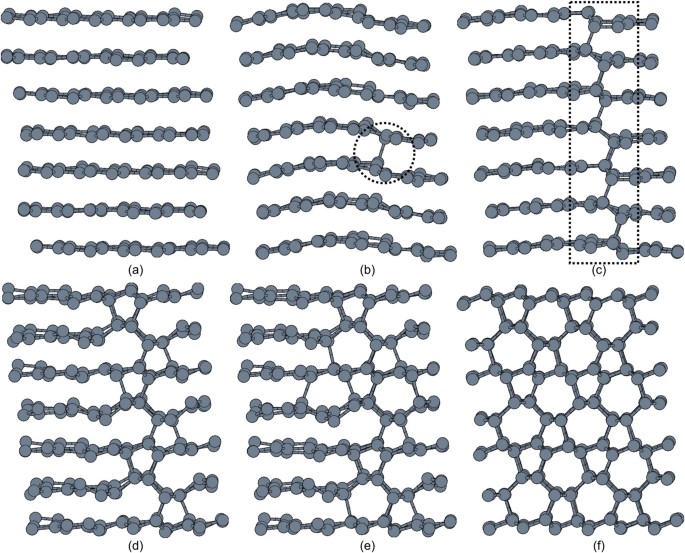
After 30–40 iterations, an inset of new structural patterns made of 5-membered and 7-membered rings appeared within 6-membered rings network (Fig. 2, (d)–(f)). These new patterns are still made of four-coordinate carbon atoms and interface very well with cubic diamond. They are characteristic of two structures suggested as metastable product for the cold compression of graphite, _M_-carbon and _W_-carbon. The small size of the inset, however, does not yet allow to discriminate between these two models (Fig. 2, (f), dotted circle).
Figure 2
Snapshots from a representative trajectory illustrating the evolution of the graphite to cubic diamond transition regime.
The mobility of graphene layers during the reconstruction creates (d)–(f) an inset of 5- and 7-membered rings (dotted circle) within a 6-membered rings network. This inset interfaces well with cubic diamond and represents the seed of the metastable phase resulting from the cold compression of graphite.
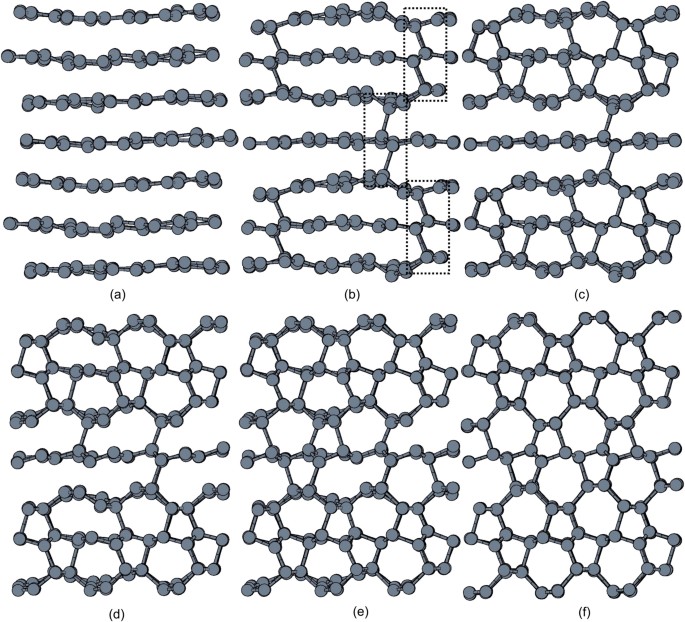
Upon further sampling of the pathway ensemble, the inset of odd-membered rings grew larger and converted the remaining cubic diamond regions into a structure identical to that of _M_-carbon. The new transition regime connects graphite to _M_-carbon (Fig. 3). Therein, the onset of the transformation is marked by the formation of interlayer single C–C bond (Fig. 3, (a)). The formation of the nucleus triggers a series of bond formation events along [001]graphite in a zigzag fashion (Fig. 3, (b)). The breaking of π-interactions within graphene layers by the emergence of the latter zigzag chain induces layer corrugation that facilitates the formation of 5-membered rings (Fig. 3, (c)–(d)). The latter rings pair up and additional 7-membered rings are formed (Fig. 3, (e)).
Figure 3
Snapshots of a representative trajectory of the stable regime corresponding to the cold compression of graphite.
A single event of (a) bond formation (dotted circle) between graphene layers triggers (b) a series of bond formation along [001]graphite in a zigzag fashion (dotted rectangle). The latter contacts facilitate the formation of (c)–(d) 5-membered rings causing the corrugation of graphene layers and inducing the formation of (d)–(e) 7-membered rings.
The degrees of freedom induced by the mobility of graphene layers under pressure theoretically allow to consider different ways of graphene layers buckling. The survival of an initial regime or the appearance of a new one is connected to the way of nucleating the high pressure modification and the corresponding free energy barrier. TPS is biased to efficiently converge to the relevant set of the path ensemble which corresponds to the real transition pathway regime29,32,33,34,35 and bypasses less-favored regions of the energy landscape corresponding to other mechanisms, graphite-bct-C4 and graphite-_W_-carbon in this case. To illustrate this feature of TPS, we performed separate simulation runs to evaluate the energy barrier of graphite to bct-C4 and to _W_-carbon transition routes and clarify the associated reconstruction mechanisms in comparison with the _M_-carbon route.
Viability of graphite to W-carbon pathway
Despite its structural and energetic closeness to the _M_-carbon phase7, the arrangement of odd-membered rings corresponding to the orthorhombic candidate structure, _W_-carbon, only appears in 20% of trajectories collected during TPS simulations performed starting from graphite to diamond trajectories, over a pressure range from 15–20 GPa.
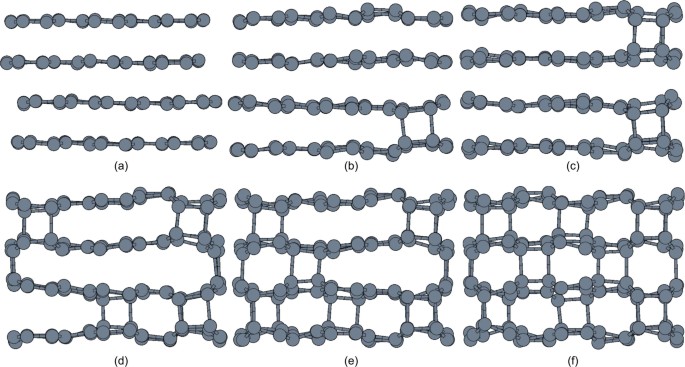
In order to investigate differences in terms of transition mechanism and kinetics, we modeled a distinct set of TPS runs. The starting trajectory was taken from the previous runs that converged to graphite to _M_-carbon. Although nucleation events triggering the transition along the graphite to _W_-carbon path (Fig. 4, (a)–(b)) are very similar to that of graphite to _M_-carbon (Fig. 3, (a)–(d)), subsequent growth of _W_-carbon slightly differs from that of _M_-carbon. The formation of a C–C bond bridging two graphene layers, in the case of _W_-carbon, induced the appearance of other bonds along [001]graphite as shown in Fig. 4. Unlike in the transition mechanism to _M_-carbon, the zigzag chain propagates in a segmented fashion (Fig. 4(b)). The key step in the evolution of graphite toward _M_-carbon or _W_-carbon is the evolution of a single nucleation event, formation of an inter-layer C-C bond, either as infinite zigzag (Fig. 3(c)) or segmented chain (Fig. 4(b)). The breaking of the chain implies different progress of phase growth and results in a higher transition barrier (see below for transition kinetics). The average length of graphite to _W_-carbon transformation is 9 ps while graphite to _M_-carbon is 8 ps or less. This reflects slower transition rate for _W_-carbon.
Figure 4
Snapshots taken from a representative graphite to _W_-carbon transformation pathway.
The buckling of graphene layers is initiated by (a)–(b) formation C–C contacts along [001]graphite in the form of finite size zigzag chains. Each zigzag chain facilitates the corrugation of graphene layers inducing the formation of (c)–(e) 5- and 7-membered rings transforming graphite into (f) _W_-carbon.
The effect of strain caused by the nucleus on the graphite lattice upon the direct conversion of graphite into diamond has been pointed out to be responsible of the formation of metastable hexagonal modification rather than cubic in agreement with experimental observations39. The nucleus formation along the graphite to _W_-carbon transition induced larger distortions of the graphite structure at the interface if compared to the path to _M_-carbon. This explains the more frequent appearance of the latter structure during the sampling.
Viability of graphite to bct-C4 pathway
As demonstrated above, the graphite to bct-C4 path is a less favored route. The set of sampling runs of such a trajectory ensemble quickly shift the transition regime toward the more favorable one, graphite-_M_-carbon. To prevent the disappearance of the bct-C4 structure, additional constraints are introduced on the order parameter to keep the transition regime as a pure graphite to bct-C4 transformation (see Methods).
A representative trajectory of the latter path is depicted in Fig. 5. Unlike the graphite-_M_-carbon transition mechanism, the onset of the reconstruction is marked by the formation of two C–C bonds defining a C4 square unit (Fig. 5, (a)–(b)). The perturbation induced by the C4 nucleus helps propagating this motif and the bct-C4 phase grows until complete reconstruction of graphite into the body-centered tetragonal structure.
Figure 5
Snapshots taken from a representative graphite to bct-C4 transformation pathway.
The transition proceeds via nucleation of C4 square units. Further growth is achieved by corrugation of graphene layers to form more C4 units and reconstruct the graphite into bct-C4.
Kinetics of graphite cold compression
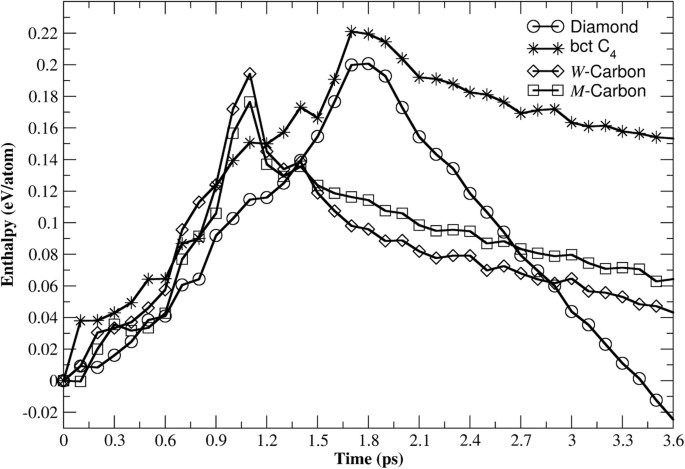
In order to shed light on the kinetics of the transformation of graphite under high-pressure and ambient temperature, enthalpy variation of different simulated transition regimes starting from graphite have been computed and depicted in Fig. 6. The graphite to bct-C4 transition barrier (Fig. 6, star line) scores as the highest with 221 meV/atom. The graphite to cubic diamond transformation (Fig. 6, circle line) has a barrier of 200 meV/atom which completely rules out the latter transition route for graphite cold compression. Despite the similarities between transformation routes to _W_-carbon (Fig. 6, diamond line) and _M_-carbon (Fig. 6, square line), the latter is favored with a barrier equal to 176 meV/atom, lower than 194 meV/atom for the former transition.
Figure 6
Enthalpy variation of different simulated transformations of graphite under pressure (15 GPa) and ambient temperature.
The graphite to _M_-carbon transformation route (square line) indicates a lower energy barrier than the transition to _W_-carbon (diamond line). The possibility of graphite transformation into bct-C4 structure (star line) on cold compression is ruled out because of higher barrier than the graphite to cubic diamond transition (circle line).
The role of graphene layers sliding
In the nucleation process, the buckling of graphene layers is realized upon their corrugation followed by formation of new C-C bonds (Fig. 1-(b), Fig. 2-(a), Fig. 3-(b), Fig. 4-(a) and Fig. 5-(b)). This is facilitated by layer sliding in graphite that brings carbon atoms into an appropriate stacking sequence for C-C bonds formation along [001]. Just prior to nucleus formation, the applied pressure causes graphene layers to slide in a fashion that determines the nature of the high pressure modification to be formed. In Fig. 6, the variation of enthalpy indicates a different evolution for graphite even before the formation of any nucleus for all investigated transformations (graphite to cubic diamond, to bct-C4, to _W_-carbon and to _M_-carbon). For t = 0 − 600 fs, graphite to bct-C4 shows a higher enthalpy kink if compared to the two other candidates, _W_- and _M_-carbon. This slight difference in enthalpy (40 meV/atom for bct-C4 and 30 meV/atom for _W_- and _M_-carbon) reflects two different layer sliding mechanisms as shown in Fig. 7. The graphite to bct-C4 transition requires an eclipsed arrangement of next-neighboring layer along [001] and changes the layers stacking sequence from …_AB_… into …_AA_… (Fig. 7, (a)–(b)) in order to trigger layers buckling.
Figure 7
Top view of graphene layers sliding fashions parallel to (001) plane.
The (a) graphite to (b) bct-C4 transition requires an eclipsed arrangement of next-neighboring layer along [001] and changes the layers stacking sequence from …_AB_… into …_AA_… in order to trigger layers buckling (only 3 graphitic layers are shown for better clarity). The (a) graphite to (d) _M_-carbon transformation implies small atomic displacements to induce (c) the onset of graphitic layers buckling.
The transformations from graphite to 5+7 structures on the contrary require a reduced amount of displacements to induce graphene layer buckling (Fig. 7, (a)–(d)). Therefore, transformation routes leading to _W_- and _M_-carbon are more favored than the path to bct-C4 in terms of layer sliding mechanism in graphite. Additionally, pathways to _W_-and _M_-carbon are less demanding in terms in-layer distortions, necessary to initiate nucleation.
The stacking sequence and atomic displacements in the case of pathways to _W_- and _M_-carbon imply minimal inlayer distortions in order to initiate nucleation. The path to _M_-carbon is favored over the one to _W_-carbon because of different growth processes making the former structure more probable to form.
The effect of simulation box size has been pointed out in large-scale molecular dynamics simulations of graphite to diamond transition39. If the simulation cell is too small, the derived transition mechanism does not feature in-layer distortions because in-layer stress implies artificial sliding of graphene layers. The size of our simulation box fairly enables the observation of relevant nucleation events and allows to capture distortions around growing nucleus without inducing artificial layer sliding as shown in Fig 3-(c) and Fig 4-(b). Our results underline the role of distortions around the nucleus and how they determine the kinetics and the overall direction of the transformation.
The very recently proposed candidate _o_C16-II deserves special notes. This orthorhombic structure can be described as an alternation of diamond and bct-C4 slabs along [100] (Fig. 3 in Supplementary Information). The existence of 4-membered rings suggests mechanistic features of buckling in a fashion similar to graphite-bct-C4 transformation. To reconstruct graphite into a structure with a topology of rings fused as 4 + 6 + 8 pattern, atomic displacements in terms of layers sliding are expected to be larger than in the mechanism leading to _M_- and _W_-carbon (Fig. 3 in Supplementary Information). On the experimental side, Zhao et al.8 demonstrated an agreement of the calculated x-ray diffraction pattern of _o_C16-II with the experimental XRD data collected for cold compression of carbon nanotube bundles. The inconsistency with experimental data on “superhard graphite” has been pointed out8. In our TPS simulations, the initial graphite to diamond pathway evolved into the graphite to _M_-carbon pathway, bypassing the _o_C16-II structure, in spite of its structural closeness to diamond.
Discussion
Unlike synthesis from the gas phase, the high-pressure synthetic route of superhard graphite depends on the particular nucleation history which would favor one pattern over another at the stage of phase growth within graphite. The viability of transformation routes is not determined by the overall stability of a particular candidate structure, but rather by the activation energy of its formation. Local events of C-C bond breaking and formation control the kinetics of the transition. In this work, an atomistic and kinetic picture of cold compression of graphite is presented using a methodology based on molecular-dynamics simulations combined with transition path sampling. It illustrates how transition path sampling can be used to assess synthesizability of metastable phases by optimizing transformation pathways. Unlike static models, we provide an unbiased description of different dynamical routes and evaluation of the associated transition barriers. The final product of this first-order transformation is unequivocally identified as a metastable monoclinic structure, _M_-carbon. Although a competitor, _W_-carbon is kinetically less favored and differences in transformation rates can be traced back to different patterns of phase nucleation and subsequent growth. We demonstrate that large distortions caused by the nucleation mechanism of _W_-carbon make it less likely to form than _M_-carbon. Another earlier proposed structures, bct-C4, is ruled out because it did not appear during trajectory sampling under cold-compression conditions.
It is worth mentioning that transformation pathways to “superhard graphite” are investigated as homogeneous nucleation processes. The presence of structural defects in the form of interlayer bonds can facilitate nucleation. Consequently, heterogeneous nucleation implies lower activation barriers. However, differences between homogeneous and heterogeneous nucleation do not alter the main features of the transformations revealed in this work. Phase growth kinetics around lattice distortions and structural defects are expected to be similar to that of homogeneous nucleation model. This investigation conducted in this work offers new insights into the understanding of materials synthesizability under high pressure, in a situation of competing phases and in the absence of high-resolution experimental data and establishes _M_-Carbon as a new carbon allotrope.
Methods
Molecular dynamics simulations
Born-Oppenheimer molecular dynamics simulations were performed in the NpT ensemble30 at temperature T = 300 K and pressures ranging from 15 to 20 GPa. Constant pressure and temperature were ensured by the Martyna-Tobias-Klein algorithm41 allowing for anisotropic shape changes of the simulation box. Interatomic forces are computed within the framework of density functional tight binding approach42,43 as implemented in the CP2K code[44](/articles/srep00471#ref-CR44 " http://cp2k.berlios.de
(2011)."). For the accuracy of the set of DFTB parameters[45](/articles/srep00471#ref-CR45 "Porezag, D., Frauenheim, Th., Köhler, Th., Seifert, G. & Kaschner, R. Construction of tight-binding-like potentials on the basis of density-functional theory: Application to carbon. Phys. Rev. B 51, 12947–12957 (1995).") used, see [ _Supplementary Information_ ](/articles/srep00471#MOESM1). The time propagation of the system was performed using the velocity Verlet algorithm[30](/articles/srep00471#ref-CR30 "Frenkel, D. & Smit, B. Understanding Molecular Simulations from algorithms to applications, Second Edition, (Academic, San Diego), 2nd Ed. (2002)."). To ensure good time-reversibility an integration timestep of 0.2 fs was used. The simulation box contained 256 carbon atoms. The starting configurations in all simulations correspond to graphite-2H structure. The presence of defects and surface effects on the transition mechanism and the nature of the product of the transition is not investigated because the main focus is set on homogenous nucleation in graphite compressed at ambient temperature.Transition path sampling
The sampling starts from an initial trajectory connecting the limiting phases. A new trajectory is generated by selecting a configuration from the existing one and slightly modifying the atomic momenta. The modifications δp are applied to randomly chosen pairs of atoms (i,j) according to: 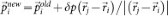 and
and 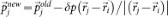 , keeping both momentum and angular momentum conserved. The resulting atomic momenta are rescaled by a factor of
, keeping both momentum and angular momentum conserved. The resulting atomic momenta are rescaled by a factor of 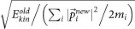 , in order to keep the total kinetic energy conserved. The propagation of the modified configuration in both directions of time (−t,+t) generates a new trajectory. Repeating this step provides a set of trajectories and the successful ones are collected and analyzed. We initialized TPS with trajectories representing four different transformation regimes: graphite–cubic diamond, graphite–bct-C4, graphite–_M_-carbon and graphite–_W_-carbon. Subsequent equilibration of the path ensemble at 300 K and pressures ranging from 15 to 20 GPa in two simulation runs for each transition regime resulted into 8 independent TPS simulation runs. The number of trial shootings for each run exceeded 500 and the acceptance ratio was between 40–60%. This production part of TPS procedure resulted in an ensemble of more than 200 successful pathways, between 8–10 ps long, of which ~70 are independent uncorrelated trajectories.
, in order to keep the total kinetic energy conserved. The propagation of the modified configuration in both directions of time (−t,+t) generates a new trajectory. Repeating this step provides a set of trajectories and the successful ones are collected and analyzed. We initialized TPS with trajectories representing four different transformation regimes: graphite–cubic diamond, graphite–bct-C4, graphite–_M_-carbon and graphite–_W_-carbon. Subsequent equilibration of the path ensemble at 300 K and pressures ranging from 15 to 20 GPa in two simulation runs for each transition regime resulted into 8 independent TPS simulation runs. The number of trial shootings for each run exceeded 500 and the acceptance ratio was between 40–60%. This production part of TPS procedure resulted in an ensemble of more than 200 successful pathways, between 8–10 ps long, of which ~70 are independent uncorrelated trajectories.
Order parameter
The average coordination number (CN) of carbon atoms is used as an order parameter to distinguish graphite (CN = 3) from high pressure modifications made of four-coordinate atoms. All structures discussed in the manuscript (_M_-carbon, _W_-carbon, bct-C4, hexagonal and cubic diamond) have an average CN = 4 within the first nearest neighbor coordination sphere. In order to narrow down the path ensemble sampling we used second and third nearest neighbors to distinguish between different four-coordinate structures Clearly, apart from a quick detection of graphite and different candidate structures (bct-C4, _M_-carbon and _W_-carbon), this order parameter is capable of accommodating not only the three possible metastable phases but allows for starting transition pathways sampling from unlikely trajectories, like graphite to cubic or hexagonal diamond. The order parameter does not impose any bias on the evolution of the trajectory sampling.
Topological models
We model transformation paths by transforming periodic nodal surfaces (PNS)1,37,38. The reciprocal space approach implements short Fourier summations to define a family of surfaces according to the formula: 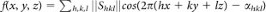 . S hkl is a geometric structure factor and α_hkl_ is the corresponding phase. The surface corresponding to f(x, y, z) = 0 is called PNS. PNS of the transforming phases (A and B) are used as starting points for the definition of a geometric model for the transition. The choice of a common cell with a constant number of atoms and the periodicity of the model ensure commensurability of the two phases. After transformation of the reflections of the new setting of the cell, the transition can be formulated as a migration from one structure to the other along a coordinate s providing weighted linear mixing of the two functions:
. S hkl is a geometric structure factor and α_hkl_ is the corresponding phase. The surface corresponding to f(x, y, z) = 0 is called PNS. PNS of the transforming phases (A and B) are used as starting points for the definition of a geometric model for the transition. The choice of a common cell with a constant number of atoms and the periodicity of the model ensure commensurability of the two phases. After transformation of the reflections of the new setting of the cell, the transition can be formulated as a migration from one structure to the other along a coordinate s providing weighted linear mixing of the two functions: 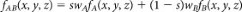 ; s ∈ [0, 1] To derive the transition state, configurations obtained from the geometric model in the range s ∈ [0, 1] are propagated in molecular dynamics simulations at 300 K. Random velocities are assigned to atoms and the system is left free to evolve toward either A or B without imposing any bias. Reversing the sign of the time coordinate allows reaching the other phase with a finite probability.
; s ∈ [0, 1] To derive the transition state, configurations obtained from the geometric model in the range s ∈ [0, 1] are propagated in molecular dynamics simulations at 300 K. Random velocities are assigned to atoms and the system is left free to evolve toward either A or B without imposing any bias. Reversing the sign of the time coordinate allows reaching the other phase with a finite probability.
References
- Oganov, A. R. (ed.) Modern Methods of Crystal Structure Prediction (WILEY-VCH, Weinheim, 2010).
- Oganov, A. R. et al. Ionic high-pressure form of elemental boron. Nature 457, 863–867 (2009).
Article ADS CAS PubMed Google Scholar - Ma, Y. et al. Transparent dense sodium. Nature 458, 182–185 (2009).
Article ADS CAS PubMed Google Scholar - Li, Q. et al. Superhard Monoclinic Polymorph of Carbon. Phys. Rev. Lett. 102, 175506–175509 (2009).
Article ADS PubMed CAS Google Scholar - Umemoto, K., Wentzcovitch, R. M., Saito, S. & Miyake, T. Body-Centered Tetragonal _C_4: A Viable _sp_3 Carbon Allotrope. Phys. Rev. Lett. 104, 125504–125507 (2010).
Article ADS PubMed CAS Google Scholar - Zhou, X.-F. et al. Ab initio study of the formation of transparent carbon under pressure. Phys. Rev. B 82, 134126–134130 (2010).
Article ADS CAS Google Scholar - Wang, J.-T., Chen, C. & Kawazoe, Y. Low-Temperature Phase Transformation from Graphite to _sp_3 Orthorhombic Carbon. Phys. Rev. Lett. 106, 075501–075504 (2011).
Article ADS PubMed CAS Google Scholar - Zhao, Z., Xu, B., Zhou, X.-F., Wang, L.-M., Wen, B., He, J., Liu, Z., Wang, H.-T. & Tian, Y. Novel Superhard Carbon: C-Centered Orthorhombic C8 . Phys. Rev. Lett. 107, 215502–215506 (2011).
Article ADS PubMed CAS Google Scholar - Selli, D., Baburin, I., Martoňák, R. & Leoni, S. Superhard _sp_3 carbon allotropes with odd and even ring topologies. Phys. Rev. B 84, 161411(R)–161415(R) (2011).
Article ADS CAS Google Scholar - Aust, R. B. & Drickamer, H. G. Carbon: A New Crystalline Phase. Science 140, 817–819 (1963).
Article ADS CAS PubMed Google Scholar - Bundy, F. P. & Kasper, J. S. Hexagonal Diamond - A New Form of Carbon. J. Chem. Phys. 46, 3437–3446 (1967).
Article ADS CAS Google Scholar - Utsumi, W. & Yagi, T. Light-transparent phase formed by room-temperature compression of graphite. Science 252, 1542–1544 (1991).
Article ADS CAS Google Scholar - Mao, W. L. et al. Bonding Changes in Compressed Superhard Graphite. Science 302, 425–427 (2003).
Article ADS CAS PubMed Google Scholar - Hanfland, M., Beister, H. & Syassen, K. Graphite under pressure: Equation of state and first-order Raman modes. Phys. Rev. B 39, 12598–12603 (1989).
Article ADS CAS Google Scholar - Hanfland, M., Syassen, K. & Sonnenschein, R. Optical reflectivity of graphite under pressure. Phys. Rev. B 40, 1951–1954 (1989).
Article ADS CAS Google Scholar - Zhao, Y. X. & Spain, I. L. X-ray diffraction data for graphite to 20 GPa. Phys. Rev. B 40, 993–997 (1989).
Article ADS CAS Google Scholar - Takano, K. J., Harashima, H. & Wakatsuki, M. New High-Pressure Phases of Carbon. Jpn. J. Appl. Phys. 30, L860–L863 (1991).
Article ADS CAS Google Scholar - Yagi, T., Utsumi, W., Yamakata, M.-A., Kikegawa, T. & Shimomura, O. High-pressure in situ x-ray-diffraction study of the phase transformation from graphite to hexagonal diamond at room temperature. Phys. Rev. B 41, 6031–6039 (1992).
Article ADS Google Scholar - Xu, J.-A., Mao, H.-K. & Hemley, R. J. The gem anvil cell: high-pressure behaviour of diamond and related materials. J. Phys. Condens. Matter 14, 11549–11552 (2002).
Article ADS CAS Google Scholar - Oganov, A. R. & Glass, C. W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J. Chem. Phys. 124, 244704–244719 (2006).
Article ADS PubMed CAS Google Scholar - Baughman, R. H., Liu, A. Y., Cui, C. & Schields, P. J. A carbon phase that graphitizes at room temperature. Synth. Met. 86, 2371–2374 (1997).
Article CAS Google Scholar - Lyakhov, A. O. & Oganov, A. R. Evolutionary search for superhard materials applied to forms of carbon and TiO2 . Phys. Rev. B 84, 092103–092106 (2011).
Article ADS CAS Google Scholar - Aryasetiawan, F. & Gunnarsson, O. The GW method. Rep. Prog. Phys. 61, 237–312 (1998).
Article ADS CAS Google Scholar - Zhu, Q., Oganov, A. R., Salvadó, M. A., Pertierra, P. & Lyakhov, A. O. Denser than diamond: Ab initio search for superdense carbon allotropes. Phys. Rev. B 83, 193410–193413 (2011).
Article ADS CAS Google Scholar - Liang, Y., Zhang, W. & Chen, L. Phase stabilities and mechanical properties of two new carbon crystals. Europhys. Lett. 87, 56003–56008 (2009).
Article ADS CAS Google Scholar - Wei, P. Y., Sun, Y., Chen, X.-Q., Li, D. Z. & Li, Y. Y. Anisotropy in electronic, optical and mechanical properties of superhard body-centered tetragonal C4 phase of carbon. App. Phys. Lett. 97, 061910–061912 (2010).
Article ADS CAS Google Scholar - O'Keeffe, M. Aspects of crystal structure prediction: some successes and some difficulties. Phys. Chem. Chem. Phys. 12, 8580–8583 (2010).
Article CAS PubMed Google Scholar - Boulfelfel, S. E., Zahn, D., Grin, Yu. & Leoni, S. Walking the Path from B4- to B1-Type Structures in GaN. Phys. Rev. Lett. 99, 125505–125508 (2007).
Article ADS CAS PubMed Google Scholar - Boulfelfel, S. E., Seifert, G. Grin, Yu. & Leoni, S. Squeezing lone pairs: The _A_17 to _A_7 pressure-induced phase transition in black phosphorus. Phys. Rev. B 85 014110–014115 (2012).
Article ADS CAS Google Scholar - Frenkel, D. & Smit, B. Understanding Molecular Simulations from algorithms to applications, Second Edition, (Academic, San Diego), 2nd Ed. (2002).
- Dellago, C., Bolhuis, P. G. & Geissler, P. L. Transition Path Sampling Methods, in Ferrario, M., Ciccotti, G. & Binder, K. (eds), Computer Simulations in Condensed Matter: From Materials to Chemical Biology Vol 1, Springer-Verlag, 349–392 (2006).
- Bolhuis, P. G., Dellago, C. & Chandler, D. Sampling ensembles of deterministic transition pathways. Faraday Discuss. 110, 421–436 (1998).
Article ADS CAS Google Scholar - Dellago, C., Bolhuis, P. G., Csajka, F. S. & Chandler, D. Transition path sampling and the calculation of rate constants. J. Chem. Phys. 108, 1964–1978 (1998).
Article ADS CAS Google Scholar - Bolhuis, P. G., Chandler, D., Dellago, C. & Geissler, P. L. Transition path sampling: Throwing ropes over rough mountain passes, in the dark. Annu. Rev. Phys. Chem. 53, 291–318 (2002).
Article ADS CAS PubMed Google Scholar - Zahn, D. & Leoni, S. Nucleation and Growth in Pressure-Induced Phase Transitions from Molecular Dynamics Simulations: Mechanism of the Reconstructive Transformation of NaCl to the CsCl-Type Structure. Phys. Rev. Lett. 92, 250201–250204 (2004).
Article ADS PubMed CAS Google Scholar - Boulfelfel, S. E. & Leoni, S. Competing intermediates in the pressure-induced wurtzite to rocksalt phase transition in ZnO. Phys. Rev. B 78, 125204–125210 (2008).
Article ADS CAS Google Scholar - Leoni, S. & Zahn, D. Putting the squeeze on NaCl: modeling and simulation of the pressure driven B1–B2 phase transition. Z. Kristallogr. 219, 339–344 (2004).
Article CAS Google Scholar - von Schnering, H. G. & Nesper, R. Nodal surfaces of Fourier series: Fundamental invariants of structured matter. Z. Phys. B 83, 407–412 (1991).
Article ADS Google Scholar - Khaliullin, R. Z., Eshet, H., Kühne, T. D., Behler, J. & Parrinello, M. Nucleation mechanism for the direct graphite-todiamond phase transition. Nat. Mat. 10, 693–697 (2011).
Article CAS Google Scholar - Scandolo, S., Bernasconi, M., Chiarotti, G. L., Focher, P. & Tosatti, E. Pressure-Induced Transformation Path of Graphite to Diamond. Phys. Rev. Lett. 74, 4015–4018 (1995).
Article ADS CAS PubMed Google Scholar - Martyna, G. J., Tobias, D. J. & Klein, M. L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 101, 4177–4190 (1994).
Article ADS CAS Google Scholar - Seifert, G., Porezag, D. & Frauenheim, Th. Calculations of molecules, clusters and solids with a simplified LCAO-DFTLDA scheme. Int. J. Quant. Chem. 58, 185–192 (1996).
Article CAS Google Scholar - Zhechkov, L., Heine, T., Patchkovskii, S., Seifert, G. & Duarte, H. A. An Efficient a Posteriori Treatment for Dispersion Interaction in Density-Functional-Based Tight Binding. J. Chem. Theo. Comp. 1, 841–847 (2005).
Article CAS Google Scholar - http://cp2k.berlios.de (2011).
- Porezag, D., Frauenheim, Th., Köhler, Th., Seifert, G. & Kaschner, R. Construction of tight-binding-like potentials on the basis of density-functional theory: Application to carbon. Phys. Rev. B 51, 12947–12957 (1995).
Article ADS CAS Google Scholar
Acknowledgements
This work is funded by DARPA (grant N66001-10-1-4037) and NSF (grant EAR-1114313). SL acknowledges support from DFG for support (SPP 1415). Calculations were performed at computing facilities of ZIH (TU-Dresden), CFN at Brookhaven National Laboratory (supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under contract No. DE-AC02-98CH10086), Joint Supercomputer Center of the Russian Academy of Sciences and Skif-MSU Supercomputer (Moscow State University).
Author information
Authors and Affiliations
- Department of Geosciences, Stony Brook University, 11794-2100, NY, USA
Salah Eddine Boulfelfel & Artem R. Oganov - Department of Geology, Moscow State University, 119899, Moscow, Russia
Artem R. Oganov - Technische Universität Dresden, Institut für Physikalische Chemie, 01062, Dresden, Germany
Stefano Leoni
Authors
- Salah Eddine Boulfelfel
You can also search for this author inPubMed Google Scholar - Artem R. Oganov
You can also search for this author inPubMed Google Scholar - Stefano Leoni
You can also search for this author inPubMed Google Scholar
Contributions
S.E.B., A.R.O. and S.L. designed research, performed simulations, analyzed data and wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Boulfelfel, S., Oganov, A. & Leoni, S. Understanding the nature of “superhard graphite”.Sci Rep 2, 471 (2012). https://doi.org/10.1038/srep00471
- Received: 25 February 2012
- Accepted: 30 April 2012
- Published: 28 June 2012
- DOI: https://doi.org/10.1038/srep00471
This article is cited by
Two-dimensional diamonds from sp2-to-sp3 phase transitions
- Francesco Lavini
- Martin Rejhon
- Elisa Riedo
Nature Reviews Materials (2022)
High-throughput systematic topological generation of low-energy carbon allotropes
- Vladislav A. Blatov
- Changhao Yang
- Artem A. Kabanov
npj Computational Materials (2021)
Structural diversity, large interlayer spacing and switchable electronic properties of graphitic systems
- Qiaoyi Han
- Kun Luo
- Julong He
Journal of Materials Science (2021)
Structure prediction drives materials discovery
- Artem R. Oganov
- Chris J. Pickard
- Richard J. Needs
Nature Reviews Materials (2019)
A new superhard carbon allotrope: tetragonal C64
- Qun Wei
- Quan Zhang
- Meiguang Zhang
Journal of Materials Science (2017)