IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity (original) (raw)
- Letter
- Published: 26 April 2001
- Hiroaki Ikeda1,
- Allen T. Bruce1,
- J. Michael White1,
- Paul E. Swanson1,
- Lloyd J. Old2 &
- …
- Robert D. Schreiber1
Nature volume 410, pages 1107–1111 (2001)Cite this article
- 30k Accesses
- 2095 Citations
- 55 Altmetric
- Metrics details
Abstract
Lymphocytes were originally thought to form the basis of a ‘cancer immunosurveillance’ process that protects immunocompetent hosts against primary tumour development1,2, but this idea was largely abandoned when no differences in primary tumour development were found between athymic nude mice and syngeneic wild-type mice3,4,5. However, subsequent observations that nude mice do not completely lack functional T cells6,7 and that two components of the immune system—IFNγ8,9 and perforin10,11,12—help to prevent tumour formation in mice have led to renewed interest in a tumour-suppressor role for the immune response. Here we show that lymphocytes and IFNγ collaborate to protect against development of carcinogen-induced sarcomas and spontaneous epithelial carcinomas and also to select for tumour cells with reduced immunogenicity. The immune response thus functions as an effective extrinsic tumour-suppressor system. However, this process also leads to the immunoselection of tumour cells that are more capable of surviving in an immunocompetent host, which explains the apparent paradox of tumour formation in immunologically intact individuals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
Similar content being viewed by others
References
- Thomas, L. in Cellular and Humoral Aspects of Hypersensitivity States (ed. Lawrence, H. S.) 529–532 (Hoeber, New York, 1959).
Google Scholar - Burnet, F. M. The concept of immunologic surveillance. Prog. Exp. Tumor Res. 13, 1–27 (1970).
Article CAS Google Scholar - Stutman, O. Spontaneous tumors in nude mice: effect of the viable yellow gene. Exp. Cell. Biol. 47, 129–135 (1979).
CAS PubMed Google Scholar - Stutman, O. Tumor development after 3-methylcholanthrene in immunologically deficient athymic-nude mice. Science 183, 534–536 (1970).
Article ADS Google Scholar - Stutman, O. Chemical carcinogenesis in nude mice: comparison between nude mice from homozygous and heterozygous matings and effect of age and carcinogen dose. J. Natl Cancer Inst. 2, 353–358 (1979).
Google Scholar - Hunig, T. T-cell function and specificity in athymic mice. Immunol. Today 4, 84–87 (1983).
Article CAS Google Scholar - Maleckar, J. R. & Sherman, L. A. The composition of the T cell receptor repertoire in nude mice. J. Immunol. 138, 3873–3876 (1987).
CAS PubMed Google Scholar - Dighe, A. S., Richards, E., Old, L. J. & Schreiber, R. D. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFNγ receptors. Immunity 1, 447–456 (1994).
Article CAS Google Scholar - Kaplan, D. H. et al. Demonstration of an IFNγ dependent tumor surveillance system in immunocompetent mice. Proc. Natl Acad. Sci. USA 95, 7556–7561 (1998).
Article ADS CAS Google Scholar - van den Broek, M. F. et al. Decreased tumor surveillance in perforin-deficient mice. J. Exp. Med. 184, 1781–1790 (1996).
Article CAS Google Scholar - Smyth, M. J. et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 191, 661–668 (2000).
Article CAS Google Scholar - Smyth, M. J. et al. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 192, 755–760 (2000).
Article CAS Google Scholar - Shinkai, Y. et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68, 855–867 (1992).
Article CAS Google Scholar - Meraz, M. A. et al. Targeted disruption of the STAT1 gene in mice reveals unexpected physiologic specificity in the Jak-STAT signaling pathway. Cell 84, 431–442 (1996).
Article CAS Google Scholar - Durbin, J. E., Hackenmiller, R., Simon, M. C. & Levy, D. E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral infection. Cell 84, 443–450 (1996).
Article CAS Google Scholar - Old, L. J. & Stockert, E. Immunogenetics of cell surface antigens of mouse leukemia. Annu. Rev. Genet. 17, 127–160 (1977).
Article Google Scholar - Marincola, F. M., Jaffee, E. M., Hicklin, D. J. & Ferrone, S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv. Immunol. 74, 181–273 (2000).
Article CAS Google Scholar - Alimonti, J. et al. TAP expression provides a general method for improving the recognition of malignant cells in vivo. Nature Biotechnol. 18, 515–520 (2000).
Article CAS Google Scholar - Marusina, K., Iyer, M. & Monaco, J. J. Allelic variation in the mouse Tap-1 and Tap-2 transporter genes. J. Immunol. 158, 5251–5256 (1997).
CAS PubMed Google Scholar - Huang, S. et al. Immune response in mice that lack the interferon-γ receptor. Science 259, 1742–1745 (1993).
Article ADS CAS Google Scholar - Dighe, A. S. et al. Tissue specific targeting of cytokine unresponsiveness in transgenic mice. Immunity 3, 657–666 (1995).
Article CAS Google Scholar
Acknowledgements
We thank J. J. Monaco for providing a cDNA encoding TAP1 and T. H. Hansen for providing TAP1 antibodies and the cDNA encoding H-2Kb. We thank M. La Regina for performing the veterinary pathology and C. Hughley and C. Arthur for expert technical assistance. We also wish to thank P. M. Allen, K. M. Murphy, A. S. Shaw and E. R. Unanue for helpful suggestions during the preparation of this manuscript and J. F. Piccirillo for assistance with the statistical analysis of the data. This work was supported by grants from the NIH and the Cancer Research Institute. V.S. was supported and A.T.B. is supported by a pre-doctoral training grant from the Cancer Research Institute (CRI) and H.I. is supported by the John Hans and Edna Alice Old Post-Doctoral Fellowship from the CRI.
Author information
Authors and Affiliations
- Department of Pathology and Immunology, Center for Immunology, Washington University School of Medicine, 660 South Euclid Avenue, St Louis, 63110, Missouri, USA
Vijay Shankaran, Hiroaki Ikeda, Allen T. Bruce, J. Michael White, Paul E. Swanson & Robert D. Schreiber - Ludwig Institute for Cancer Research, New York Branch at Memorial Sloan-Kettering Cancer Center, New York, 10021, New York, USA
Lloyd J. Old
Authors
- Vijay Shankaran
You can also search for this author inPubMed Google Scholar - Hiroaki Ikeda
You can also search for this author inPubMed Google Scholar - Allen T. Bruce
You can also search for this author inPubMed Google Scholar - J. Michael White
You can also search for this author inPubMed Google Scholar - Paul E. Swanson
You can also search for this author inPubMed Google Scholar - Lloyd J. Old
You can also search for this author inPubMed Google Scholar - Robert D. Schreiber
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toRobert D. Schreiber.
Supplementary information
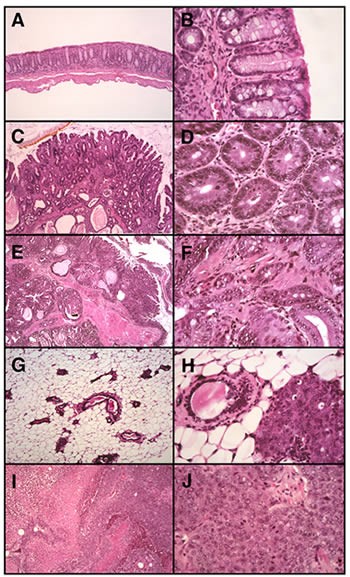
Figure 1.
Histology of spontaneous neoplasia in immunodeficient mice. A. Normal colon mucosa (100x) of a RAG2-/- mouse is a flat profile mucosa composed of straight tubular crypts in a variously cellular lamina propria underlain by a delicate muscularis mucosae. B. At higher magnification (400x) the columnar cells of the mucosal crypts are uniform in appearance, with small ovoid basal nuclei and abundant eosinophilic cytoplasm or mucin droplets (goblet cells). C. In intestinal adenoma (50x), the RAG2-/- mucosa is irregularly thickened, predominantly populated in this example by tubular crypts seen variously in longitudinal- or cross-section; small villi occupy a small portion of this "tubulovillous adenoma". D. At higher magnification (400x), the constituent glands are closely spaced in a stroma similar to that of normal mucosa, but increased in number relative to normal mucosa and containing cells less obviously columnar with mitotically active nuclei and decreased numbers of goblet cells. E. Intestinal adenocarcinoma (50x) in RAG2-/- mice is a more complex lesion at low magnification, with expansion of the lamina propria and evidence of invasion into submucosa or deeper structures. F. At higher magnification (400x), the glands in adenocarcinoma are more irregular in shape, have greater degrees of nuclear pleomorphism, and occupy a stroma that is more fibrous than that seen in benign proliferations. G. In normal breast tissue of RkSk mice (20x), ducts and small acini rest in an adipose tissue rich stroma. H. Early malignant change is manifest as in-situ ductal carcinoma (20x), a process that arises in pre-existing ducts, partially replacing the normal architecture by a space-filling proliferation of larger, pleomorphic polygonal or cuboidal cells. These cells, when compared with the normal ductal elements in this image, have vesicular nuclei and prominent nucleoli, as well as foci of increased mitotic activity and apoptosis. I. In a typical example of mammary carcinoma in RkSk mice (20x), invasive sheets of cells (often with conspicuous necrosis) form a solid mass. J. Higher magnification (400x) reveals cytoplasmic and nuclear features similar to those of in-situ disease. The lack of overt glandular or ductal differentiation in most fields of these breast tumors defines these as high grade (poorly differentiated) carcinomas. Mitotic activity is conspicuous. (JPG 73.3 KB)
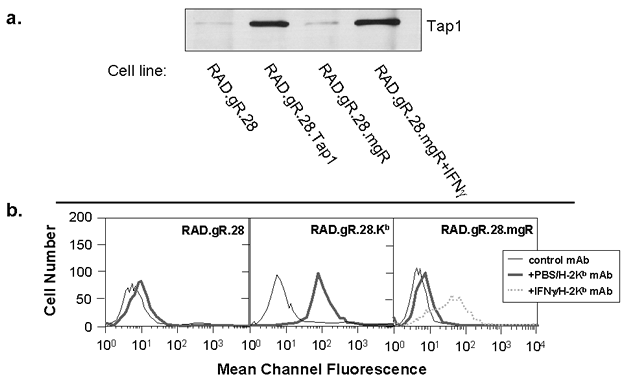
Figure 2.
Demonstration of enforced expression of Tap1 and H-2Kb in RAD.gR28. A. Tap1 expression in tumor cells was analyzed by immunoprecipitation and western blotting utilizing rabbit antisera specific for Tap1 (a gift from Dr. Ted Hansen, Washington University School of Medicine). Each lane represents precipitates formed using 5 x 106 cells. B. MHC class I expression on tumor cell surfaces was determined by flow cytometry. Thin lines represent staining with an irrelevant anti-mouse CD2 mAb, thick lines represent constitutive H-2Kb expression on unstimulated cells (anti-H-2Kb purchased from Pharmingen) and dotted lines represent H-2Kb expression on cells stimulated with IFNg. (GIF 28.7 KB)
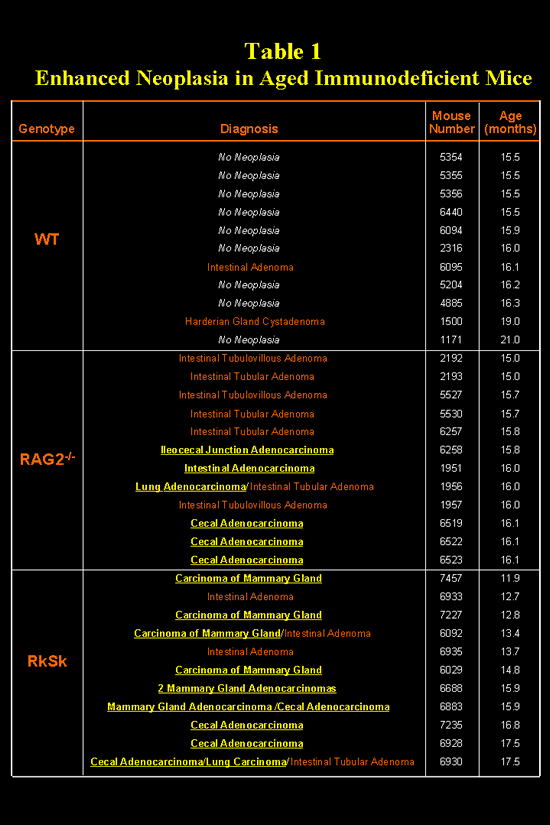
Table 1.
Enhanced Neoplasia in Aged Immunodeficient Mice (GIF 53.5 KB)
Rights and permissions
About this article
Cite this article
Shankaran, V., Ikeda, H., Bruce, A. et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity.Nature 410, 1107–1111 (2001). https://doi.org/10.1038/35074122
- Received: 12 January 2001
- Accepted: 28 February 2001
- Issue Date: 26 April 2001
- DOI: https://doi.org/10.1038/35074122