Docking IκB kinases (original) (raw)
- News & Views
- Published: 17 September 1998
Signal transduction
Nature volume 395, pages 225–226 (1998)Cite this article
- 390 Accesses
- 59 Citations
- 3 Altmetric
- Metrics details
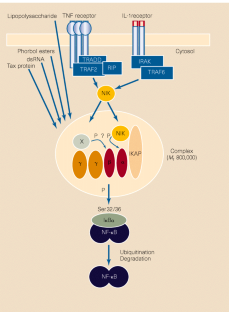
In response to stress or inflammatory and immune reactions, mammalian cells rapidly send a transcription factor called NF-κB into the nucleus. Once there, this protein activates the transcription of specific genes, eliciting various cellular responses. Without the right extracellular signals, however, NF-κB is kept under tight control in the cytoplasm, coupled to inhibitory IκB proteins (IκBα, β and ε), which block its transport into the nucleus. NF-κB is critical for proper immune function, cell growth and survival, and anomalous activation is associated with inflammatory and neoplastic diseases and viral infection.
Several laboratories have established that signal-induced phosphorylation is accomplished by an intriguingly large IKK complex. Two IKKs — IKK-α and IKK-β — have been cloned3,4, and shown to be part of multi-protein complexes in the appropriate size range (relative molecular mass, _M_r, 800,000). To date, they are the only kinases known to phosphorylate IκBα at the same residues that are modified in response to agents that activate NF-κB (such as tumour-necrosis factor-α (TNF-α), interleukin-1 (IL-1) and lipopolysaccharide). Moreover, IKK-α and IKK-β are both stimulated by TNF-α or IL-1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
Figure 1: Putative connections between IκB kinases, scaffold proteins and signalling molecules in the activation of NF-κB.
References
- Cohen, L., Henzel, W. J. & Baeuerle, P. A. Nature 395, 292–296 (1998).
Article ADS CAS Google Scholar - Rothwarf, M. K., Zandi, E., Natoli, G. & Karin, M. Nature 395, 297–300 (1998).
Article ADS CAS Google Scholar - Maniatis, T. Science 278, 818–819 (1997).
Google Scholar - Stancovski, I. & Baltimore, D. Cell 91, 299–302 (1997).
Google Scholar - Yamaoka, S.et al. Cell 93, 1231–1240 (1998).
Google Scholar - Mercurio, F.et al. Science 278, 860–866 (1997).
Google Scholar - Malinin, N. L., Boldin, M. P., Kovalenk, A. V. & Wallach, D. Nature 385, 540–544 (1997).
Article ADS CAS Google Scholar - Lee, F. S., Peters, R. T., Dang, L. C. & Maniatis, T. Proc. Natl Acad. Sci. USA 95, 9319–9324 (1998).
Google Scholar - Karin, M. & Delhase, M. Proc. Natl Acad. Sci. USA 95, 9067–9069 (1998).
Google Scholar - Zandi, E. & Karin, M. Science 281, 1360–1363 (1988).
Google Scholar
Author information
Authors and Affiliations
- the Max-Delbrück-Centrum für Molekulare Medizin, Robert-Rössle-Strasse 10, 13122, Berlin, Germany
Claus Scheidereit
Rights and permissions
About this article
Cite this article
Scheidereit, C. Docking IκB kinases.Nature 395, 225–226 (1998). https://doi.org/10.1038/26121
- Issue Date: 17 September 1998
- DOI: https://doi.org/10.1038/26121