Extreme convergence in egg-laying strategy across insect orders (original) (raw)
- Article
- Open access
- Published: 16 January 2015
- Joachim Bresseel2,
- Jerome Constant2,
- Bruno Kneubühler3,
- Fanny Leubner1,
- Peter Michalik4 &
- …
- Sven Bradler1
Scientific Reports volume 5, Article number: 7825 (2015)Cite this article
- 12k Accesses
- 70 Citations
- 63 Altmetric
- Metrics details
Subjects
Abstract
The eggs of stick and leaf insects (Phasmatodea) bear strong resemblance to plant seeds and are commonly dispersed by females dropping them to the litter. Here we report a novel egg-deposition mode for Phasmatodea performed by an undescribed Vietnamese species of the enigmatic subfamily Korinninae that produces a complex egg case (ootheca), containing numerous eggs in a highly ordered arrangement. This novel egg-deposition mode is most reminiscent of egg cases produced by members of unrelated insect orders, e.g. by praying mantises (Mantodea) and tortoise beetles (Coleoptera: Cassidinae). Ootheca production constitutes a striking convergence and major transition in reproductive strategy among stick insects, viz. a shift from dispersal of individual eggs to elaborate egg concentration. Adaptive advantages of ootheca formation on arboreal substrate are likely related to protection against parasitoids and desiccation and to allocation of specific host plants. Our phylogenetic analysis of nuclear (28S, H3) and mitochondrial (COI, COII) genes recovered Korinninae as a subordinate taxon among the species-rich Necrosciinae with Asceles as sister taxon, thus suggesting that placement of single eggs on leaves by host plant specialists might be the evolutionary precursor of ootheca formation within stick insects.
Similar content being viewed by others
Introduction
Predation is a primary driving force in the evolution of insects, triggering elaborate anti-predator adaptations that involve diverse camouflage and reproductive strategies1,2,3. Among terrestrial arthropods, the herbivorous stick and leaf insects or Phasmatodea exhibit an exceptionally high degree of plant mimicry, imitating various parts of plants such as leaves, twigs and bark4. Camouflage, or more precisely masquerade3, already played a crucial role in the early evolution of this insect group5,6 and does not stop short at the insects' eggs, which have a strong resemblance to plant seeds7,8. The phasmatodean egg capsule is remarkably hard-shelled and diversely sculptured (Fig. 1a), bearing a lid-like operculum at its anterior pole through which the offspring emerges (Fig. 1b). Adult females lay eggs over a period of several months at a rate of one (or less) to several per day4,8. During oviposition females of most species remain in the foliage and drop or flick single eggs from their ovipositor to the ground8,9. Some species place their eggs more carefully by inserting them into crevices or soil, glue them to substrate or pierce them into leaves8,9,10,11. One common feature of these diverse egg-laying modes is that eggs are laid singly, with very few exceptions where separate eggs are arranged in loose clutches or in a small row9. Here we report the first stick insect to produce a complex egg case or ootheca that contains numerous eggs in a highly ordered arrangement. This unknown mode of egg deposition constitutes an unexpected evolutionary novelty and a major shift in the reproductive strategy of phasmatodeans, i.e. a switch from dispersal of individual eggs to sophisticated egg concentration.
Figure 1
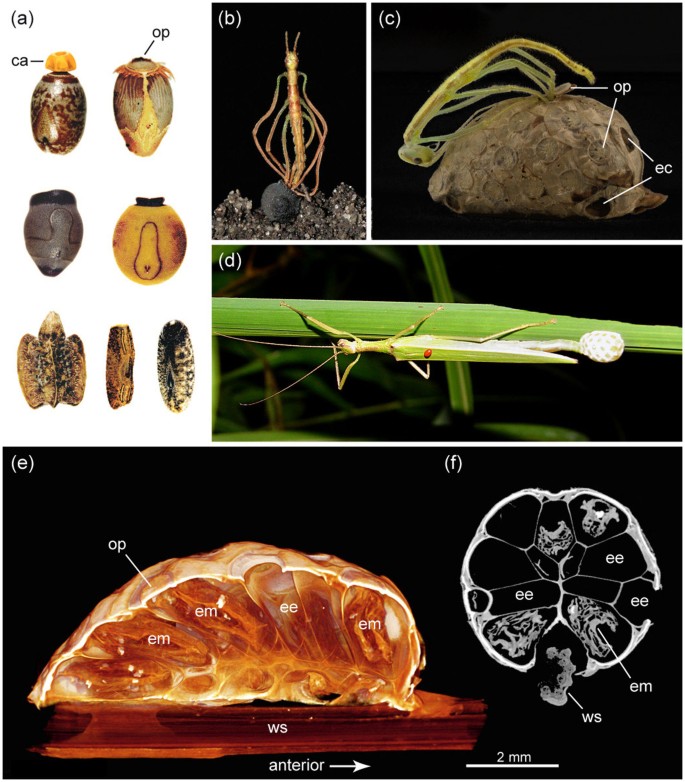
(a) single eggs of various stick insects (not to scale); (b) juvenile (Anchiale spec.) hatching from egg; (c) juvenile Korinninae spec. hatching from ootheca, please note that the opened operculum has a 2nd outer layer consisting of ootheca material; (d) female Korinninae spec. while producing an ootheca; (e,f) ootheca scans: (e) longitudinal section of volume rendered ootheca showing arrangements of eggs, abandoned or with embryos inside; (f) cross section. ca, capitulum; ec, egg-like chamber; ee, empty egg; em, embryo; op, operculum; ws, wooden stick. Photographs taken by the authors.
Results
Egg case morphology
The oothecae (Fig. 1c–f) have an oval general appearance. The scanned ootheca consists of 34 eggs oriented radially around the substrate center (twig or leaf) to which it was attached. Each egg's anterior end is directed to the ootheca's surface bearing the operculum. The posterior end of each egg is tapered and directed towards the ootheca's center, forming a honeycomb-like lattice with eggs appearing hexagonal or pentagonal in cross section. The egg capsule appears to be extraordinarily thin and must have been soft-walled during oviposition. There is some material of unknown origin filling the minor space between the tightly packed eggs and providing a fine but dense layer on the ootheca's surface, particularly around the opercular rim. There are four small chambers at the anterior and two at the posterior pole of the ootheca, which have an operculum-like opening but do not harbour eggs. The function of these egg-like chambers is enigmatic; ventilation is unlikely as their openings appear sealed by a thin layer of ootheca material (for further details see electronic supplementary movie).
Identification of the ootheca-producing stick insect species
Based on the following combination of characters, we identified this ootheca-producing species as an undescribed member of the subfamily Korinninae, the most species-poor subfamily recognised among stick insects12,13,14,15,16: Legs with area apicalis, a demarcated triangular area located ventrally on the apex of the tibiae and with non-pectinate ungues; hind wings fully developed with unbranched radius vein; female operculum with deeply notched hind margin. The subfamily was erected by Günther12 based on the genera Korinnis and Kalokorinnis, which currently comprise only seven described species from Borneo, Thailand and the Philippines13,14,15,16.
Phylogenetic analysis
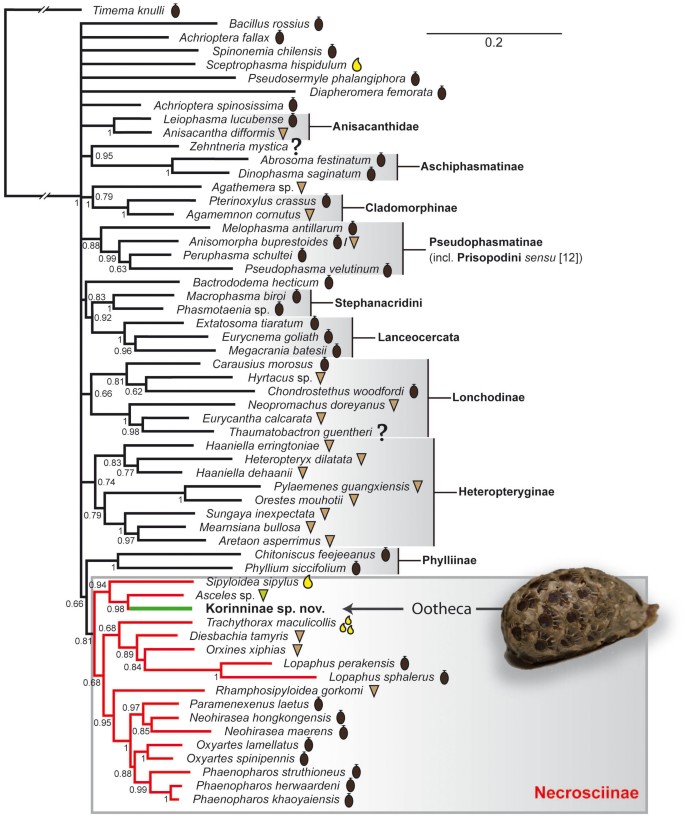
Our analyses of the concatenated molecular data using likelihood (Fig. 2) and Bayesian (Fig. 3) methods yielded similar phylogenies consistent with previous studies11,17,18 including well-supported monophyletic Aschiphasmatinae, Cladomorphinae, Diapheromerinae, Heteropteryginae, Lanceocercata, Lonchodinae, Pseudophasmatinae (including Melophasma) and Stephanacridini. Furthermore, we found good support for Anisacanthidae and Achriopterini. The undescribed Korinninae species was recovered as a subordinate taxon within Necrosciinae with Asceles as sister taxon (MLB = 86; BPP = 0.98).
Figure 2
Maximum likelihood tree of the Phasmatodea based on combined molecular data with egg-deposition modes mapped on taxa according to symbol legend.
Bootstrap values >50 are given below nodes. Ootheca photographed by Bruno Kneubühler.
Figure 3
Majority-rule consensus tree of post-burn trees of Phasmatodea resulting from Bayesian analysis of the combined molecular data.
Posterior probabilities are given below nodes. Symbols of egg-deposition modes as in Fig. 2. Ootheca photographed by Bruno Kneubühler.
Discussion
Concentrated egg-deposition in form of an ootheca is unique and highly unusual for stick insects. In contrast, ootheca formation is a defining groundplan feature of the Dictyoptera (cockroaches, termites and praying mantises)19,20,21, but is also found in various other insect groups such as grasshoppers and locusts (Orthoptera: Caelifera)19,22,[23](/articles/srep07825#ref-CR23 "Salas-Araiza, M. D., Mackay, W. P., Valdez-Carrasco, J., Salazar-Solís, E. & Martínez-Jaime, O. A. Characterization and comparison of the eggs of seven species of mexican grasshoppers. Southwestern Ent. 38, 267–274; doi: http://dx.doi.org/10.3958/059.038.0210
(2013)."), heelwalkers (Mantophasmatodea)[24](/articles/srep07825#ref-CR24 "Klass, K.-D., Picker, M. D., Damgaard, J., van Noort, S. & Tojo, K. The taxonomy, genitalic morphology and phylogenetic relationships of Southern African Mantophasmatodea (Insecta). Ent. Abh. 61, 3–67 (2003)."),[25](/articles/srep07825#ref-CR25 "Roth, S., Molina, J. & Predel, R. Biodiversity, ecology and behavior of the recently discovered insect order Mantophasmatodea. Front. Zool. 11, 70; 10.1186/s12983-014-0070-0 (2014).") and even some chrysomelid beetles (Coleoptera: Cassidinae)[19](/articles/srep07825#ref-CR19 "Grimaldi, D. & Engel, M. S. Evolution of the insects (Cambridge University Press, New York, 2005)."),[26](/articles/srep07825#ref-CR26 "Gomes, P. A. A., Prezoto, F. & Frieiro-Costa, F. A. Biology of Omaspidespallidipennis Boheman, 1854 (Coleoptera: Chrysomelidae: Cassidinae). Psyche 2012, 1–8; 10.1155/2012/290102 (2012)."). Oothecae, also referred to as egg pods or egg cases, likely evolved to protect the eggs from desiccation, predators and parasitoids[19](/articles/srep07825#ref-CR19 "Grimaldi, D. & Engel, M. S. Evolution of the insects (Cambridge University Press, New York, 2005)."),[20](/articles/srep07825#ref-CR20 "Ware, J. L., Litman, J., Klass, K.-D. & Spearman, L. A. Relationships among the major lineages of Dictyoptera: the effect of outgroup selection on dictyopteran tree topology. Sys. Ent. 33, 429–450; 10.1111/j.1365-3113.2008.00424.x (2008)."),[23](/articles/srep07825#ref-CR23 "Salas-Araiza, M. D., Mackay, W. P., Valdez-Carrasco, J., Salazar-Solís, E. & Martínez-Jaime, O. A. Characterization and comparison of the eggs of seven species of mexican grasshoppers. Southwestern Ent. 38, 267–274; doi:
http://dx.doi.org/10.3958/059.038.0210
(2013)."),[25](/articles/srep07825#ref-CR25 "Roth, S., Molina, J. & Predel, R. Biodiversity, ecology and behavior of the recently discovered insect order Mantophasmatodea. Front. Zool. 11, 70; 10.1186/s12983-014-0070-0 (2014)."). In arid environment, the eggs of grasshoppers are laid into the ground protected by a hardening foamy substance that is also adhesive to surrounding habitat material like sand and soil[23](/articles/srep07825#ref-CR23 "Salas-Araiza, M. D., Mackay, W. P., Valdez-Carrasco, J., Salazar-Solís, E. & Martínez-Jaime, O. A. Characterization and comparison of the eggs of seven species of mexican grasshoppers. Southwestern Ent. 38, 267–274; doi:
http://dx.doi.org/10.3958/059.038.0210
(2013)."). A similar mode of egg deposition is found in Mantophasmatodea who produce egg pods only when soil is provided[25](/articles/srep07825#ref-CR25 "Roth, S., Molina, J. & Predel, R. Biodiversity, ecology and behavior of the recently discovered insect order Mantophasmatodea. Front. Zool. 11, 70; 10.1186/s12983-014-0070-0 (2014)."). The reported stick-insect ootheca is most reminiscent of those found in praying mantises (Mantodea) or tortoise beetles (Cassidinae) whose eggs do not bear a operculum, but who also build the egg case externally upon certain substrate, e.g. against plant parts or rocky underground, including external application of coating during and after highly ordered egg placement[19](/articles/srep07825#ref-CR19 "Grimaldi, D. & Engel, M. S. Evolution of the insects (Cambridge University Press, New York, 2005)."),[20](/articles/srep07825#ref-CR20 "Ware, J. L., Litman, J., Klass, K.-D. & Spearman, L. A. Relationships among the major lineages of Dictyoptera: the effect of outgroup selection on dictyopteran tree topology. Sys. Ent. 33, 429–450; 10.1111/j.1365-3113.2008.00424.x (2008)."),[26](/articles/srep07825#ref-CR26 "Gomes, P. A. A., Prezoto, F. & Frieiro-Costa, F. A. Biology of Omaspidespallidipennis Boheman, 1854 (Coleoptera: Chrysomelidae: Cassidinae). Psyche 2012, 1–8; 10.1155/2012/290102 (2012).").Stick insects in general are well adapted to disperse their hard-shelled, seed-like eggs by dropping them individually to the ground, which is considered to represent the ground pattern in Phasmatodea27 and to be an advantageous strategy for cryptic animals2. On the contrary, in webspinners (Embioptera), which are the sister group of stick and leaf insects18,28,29,30, eggs are often deposited in tight or loose clusters within the silk galleries the individuals inhabit, usually attached to a substrate31,32. This behaviour also involves brood care and embedment in a hardened paste consisting of pulverised plant substrate and fecal pellets supplemented by salivary secretions31,32. In the Californian stick insect Timema, which represents one of the two basal lineages among extant Phasmatodea18,27, females also coat their eggs in pulverised substrate before dropping or placing single eggs onto the ground33.
In all remaining stick insects, the species-rich Euphasmatodea, females that lay single, non-adhesive eggs represent the plesiomorphic condition27 (see Fig. 2). Numerous phasmid species throw the eggs some distance in order to further disperse them and avoid clumping in the litter, thus decreasing susceptibility to predators and egg-parasitoids4. Additional adaptations promoting dispersal involve specialized structures attached to the egg's operculum, the capitulum (Fig. 1a), which induces egg removal and transportation by ants7,8. Density-responsive egg-parasitoids and predation by granivorous birds are considered to be significant driving forces for acquisition of these elaborate dispersal strategies34,[35](/articles/srep07825#ref-CR35 "Shelomi, M. Phasmid eggs do not survive digestion by quails and chick. J. Orth. Res. 20, 159–163; doi: http://dx.doi.org/10.1665/034.020.0203
(2011)."). Two subgroups of the chrysidids or cuckoo wasps, Amiseginae and Loboscelidiinae, are obligatory parasitoids to stick insect eggs[36](/articles/srep07825#ref-CR36 "Krombein, K. V. Biosystematic studies of Ceylonese wasps, XI: A monograph of the Amiseginae and Loboscelidiinae (Hymenoptera: Chrysididae). Smithson. Contr. Zool. 376, 1–79 (1983)."). The predominantly flightless female wasps search for eggs in low vegetation and leaf litter. They chew holes into the egg capsule with their specialised mouthparts and oviposit into the phasmatodean egg. The operculum of the egg, which is not damaged during this process, is burst open by the juvenile wasp after development is finished[36](/articles/srep07825#ref-CR36 "Krombein, K. V. Biosystematic studies of Ceylonese wasps, XI: A monograph of the Amiseginae and Loboscelidiinae (Hymenoptera: Chrysididae). Smithson. Contr. Zool. 376, 1–79 (1983)."). The geographic distribution of these wasps overlaps worldwide with those of Phasmatodea[36](/articles/srep07825#ref-CR36 "Krombein, K. V. Biosystematic studies of Ceylonese wasps, XI: A monograph of the Amiseginae and Loboscelidiinae (Hymenoptera: Chrysididae). Smithson. Contr. Zool. 376, 1–79 (1983).") and parasitisation rates of eggs between 40 and 80% have been reported[8](/articles/srep07825#ref-CR8 "Hughes, L. & Westoby, M. Capitula on stick insect eggs and elaiosomes on seeds: convergent adaptations for burial by ants. Funct. Ecol. 6, 642–648 (1992)."). Egg deposition on arboreal substrate as performed by Korinninae and few other stick insect taxa might reduce parasitisation rates significantly[36](/articles/srep07825#ref-CR36 "Krombein, K. V. Biosystematic studies of Ceylonese wasps, XI: A monograph of the Amiseginae and Loboscelidiinae (Hymenoptera: Chrysididae). Smithson. Contr. Zool. 376, 1–79 (1983)."). Furthermore, the dense layer coating the surface of the ootheca likely provides further protection as does the tight egg arrangement that largely decreases the capsule surface which can be accessed by the parasitoids, since egg opercula remain unaffected. The reduced capsule surface might also reduce desiccation of eggs although the oothecae are not found in a particularly arid environment.A further adaptive advantage of arboreal concentration of eggs is probably related to the insects' diet. Flightless phasmatodeans are exceedingly polyphagous, linked to their limited motility in diversely structured forests37. In contrast, volant forms as found in the species-rich Southeast Asian Necrosciinae who can more easily reach dispersed plants have a more restricted diet and are often regarded as host plant specialists38.
These potential advantages obviously compensate for the parental investment of a time-consuming ootheca production and for the drawback of synchronous egg hatch that places newly-hatched offspring in greater risk of being detected by predators.
We recovered the Korinninae species as a subordinate taxon within the Necrosciinae. This is particularly noteworthy, since Korinninae and Necrosciinae are considered to belong to the two different suborders of Phasmatodea, Areolatae and Anareolatae12. This traditional subdivision is based on the presence (areolate) or absence (anareolate) of the area apicalis on the tibiae, but neither Areolatae nor Anareolatae appear to be monophyletic11,17,18. Furthermore, the phylogenetic placement of the areolate Korinninae within the anareolate Necrosciinae suggests an atavistic origin or reversal of this trait in Korinninae, i.e. a recovery of the area apicalis after former loss, a phenomenon described before for wings and other morphological traits18,39. Originally considered to be an “isolated” areolate taxon without obvious relationships to other phasmatodean subfamilies12, recent classifications placed Korinninae either as sister group to the areolate Southeast Asian Aschiphasmatinae13 or as sister to the likewise areolate Neotropical Prisopodinae14. The results of our molecular analysis refute any close relationship of Korinninae to either of these groups, namely to Abrosoma + Dinophasma (Aschiphasmatinae) or to Melophasma (Prisopodinae).
A strong overall resemblance between Korinninae and certain Necrosciinae, both gracile winged stick insects with long antennae, was indicated before12. Flighted necrosciines can effectively distribute their offspring and often place eggs onto or near host plant leaves, sometimes even in small rows or batches4,9,38. The ootheca-forming Korinninae female exhibits good flight capability (pers. obs. J.B., J.C.) and appears to be a diet specialist as well since the offspring did not accept any plant offered in captivity (pers. obs. B.K.). Yet, the natural host plant range of Korinninae remains to be identified. The plant specialist Asceles, which was recovered as sister taxon to the Korinninae, pierces single eggs onto leaves10. Consequently, we consider the careful placement of eggs on host plants to be the evolutionary precursor of ootheca formation. However, the few oothecae collected were not found associated with a certain potential host plant.
The biology of Korinninae is virtually unknown and the egg deposition mode hitherto unreported. However, single eggs of Kalokorinnis were described before based on immature eggs removed from the dissected abdomen of a female13,15. The eggs were described and illustrated as being tapered towards their polar end, which might be an adaptation to the arrangement in egg cases. Thus, formation of an ootheca might apply to all Korinninae and constitute an apomorphic trait of this group.
Moreover, the subordinate phylogenetic placement of Korinninae within Necrosciinae provides insight into the evolutionary speed of this convergent transition of ootheca formation in stick insects, which requires numerous physiological and behavioural adaptations, such as synchronized egg maturation, development of specific accessory glands and ootheca-building capability. Females of some Necrosciinae genera such as Calvisia, Marmessoidea and Trachythorax glue their eggs in loose single-layer clusters without any coating on parts of plants9. With synchronous egg maturation and sticky glandular secretion already developed in these taxa, it would be straightforward to assume that these forms preceded the ootheca formation. However, we found no support for this assumption as our molecular phylogeny recovered Trachythorax to be unrelated to Korinninae (Figs. 2,3). Consequently, egg clumping on arboreal substrate, which is also reported for at least one member of Pseudophasmatinae (Metriophasma diocles)9, evolved several times independently within Phasmatodea. This mode of egg deposition is probably advantageous in the foliage in absence of flightless wasps that search for dispersed phasmid eggs on the ground. However, at least some winged Amiseginae were also found on trees9.
Admittedly, we do not know whether novel glands have been developed in Korinninae. The internal anatomy of the new species is not known and a broad comparative investigation of female internal genitalia of phasmatodeans is lacking. In dictyopterans, the produced egg mass is coated with secretions from genitalic accessory glands of the ninth abdominal segment40. These glands might be part of the ground pattern of pterygote insects41, though absent in some taxa, i.e. Mantophasmatodea24, which appear closely related to Phasmatodea30 and also produce egg pods25. Paired glands leading into the bursa copulatrix (genital chamber) were described for some stick insects42, but were not found in Eurycantha42 and not reported for Timema43. Noteworthy, these accessory glands are also absent in Calvisia and Trachythorax, two taxa that glue their eggs in batches. Both species possess various other glands associated with the oviduct instead42, thus indicating that a derived egg deposition mode might indeed require anatomical modifications.
Necrosciinae has started a rapid radiation approximately 30 million years ago11, most probably giving rise to Korinninae and ootheca formation within less than 10 million years. Further phylogenetic investigations of phasmatodeans based on more densely sampled Necrosciinae diversity will become necessary to adequately describe the evolutionary transitional steps leading to ootheca formation.
In summary, parasitisation and host-plant specificity have most probably triggered the rapid evolution of this unique oviposition strategy among stick insects. Yet, stick insects remain poorly studied in their natural environment and our explanations await further investigations, particularly in regard of the ecology and reproductive anatomy of this enigmatic new species.
Methods
Collection of material
Three oothecae, four male and three female stick insects were collected during a field trip to Cat Tien National Park and Dong Nai Biosphere Reserve South Vietnam, July 2013. The oothecae of the undescribed species were found glued to different plant species and also on the wall of a guest house. Specimens are housed in the Royal Belgian Institute of Natural Sciences, Brussels, Belgium and in the Institute of Ecology and Biological Resources, Hanoi, Vietnam.
Micro-computed tomography
The dry ootheca was mounted on a wooden stick and scanned with a Xradia MicroXCT-200 X-ray imaging system (Carl Zeiss X-ray Microscopy Inc., Pleasanton, USA) at 30 KV and 6 W (0.39 scintillator-objective lens unit, 3 s exposure time, 13.5 µm pixel size). The obtained data were processed using the 3D analysis software AMIRA v. 5.4.3 (Visage Imaging, Berlin, Germany).
Phylogenetic analyses
The molecular analyses targeted mitochondrial (COI, COII) and nuclear gene regions (H3, 28S) that were used in previous Phasmatodea studies11,17,18. PCR cycling, purification, sequencing conditions and sequence alignments followed11,44. Phylogenetic analyses included 59 phasmatodean taxa (supplementary table S1) with the Californian Timema used as outgroup11,17 and were performed using the program Geneious (Geneious v7.0.1. Available at http://www.geneious.com). Alignments of different genetic markers were concatenated and subsequent analyses were performed using the combined dataset. We utilized likelihood (ML) and Bayesian algorithms (BI) for analyses. We employed the Akaike information criteria (AIC) as implemented in Modeltest v3.745 to select a suitable model of sequence evolution for the combined data.
ML analysis used PHyML46 incorporating a GTR model with gamma-distributed rate variation across sites and a proportion of invariable sites. Bootstrap re-sampling used 500 iterations and resulting ML bootstrap values (MLB) were recorded.
MrBayes 3.1.247 was utilized to implement Bayesian analysis, applying the GTR model with gamma-distributed rate variation across sites and a proportion of invariable sites. Analyses with MrBayes used four independent Markov Chain Monte Carlo (MCMC) runs for ten million generations with a burn-in of 25% and a tree sampling frequency of 1000. Resulting posterior probabilities on the nodes were recorded. Results were then checked for convergence. Trees sampled after burn-in of the four different MCMC runs were merged and used to construct a 50% majority rule consensus tree. Resulting posterior probabilities (BPP) were recorded. Novel sequence data generated in the present study are deposited on GenBank under the accession numbers KP300885-KP300930. Sequence data from previous studies can be accessed under accession numbers FJ474100–FJ47440311, KJ024376–KJ02457517, AY121129–AY121186 and AY125216–AY12532618.
References
- Edmunds, M. Defence in animals (Longman Group Limited, HarlowNew York, 1974).
- Taylor, J. The advantage of spacing-out. J. theor. Biol. 59, 485–490 (1976).
Article CAS PubMed Google Scholar - Stevens, M. & Merilaita, S. Animal camouflage: current issues and new perspectives. Phil. Trans. R. Soc. B 364, 423–427; 10.1098/rstb.2008.0217 (2009).
Article PubMed Google Scholar - Bedford, G. O. Biology and ecology of the Phasmatodea. Ann. Rev. Ent. 23, 125–149 (1978).
Article Google Scholar - Wedmann, S., Bradler, S. & Rust, J. The first fossil leaf insect: 47 million years of specialized cryptic morphology and behavior. Proc. Natl. Acad. Sci. USA 104, 565–569; 10.1073/pnas.0606937104 (2007).
Article CAS ADS PubMed Google Scholar - Wang, M. et al. Under cover at pre-angiosperm times: a cloaked phasmatodean insect from the Early Cretaceous Jehol biota. PLOS ONE 9, e91290; 10.1371/journal.pone.0091290 (2014).
Article CAS ADS PubMed PubMed Central Google Scholar - Compton, S. G. & Ware, A. B. Ants disperse the elaiosome-bearing eggs of an African stick insect. Psyche 98, 207–213 (1991).
Article Google Scholar - Hughes, L. & Westoby, M. Capitula on stick insect eggs and elaiosomes on seeds: convergent adaptations for burial by ants. Funct. Ecol. 6, 642–648 (1992).
Article Google Scholar - Carlberg, U. A review of different types of egglaying in the Phasmida in relation to the shape of the eggs and with a discussion on their taxonomic importance (Insecta). Biol. Zbl. 102, 587–602 (1983).
Google Scholar - Sellick, J. The range of egg capsule morphology within the Phasmatodea and its relevance to the taxonomy of the order. Ital. J. Zool. 64, 97–104 (1997).
Article Google Scholar - Buckley, T. R., Attanayake, D. & Bradler, S. Extreme convergence in stick insect evolution: phylogenetic placement of the Lord Howe Island tree lobster. Proc. R. Soc. B 276, 1055–1062; 10.1098/rspb.2008.1552 (2009).
Article CAS PubMed Google Scholar - Günther, K. Über die taxonomische Gliederung und geographische Verbreitung der Insektenordnung der Phasmatodea. Beitr. Ent. 3, 541–563 (1953).
Google Scholar - Bragg, P. E. Phasmids of Borneo (Natural History Publications Borneo, Kota Kinabalu, 2001).
- Zompro, O. Revision of the genera of the Areolatae, including the status of Timema and Agathemera (Insecta, Phasmatodea). Verh. Naturwiss. Ver. Hamburg (NF) 37, 1–327 (2004).
Google Scholar - Bragg, P. E. A review of the subfamily Korinninae (Phasmida: Pseudophasmatidae), with the description of a new species. Tijdschr. v. Ent. 138, 45–50 (1995).
Google Scholar - Gottardo, M. A new species of Korinnis Günther from the Philippines (Phasmatodea: Prisopodidae: Korinninae). Zootaxa 1917, 61–64 (2008).
Article Google Scholar - Bradler, S., Robertson, J. A. & Whiting, M. F. A molecular phylogeny of Phasmatodea with emphasis on Necrosciinae, the most species-rich subfamily of stick insect. Sys. Ent. 39, 205–222; 10.1111/syen.12055 (2014).
Article Google Scholar - Whiting, M. F., Bradler, S. & Maxwell, T. Loss and recovery of wings in stick insects. Nature 421, 264–267; 10.1038/nature01313 (2003).
Article CAS ADS PubMed Google Scholar - Grimaldi, D. & Engel, M. S. Evolution of the insects (Cambridge University Press, New York, 2005).
- Ware, J. L., Litman, J., Klass, K.-D. & Spearman, L. A. Relationships among the major lineages of Dictyoptera: the effect of outgroup selection on dictyopteran tree topology. Sys. Ent. 33, 429–450; 10.1111/j.1365-3113.2008.00424.x (2008).
- Bohn, H. & Klass, K.-D. in Lehrbuch der Speziellen Zoologie I, 5. Teil: Insecta (ed. Dathe H. H.) 181–182 (Spektrum Akademischer Verlag, Gustav Fischer, Heidelberg, Berlin, 2003).
- Baldacchino, F., Sciarretta, A. & Addante, R. Evaluating the spatial distribution of Dociostaurus maroccanus egg pods using different sampling designs. Bull. Insectol. 65, 223–231 (2012).
Google Scholar - Salas-Araiza, M. D., Mackay, W. P., Valdez-Carrasco, J., Salazar-Solís, E. & Martínez-Jaime, O. A. Characterization and comparison of the eggs of seven species of mexican grasshoppers. Southwestern Ent. 38, 267–274; doi: http://dx.doi.org/10.3958/059.038.0210 (2013).
Article Google Scholar - Klass, K.-D., Picker, M. D., Damgaard, J., van Noort, S. & Tojo, K. The taxonomy, genitalic morphology and phylogenetic relationships of Southern African Mantophasmatodea (Insecta). Ent. Abh. 61, 3–67 (2003).
Google Scholar - Roth, S., Molina, J. & Predel, R. Biodiversity, ecology and behavior of the recently discovered insect order Mantophasmatodea. Front. Zool. 11, 70; 10.1186/s12983-014-0070-0 (2014).
Article Google Scholar - Gomes, P. A. A., Prezoto, F. & Frieiro-Costa, F. A. Biology of Omaspides pallidipennis Boheman, 1854 (Coleoptera: Chrysomelidae: Cassidinae). Psyche 2012, 1–8; 10.1155/2012/290102 (2012).
Article Google Scholar - Bradler, S. Die Phylogenie der Stab- und Gespenstschrecken (Insecta: Phasmatodea). Spec. Phyl. Evol. 2, 3–139 (2009).
Google Scholar - Ishiwata, K., Sasaki, G., Ogawa, J., Miyata, T. & Su, Z.-H. Phylogenetic relationships among insect orders based on three nuclear protein-coding gene sequences. Mol. Phyl. Evol. 58, 169–180; 10.1016/j.ympev.2010.11.001 (2011).
Article CAS Google Scholar - Simon, S. & Letsch, H. Insect phylogenomics: new insights on the relationships of lower neopteran orders (Polyneoptera). Sys. Ent. 38, 783–793; 10.1111/syen.12028 (2013).
Article Google Scholar - Misof, B. et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767; 10.1126/science.1257570 (2014).
Article CAS ADS PubMed Google Scholar - Ross, E. S. EMBIA. Contributions to the biosystematics of the insect order Embiidina. Part 2. A review of the biology of Embiidina. Occas. Pap. Calif. Acad. Sci. 149, 1–35 (2000).
Google Scholar - Matzke, D. & Klass, K.-D. Reproductive biology and nymphal development in the basal earwig Tagalina papua (Insecta: Dermaptera: Pygidicranidae), with a comparison of brood care in Dermaptera and Embioptera. Ent. Abh. 62, 99–116 (2005).
Google Scholar - Henry, L. M. Biological notes on Timema californica Scudder (Phasmoidea: Timemidae). Pan-Pac. Ent. 13, 137–141 (1937).
Google Scholar - Windsor, D. M., Trapnell, D. W. & Amat, G. The egg capitulum of a Neotropical walkingstick, Calynda bicuspis, induces aboveground egg dispersal by the ponerine ant, Ectatomma ruidum. J. Ins. Behav. 9, 353–367 (1996).
Article Google Scholar - Shelomi, M. Phasmid eggs do not survive digestion by quails and chick. J. Orth. Res. 20, 159–163; doi: http://dx.doi.org/10.1665/034.020.0203 (2011).
Article Google Scholar - Krombein, K. V. Biosystematic studies of Ceylonese wasps, XI: A monograph of the Amiseginae and Loboscelidiinae (Hymenoptera: Chrysididae). Smithson. Contr. Zool. 376, 1–79 (1983).
Article Google Scholar - Blüthgen, N., Metzner, A. & Ruf, D. Food plant selection by stick insects (Phasmida) in a Bornean rain forest. J. Trop. Ecol. 22, 35–40; 10.1017/S0266467405002749 (2006).
Article Google Scholar - Junker, R. R., Itioka, T., Bragg, P. E. & Blüthgen, N. Feeding preferences of phasmids (Insecta: Phasmida) in a Bornean dipterocarp forest. Raffl. Bull. Zool. 56, 445–452 (2008).
Google Scholar - Wiens, J. J. Re-evolution of lost mandibular teeth in frogs after more than 200 million years and re-evaluating Dollo's law. Evolution 65, 1283–1296; 10.1111/j.1558-5646.2011.01221.x (2011).
Article PubMed Google Scholar - Klass, K.-D. & Ehrmann, R. in Lehrbuch der Speziellen Zoologie I, 5. Teil: Insecta (ed. Dathe, Dathe H. H.) 182–197 (Spektrum Akademischer Verlag, Gustav Fischer, Heidelberg, Berlin, 2003).
- Klass, K.-D. The female abdomen of ovipositor-bearing Odonata (Insecta: Pterygota). Arthropod Syst. Phyl. 66, 45–142 (2008).
Google Scholar - Günther, K. Funktionell-anatomische Untersuchungen über die Bursa copulatrix, den Ovipositor und den männlichen Kopulationsapparat bei Phasmiden. Jenaische Z. Naturw. 68, 403–462 (1933).
Google Scholar - Tilgner, E. H., Kiselyova, T. G. & McHugh, J. V. A morphological study of Timema cristinae Vickery with implications for the phylogenetics of Phasmida. Dtsch. Ent. Z. 46, 149–162 (1999).
Article Google Scholar - Goldberg, J., Knapp, M., Emberson, R. M., Townsend, J. I. & Trewick, S. A. Species Radiation of Carabid Beetles (Broscini: Mecodema) in New Zealand. PLOS ONE 9, e86185; 10.1371/journal.pone.0086185 (2014).
Article CAS ADS PubMed PubMed Central Google Scholar - Posada, D. & Crandall, K. A. Modeltest: Testing the model of DNA substitution. Bioinformatics 14, 817–818; 10.1093/bioinformatics/14.9.817 (1998).
Article CAS PubMed Google Scholar - Guindon, S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321; 10.1093/sysbio/syq010 (2010).
Article CAS PubMed Google Scholar - Ronquist, F. & Huelsenbeck, J. P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574; 10.1093/bioinformatics/btg180 (2003).
Article CAS PubMed Google Scholar
Acknowledgements
We thank Christoph Seiler, Cecilia Dominguez and Alejandro Vera Sánchez for providing information about egg-deposition modes of certain taxa. This study was supported by the German Science Foundation (DFG grant BR 2930/2-1 to S.B.) and by the capacity building Programme of the Belgian Global Taxonomic Initiative National Focal Point that runs with financial support from the Belgian Directorate-General for Development Cooperation.
Author information
Authors and Affiliations
- Johann-Friedrich-Blumenbach-Institute of Zoology and Anthropology, Georg-August-University Göttingen, Berliner Str. 28, 37073, Göttingen, Germany
Julia Goldberg, Fanny Leubner & Sven Bradler - Royal Belgian Institute of Natural Sciences, Vautier Street 29, Brussels, 1000, Belgium
Joachim Bresseel & Jerome Constant - Schädrütihalde 47c, 6006, Lucerne, Switzerland
Bruno Kneubühler - Zoological Institute and Museum, Ernst-Moritz-Arndt-University, Johann-Sebastian-Bach-Str. 11/12, 17489, Greifswald, Germany
Peter Michalik
Authors
- Julia Goldberg
You can also search for this author inPubMed Google Scholar - Joachim Bresseel
You can also search for this author inPubMed Google Scholar - Jerome Constant
You can also search for this author inPubMed Google Scholar - Bruno Kneubühler
You can also search for this author inPubMed Google Scholar - Fanny Leubner
You can also search for this author inPubMed Google Scholar - Peter Michalik
You can also search for this author inPubMed Google Scholar - Sven Bradler
You can also search for this author inPubMed Google Scholar
Contributions
S.B. and J.G. designed research. J.B. and J.C. collected material. B.K. conducted culturing experiments. J.G. obtained and analysed molecular data. P.M. and F.L. carried out x-ray scanning and 3D reconstruction. S.B., J.G. and P.M. wrote the paper. J.B., J.C. and F.L. contributed to manuscript editing. All authors have approved of the final version of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Goldberg, J., Bresseel, J., Constant, J. et al. Extreme convergence in egg-laying strategy across insect orders.Sci Rep 5, 7825 (2015). https://doi.org/10.1038/srep07825
- Received: 14 August 2014
- Accepted: 12 December 2014
- Published: 16 January 2015
- DOI: https://doi.org/10.1038/srep07825