Fluctuations caught in the act (original) (raw)
- Brief Communication
- Published: 23 March 2000
Critical phenomena
Nature volume 404, page 352 (2000)Cite this article
- 939 Accesses
- 85 Citations
- Metrics details
Abstract
When a system approaches a critical point, strong fluctuations develop on every scale, from molecules to the entire system. Here we show that critical fluctuations in the domains in a lipid monolayer can be captured and measured by immobilizing it on a solid support and visualizing the pattern using atomic-force microscopy. Such fluctuations in physical properties may reflect the nanometre-scale domain organization in the lipid-bilayer component of biological membranes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
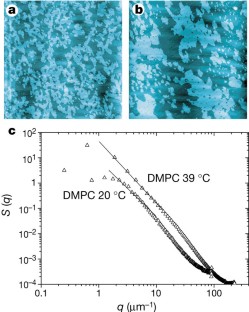
Figure 1: Critical fluctuations in phospholipid monolayers imaged by atomic-force microscopy as a height-difference map.
Similar content being viewed by others
References
- Stanley, H. E. Rev. Mod. Phys. 71, S358–S366 (1999).
Article CAS Google Scholar - Andrews, T. Phil. Trans. R. Soc. Lond. 159, 575– 590 (1869).
Article ADS Google Scholar - Albrecht, O., Gruler, H. & Sackmann, E. J. Phys. (Paris) 39, 301– 313 (1978).
Article CAS Google Scholar - Knobler, C. M. & Schwartz, D. K. Curr. Opin. Colloid Interface Sci. 4, 46–51 ( 1999).
Article CAS Google Scholar - Nielsen, L. K., Bjørnholm, T. & Mouritsen, O. G. J. Phys. Cond. Mat. 12, 309–314 (2000).
Article ADS Google Scholar - Mouritsen, O. G. & Andersen, O. S. (eds) Biol. Skr. Kgl. Dan. Vid. Selsk. 49, 1–214 (1998).
Google Scholar - Hønger, T. et al. Biochemistry 35, 9003– 9006 (1996).
Article Google Scholar - Mustonen, P. et al. Biochemistry 26, 2991– 2997 (1987).
Article CAS Google Scholar - Mouritsen, O. G. & Jørgensen, K. Curr. Opin. Struct. Biol. 7, 518–527 (1997).
Article CAS Google Scholar - Edidin, M. Curr. Opin. Struct. Biol. 7, 528–532 (1997).
Article CAS Google Scholar - Korlach, J., Schwille, P., Webb, W. W. & Feigenson, G. W. Proc. Natl Acad. Sci. USA 96, 8461– 8466 (1999).
Article CAS ADS Google Scholar - Simons, K. & Ikonen, E. Nature 387, 569–572 (1997).
Article CAS ADS Google Scholar
Author information
Authors and Affiliations
- Department of Chemistry, Technical University of Denmark, Lyngby, DK-2800, Denmark
Lars K. Nielsen & Ole G. Mouritsen - Laboratory for Materials Science Department of Chemistry, CISMI, University of Copenhagen, Fruebjergvej 3, Copenhagen, DK-2100, Denmark
Thomas Bjørnholm
Authors
- Lars K. Nielsen
- Thomas Bjørnholm
- Ole G. Mouritsen
Corresponding author
Correspondence toOle G. Mouritsen.
Rights and permissions
About this article
Cite this article
Nielsen, L., Bjørnholm, T. & Mouritsen, O. Fluctuations caught in the act.Nature 404, 352 (2000). https://doi.org/10.1038/35006162
- Issue Date: 23 March 2000
- DOI: https://doi.org/10.1038/35006162