Scalable architecture in mammalian brains (original) (raw)
- Letter
- Published: 10 May 2001
Nature volume 411, pages 189–193 (2001)Cite this article
- 2946 Accesses
- 258 Citations
- 9 Altmetric
- Metrics details
Abstract
Comparison of mammalian brain parts has often focused on differences in absolute size1,2,3, revealing only a general tendency for all parts to grow together2. Attempts to find size-independent effects using body weight as a reference variable1 obscure size relationships owing to independent variation of body size4 and give phylogenies of questionable significance5. Here we use the brain itself as a size reference to define the cerebrotype, a species-by-species measure of brain composition. With this measure, across many mammalian taxa the cerebellum occupies a constant fraction of the total brain volume (0.13 ± 0.02), arguing against the hypothesis that the cerebellum acts as a computational engine principally serving the neocortex3. Mammalian taxa can be well separated by cerebrotype, thus allowing the use of quantitative neuroanatomical data to test evolutionary relationships. Primate cerebrotypes have progressively shifted and neocortical volume fractions have become successively larger in lemurs and lorises, New World monkeys, Old World monkeys, and hominoids, lending support to the idea that primate brain architecture has been driven by directed selection pressure4. At the same time, absolute brain size can vary over 100-fold within a taxon, while maintaining a relatively uniform cerebrotype. Brains therefore constitute a scalable architecture.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
Similar content being viewed by others
References
- Jerison, H. J. Brain Size and the Evolution of Mind (American Museum of Natural History, New York, 1991).
Google Scholar - Finlay, B. L. & Darlington, R. B. Linked regularities in the development and evolution of mammalian brains. Science 268, 1578–1584 (1995).
Article ADS CAS Google Scholar - Barton, R. A. & Harvey, P. H. Mosaic evolution of brain structure in mammals. Nature 405, 1055–1058 (2000).
Article ADS CAS Google Scholar - Barton, R. A. & Dunbar, R. I. M. in Machiavellian Intelligence II (eds Whiten, A. W. & Byrne, R. W.) 240–263 (Cambridge Univ. Press, New York, 1992).
Google Scholar - Douglas, R. J. & Marcellus, D. The ascent of man: deductions based on a multivariate analysis of the brain. Brain Behav. Evol. 11, 179–213 (1975).
Article CAS Google Scholar - Stephan, H., Frahm, H. & Baron, G. New and revised data on volumes of brain structures in insectivores and primates. Folia Primatol. 35, 1–29 (1981).
Article CAS Google Scholar - Aiello, L. C. & Wheeler, P. The expensive-tissue hypothesis: the brain and digestive system in primate evolution. Curr. Anthropol. 36, 199–221 (1995).
Article Google Scholar - Airapet’yants, E. S. & Konstantinov, A. I. Echolocation in Animals (Keter, Jerusalem, 1973).
Google Scholar - Jansen, J. On the whale brain with special reference to the weight of the fin whale (Balaenoptera physalus). Norsk Hvalfangst-tidende 9, 480–486 (1952).
Google Scholar - Pilleri, G. Morphologie des gehirnes des “Southern Right Whale”. Acta Zool. 46, 245–272 (1964).
Article Google Scholar - Jansen, J. & Jansen, J. K. S. in The Biology of Marine Mammals (ed. Andersen, H. T.) 175–252 (Academic, New York, 1969).
Google Scholar - Ridgway, S. H. in The Bottlenosed Dolphin, Tursiops spp. (eds Leatherwood, J. S. & Reeves, R.) 69–97 (Academic, San Diego, 1989).
Google Scholar - Baron, G., Stephan, H. & Frahm, H. D. Comparative Neurobiology in Chiroptera: Macromorphology, Brain Structures, Tables, and Atlases (Birkhaeuser, Basel, 1996).
Google Scholar - Paulin, M. G. The role of the cerebellum in motor control and perception. Brain Behav. Evol. 41, 39–50 (1993).
Article CAS Google Scholar - Mouchaty, S. K., Gullberg, A., Janke, A. & Arnason, U. The phylogenetic position of the Talpidae within Eutheria based on analysis of complete mitochondrial sequences. Mol. Biol. Evol. 17, 60–67 (2000).
Article CAS Google Scholar - Purvis, A. A composite estimate of primate phylogeny. Phil. Trans. R. Soc. Lond. B 348, 405–421 (1995).
Article ADS CAS Google Scholar - Kinzey, W. G. New World Primates: Ecology, Evolution, and Behavior (Aldine de Gruyter, New York, 1997).
Google Scholar - Harvey, P. H. & Pagel, M. The Comparative Method in Evolutionary Biology (Oxford Univ. Press, Oxford, 1991).
Google Scholar - Gura, T. Bones, molecules...or both? Nature 406, 230–233 (2000).
Article ADS CAS Google Scholar - Xuan, S. et al. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron 14, 1141–1152 (1995).
Article CAS Google Scholar - Kornack, D. R. & Rakic, P. Changes in cell-cycle kinetics during the development and evolution of primate neocortex. Proc. Natl Acad. Sci. USA 95, 1242–1246 (1998).
Article ADS CAS Google Scholar - Braitenberg, V. & Schüz, A. Cortex: Statistics and Geometry of Neuronal Connectivity (Springer, Berlin, 1998).
Book Google Scholar - Kruska, D. & Rohrs, M. Comparative-quantitative investigations on brains of feral pigs from the Galapagos Islands and of European domestic pigs. Z. Anat. Entwicklungsgesch 144, 61–73 (1974).
Article CAS Google Scholar - Meyer, J. A quantitative comparison of the parts of the brains of two Australian marsupials and some eutherian mammals. Brain Behav. Evol. 18, 60–71 (1981).
Article CAS Google Scholar - Pirlot, P. & Kamiya, T. Quantitative brain organization in anteaters (Edentata-Tubilidentata). J. Hirnforsch. 24, 677–689 (1983).
CAS PubMed Google Scholar - Fox, J. H. & Wilczynski, W. Allometry of major CNS divisions: towards a reevaluation of somatic brain–body scaling. Brain Behav. Evol. 28, 157–169 (1986).
Article CAS Google Scholar - Frahm, H. D., Rehkämper, G. & Nevo, E. Brain structure volumes in the mole rat, Spalax ehrenbergi (Spalacidae, Rodentia) in comparison to the rat and subterrestrial insectivores. J. Brain Res. 38, 209–222 (1997).
CAS Google Scholar - Reep, R. L. & O'Shea, T. J. Regional brain morphometry and lissencephaly in the Sirenia. Brain Behav. Evol. 35, 185–194 (1990).
Article CAS Google Scholar - Pirlot, P. & Kamiya, T. Qualitative and quantitative brain morphology in the Sirenian Dugong dugong Erxl. Z. Zool. Syst. Evol.-forsch. 23, 147–155 (1985).
Article Google Scholar - Zilles, K. & Rehkämper, G. in Orang-utan Biology (ed. Schwartz, J. H.) 157–176 (Oxford Univ. Press, New York, 1988).
Google Scholar
Acknowledgements
We thank J. M. Allman and M. J. Berry for discussions, R. Kasthuri for research assistance, and T. A. Barney for secretarial assistance. D.A.C. is in the Princeton University Program in Biophysics. S.S.-H.W. is supported by the Alfred P. Sloan Foundation.
Author information
Authors and Affiliations
- Department of Molecular Biology, Princeton University, Princeton, 08544, New Jersey, USA
Damon A. Clark & Samuel S.-H. Wang - Department of Physics, Princeton University, Princeton, 08544, New Jersey, USA
Damon A. Clark - Bell Laboratories, Lucent Technologies, 600 Mountain Avenue, Murray Hill, 07974, New Jersey, USA
Partha P. Mitra
Authors
- Damon A. Clark
- Partha P. Mitra
- Samuel S.-H. Wang
Corresponding author
Correspondence toSamuel S.-H. Wang.
Supplementary information
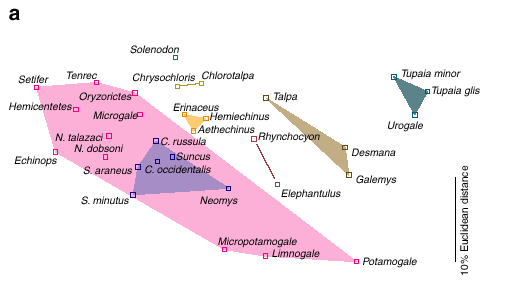
Figure a (GIF 10.6 KB)

Figure b (GIF 7.85 KB)

Figure c (GIF 8.35 KB)
Primate key
- sp32 Cheirogaleus major
- sp33 Cheirogaleus medius
- sp34 Microcebus murinus
- sp35 Lepilemur ruficaudatus
- sp36 Lemur fulvus
- sp37 Lemur variegatus
- sp38 Avahi l. laniger
- sp39 Avahi l. occidentalis
- sp40 Propithecus verreauxi
- sp41 Indri indri
- sp42 Daubentonia madagasc.
- sp43 Loris tardigradus
- sp44 Nycticebus coucang
- sp45 Perodicticus potto
- sp46 Galago crassicaudatus
- sp47 Galago demidoff
- sp48 Galago senegalensis
- sp49 Tarsius sp.
- sp50 Callithrix jacchus
- sp51 Cebuella pygmaea
- sp52 Saguinus oedipus
- sp53 Saguinus tamarin
- sp54 Callimico goeldii
- sp55 Aotus trivirgatus
- sp56 Pithecia monachus
- sp57 Alouatta sp.
- sp58 Ateles geoffroyi
- sp59 Lagothrix lagotricha
- sp60 Cebus sp.
- sp61 Saimiri sciureus
- sp62 Macaca mulatta
- sp63 Cercocebus albigena
- sp64 Papio anubis
- sp65 Cercopithecus mitis
- sp66 Cercopithecus ascan.
- sp67 Cercopithecus talap.
- sp68 Erythrocebus patas
- sp69 Pygathrix nemaeus
- sp70 Nasalis larvatus
- sp71 Colobus badius
- sp72 Hylobates lar
- sp73 Pan troglodytes
- sp74 Gorilla gorilla
- sp75 Homo sapiens sapiens
- sp76 Pongo pygmaeus
Clark_data (TXT 22.4 KB)
What's what in 'Clark_data.txt': The file is tab-delimited. Raw volume data from Stephan et al. (1981). All volumes given are in mm^3 unless otherwise noted.
- Rows indicate regions as follows:
- 1 body weight in grams
- 2 brain weight in mg
- 3 ventricles
- 4 meninges, hypophysis, nerves, etc.
- 5 total brain
- 6 medulla oblongata
- 7 cerebellum
- 8 mesencephalon
- 9 diencephalon
- 10 telencephalon
- 11 bulbus olfactorius
- 12 bulbus olfactorius accessorius
- 13 lobus piriformis
- 14 septum
- 15 striatum
- 16 schizocortex
- 17 hippocampus
- 18 neocortex
- Columns are species as follows (The ordering is the same as in Stephan et al. Callicebus moloch is excluded because of a checksum error.):
- The ordering is the same as in Stephan et al.
- Callicebus moloch is excluded because of a checksum error.
- 1 Solenodon paradoxus
- 2 Tenrec ecaudatus
- 3 Setifer setosus
- 4 Hemicentetes semispin.
- 5 Echinops telfairi
- 6 Oryzorictes talpoides
- 7 Microgale cowani
- 8 Limnogale mergulus
- 9 Nesogale dobsoni
- 10 Nesogale talazaci
- 11 Micropotamogale lamottei
- 12 Potamogale velox
- 13 Chlorotalpa stuhlmanni
- 14 Chrysochloris asiatica
- 15 Aethechinus algirus
- 16 Erinaceus europaeus
- 17 Hemiechinus auritus
- 18 Elephantulus fuscipes
- 19 Rhynchocyon stuhlmanni
- 20 Sorex minutus
- 21 Sorex araneus
- 22 Neomys fodiens
- 23 Crocidura occidentalis
- 24 Crocidura russula
- 25 Suncus murinus
- 26 Talpa europaea
- 27 Desmana moschata
- 28 Galemys pyrenaicus
- 29 Tupaia glis
- 30 Tupaia minor
- 31 Urogale everetti
- 32 Cheirogaleus major
- 33 Cheirogaleus medius
- 34 Microcebus murinus
- 35 Lepilemur ruficaudatus
- 36 Lemur fulvus
- 37 Lemur variegatus
- 38 Avahi l. laniger
- 39 Avahi l. occidentalis
- 40 Propithecus verreauxi
- 41 Indri indri
- 42 Daubentonia madagasc.
- 43 Loris tardigradus
- 44 Nycticebus coucang
- 45 Perodicticus potto
- 46 Galago crassicaudatus
- 47 Galago demidoff
- 48 Galago senegalensis
- 49 Tarsius sp.
- 50 Callithrix jacchus
- 51 Cebuella pygmaea
- 52 Saguinus oedipus
- 53 Saguinus tamarin
- 54 Callimico goeldii
- 55 Aotus trivirgatus
- 56 Pithecia monachus
- 57 Alouatta sp.
- 58 Ateles geoffroyi
- 59 Lagothrix lagotricha
- 60 Cebus sp.
- 61 Saimiri sciureus
- 62 Macaca mulatta
- 63 Cercocebus albigena
- 64 Papio anubis
- 65 Cercopithecus mitis
- 66 Cercopithecus ascan.
- 67 Cercopithecus talap.
- 68 Erythrocebus patas
- 69 Pygathrix nemaeus
- 70 Nasalis larvatus
- 71 Colobus badius
- 72 Hylobates lar
- 73 Pan troglodytes
- 74 Gorilla gorilla
- 75 Homo sapiens sapiens
Clark_tables (XLS 25.0 KB)
Clark_data (PDF 551 kb)
Rights and permissions
About this article
Cite this article
Clark, D., Mitra, P. & Wang, SH. Scalable architecture in mammalian brains.Nature 411, 189–193 (2001). https://doi.org/10.1038/35075564
- Received: 28 November 2000
- Accepted: 12 March 2001
- Issue Date: 10 May 2001
- DOI: https://doi.org/10.1038/35075564