De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii (original) (raw)
- Letter
- Published: 21 February 2002
Nature volume 415, pages 926–929 (2002)Cite this article
- 4862 Accesses
- 195 Citations
- 4 Altmetric
- Metrics details
Abstract
Toxoplasma gondii is a ubiquitous protozoan parasite that is responsible for severe congenital birth defects and fatal toxoplasmic encephalitis in immunocompromized people1. Fundamental aspects of obligate intracellular replication and pathogenesis are only now beginning to emerge for protozoan parasites. T. gondii has a fragmented pathway for salvaging pyrimidine nucleobases derived from the parasite or host cell, and this limited pyrimidine salvage capacity is funnelled exclusively through uracil phosphoribosyltransferase2,3. Disrupting the function of this enzyme does not affect the growth of T. gondii tachyzoites4, which suggests that the de novo pyrimidine biosynthesis pathway may be necessary for growth. We have examined the virulence of T. gondii mutants that lack carbamoyl phosphate synthetase II (uracil auxotrophs) to determine whether de novo pyrimidine biosynthesis is required in vivo. Here we show that T. gondii uracil auxotrophs are completely avirulent not only in immune-competent BALB/c mice but also in mice that lack interferon-γ. A single injection of the uracil auxotroph into BALB/c mice induces long-term protective immunity to toxoplasmosis. Our findings indicate the significance of the de novo pyrimidine biosynthesis pathway for the virulence of parasitic protozoa, and suggest routes for developing vaccines and chemotherapy.
Similar content being viewed by others
Main
Because of its genetic accessibility5,6,7 and natural virulence in mice, T. gondii is an excellent model for the discovery and evaluation of auxotrophic mutants that capitalize on differences in metabolism between protozoan parasites and their hosts. Like its host, T. gondii has an intact pathway for de novo pyrimidine biosynthesis, but differs in that it has only a limited pyrimidine salvage pathway (Fig. 1). In T. gondii, as in other parasites of the phylum Apicomplexa, the key regulatory enzyme of de novo pyrimidine biosynthesis is carbamoyl phosphate synthetase II (CPSII), a parasite enzyme that has distinctive properties compared with the CPSII activity of the mammalian host cell8. In addition, apicomplexan parasites have a monofunctional CPSII domain fused onto the same polypeptide as the glutamine amidotransferase domain, which produces an enzyme architecture that is not observed in bacteria, fungi or mammals9,10. These unique features of T. gondii CPSII make this activity an attractive target to disrupt to obtain null mutants.
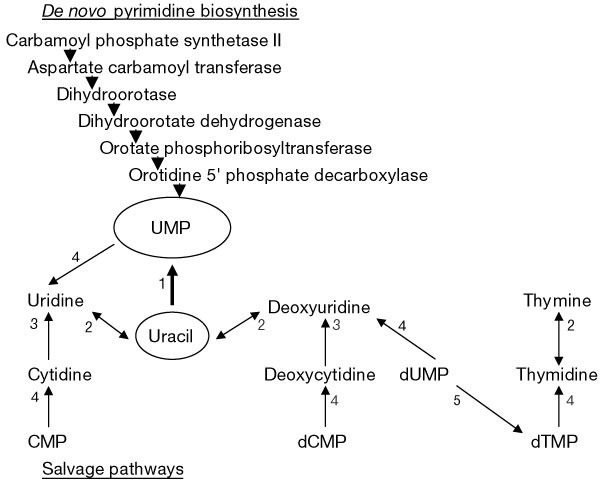
Figure 1: Pyrimidine salvage and de novo pyrimidine biosynthesis pathways in T. gondii.
Pathways that produce UMP, the precursor of all pyrimidines used by T. gondii, are shown. The six steps of the de novo pyrimidine biosynthesis pathway and their corresponding enzymes are shown. Salvage pathway2,3 steps that have been detected in T. gondii are shown in solid lines. Double arrowheads indicate that the activity is capable of both conversions. Enzyme activities: uracil phosphoribosyltransferase (1); nucleoside phosphorylase (2); nucleoside deaminase (3); nucleoside 5′-monophosphate phosphohydrolase (4); and thymidylate synthase (5; part of the bifunctional DHFR-TS6,19). Thymidylate synthase is not considered a salvage enzyme; it is a key enzyme in the interconversion of pyrimidine nucleotides. Many potential salvage enzyme activities have not been detected in _T. gondii_3.
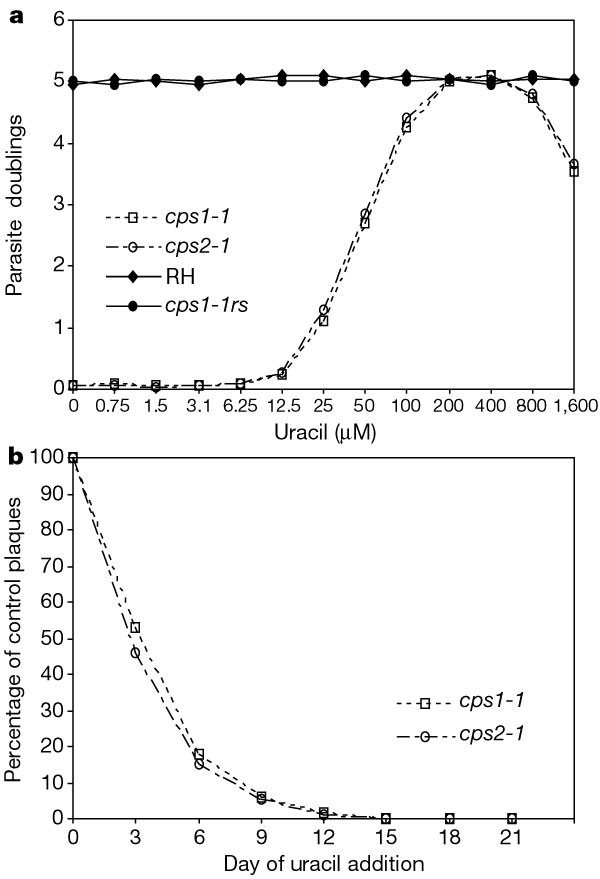
We cloned a 6.6-kilobase (kb) _Hin_dIII CPSII genomic DNA fragment from strain RH that contains exons with significant similarity to CPSII from fungi, plants and mammals (Supplementary Information). The single chromosomal copy of this 6.6-kb _Hin_dIII fragment was disrupted to obtain uracil auxotroph strains cps1-1 and cps2-1. Neither strain had detectable CPSII activity compared with the activity measured in the wild-type RH strain (57 nmol h-1 mg-1). Without uracil supplementation the parasite invaded normally, but it failed to replicate once intracellular. Essentially, no growth was observed at uracil concentrations lower than 20 µM (Fig. 2a). Normal parasite growth was seen between about 0.2 and 0.6 mM uracil; however, growth was suppressed in uracil concentrations higher than 0.8 mM, which indicates that normal regulation of pyrimidine pools or salvage mechanisms may be disrupted in the auxotrophic mutants. The ability of added uracil to rescue this nonreplicating uracil auxotroph declined with time (Fig. 2b).
Figure 2: Growth and viability of uracil auxotrophs of T. gondii.
a, Growth rate of uracil auxotroph mutants was measured as a function of the uracil concentration of the infection medium. Parasites were allowed to invade HFFs for 3 h, the cells were then washed and incubated with infection medium containing various concentrations of uracil. Host cells were microscopically inspected 36 h later to identify individual vacuoles containing parasites. The average number of tachyzoites per vacuole was determined by scoring 50 independent vacuoles for each data point. The average number of parasites per vacuole was converted into ‘parasite doublings’ (for example, 1 parasite doubling = 2 parasites per vacuole, and 5 parasite doublings = 32 parasites per vacuole). Growth responses for auxotrophic mutants cps1-1 and cps2-1 are compared with that of the RH parent and a representative clone (cps1-1rs) of a rescued parasite obtained after transfection of cps1-1 with the wild-type 6.6-kb CPSII _Hin_dIII fragment (see Table 1). b, Ability of added uracil to rescue the nonreplicating intracellular parasites was determined by a plaque assay (Methods). For uracil addition on day 15, 18 and 21, plaques from the auxotrophic mutants (cps1-1 and cps2-1) were reduced to about 0.2%, 0.02% and 0.003%, respectively, of the control p.f.u.
We verified that disruption of the CPSII gene was the genetic lesion responsible for the uracil auxotrophy of cps1-1 and cps2-1. Transfection of cps1-1 and cps2-1 with a linearized 6.6-kb CPSII _Hin_dIII fragment from the wild-type strain RH rescued significant numbers of parasites (scored as plaques), which then grew normally in the absence of uracil (Table 1). Rescued isolates (cps1-1rs) all had the wild-type RH CPSII genotype and phenotype on the basis of Southern blot (data not shown), CPSII activity (61 nmol h-1 mg-1 protein), and growth responses in uracil (Fig. 2a). In contrast, transfection of cps1-1 or cps2-1 with only plasmid DNA, or with the Δ6.6-kb CPSII _Hin_dIII plasmid containing an internal 1.1-kb _Bam_HI-generated deletion failed to rescue any additional plaques in the absence of uracil (Table 1).
Table 1 Frequency of rescue of uracil auxotroph mutants after transfection of plasmid DNA
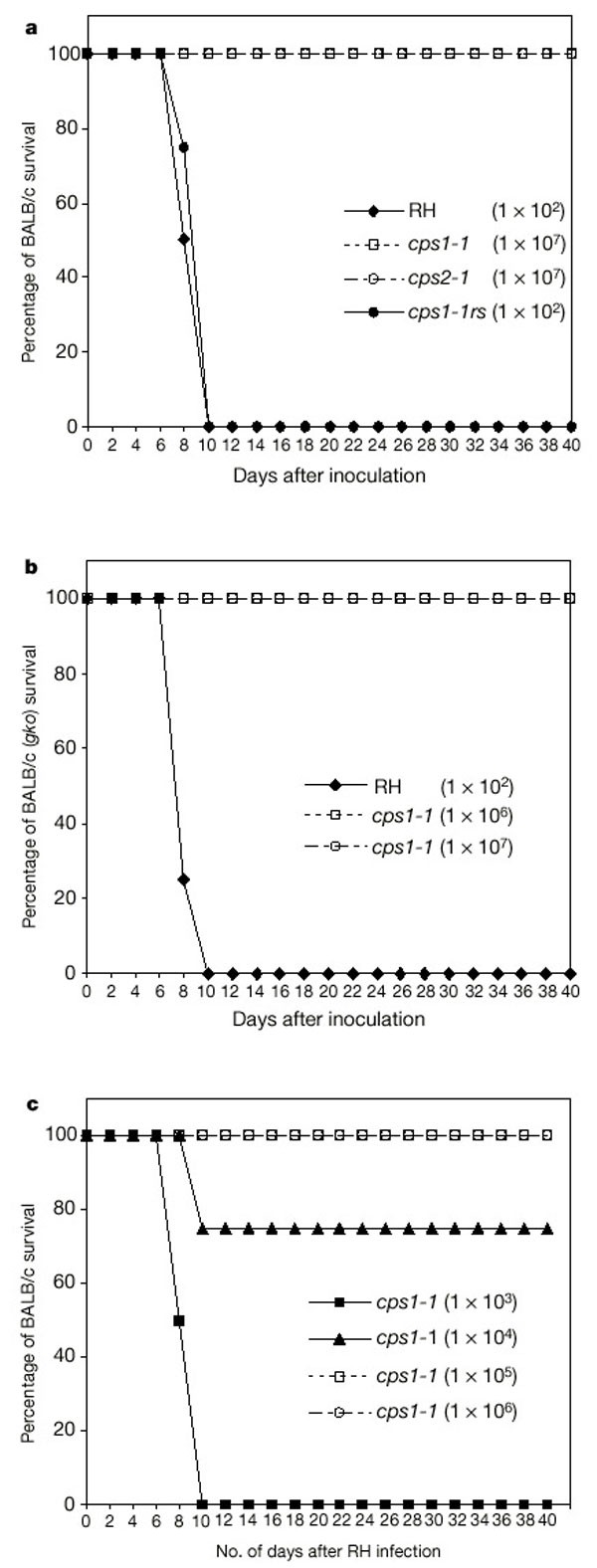
We tested the virulence of the T. gondii CPSII gene knockout mutants in a BALB/c mouse model of lethal toxoplasmosis. The parental RH strain is hypervirulent in all mice strains, with an estimated 100% lethal dose of fewer than 10 parasites11. Mice injected intraperitoneally with only a low dose of the parental wild-type RH strain succumb rapidly to the infection (median survival of 9 d). In contrast, mice injected with the cps1-1 or cps2-1 mutant survived the infection at inoculating doses of 103, 104, 105, 106 (data not shown) and 107 tachyzoites (Fig. 3a). Parasites could not be recovered from the intraperitoneal cavity of mice at 3 weeks after inoculation. Mice inoculated with the uracil auxotroph mutants survived longer than 12 months with no evidence of any phenotype of parasite persistence (tachyzoites or brain cysts). In contrast, parasites such as cps1-1rs, which were rescued by transfection of the uracil auxotroph mutants with the wild-type 6.6-kb CPSII _Hin_dIII fragment (Table 1), were highly virulent in BALB/c mice (Fig. 3a).
Figure 3: Uracil auxotroph mutants are avirulent in mice and confer long-term protective immunity.
a, Tachyzoites of the indicated strains were injected i.p. into BALB/c mice (n = 4) and monitored for more than 40 d (Methods). The inoculation doses are shown. b, Tachyzoites of the indicated strains were injected i.p. into homozygous IFN-γ knockout (BALB/c background) mice (n = 4) and monitored for more than 40 d. The inoculation doses are shown. c, Various doses of tachyzoites of cps1-1 were injected i.p. into BALB/c mice (n = 4). Mice were challenged 40 days later with 200 p.f.u. of wild-type strain RH tachyzoites administered by the i.p. route.
We examined the virulence of cps1-1 in homozygous interferon-γ (IFN-γ) knockout (gko) mice on a BALB/c background. Because the cytokine IFN-γ is necessary for host control of T. gondii infection, IFN-γ knockout mice rapidly succumb to toxoplasmosis even after infection with normally avirulent strains12. As expected, gko mice rapidly succumbed to the RH strain (median survival of 9 d; Fig. 3b). Unexpectedly, gko mice injected with the cps1-1 mutant survived the infection at inoculating doses of 103, 104, 105 (data not shown), 106 and 107 tachyzoites (Fig. 3b). The _cps1-1_-inoculated gko mice showed long-term survival (> 6 months), with no apparent phenotype of parasite persistence.
We examined whether the avirulent uracil auxotrophic mutants could protect mice from a lethal toxoplasma challenge infection. Groups of BALB/c mice were injected once with various doses of cps1-1 tachyzoites. These mice were injected 40 d later with a lethal challenge dose of strain RH. Parasite doses greater than 104 cps1-1 tachyzoites were highly effective in inducing a long-term protective immunity in BALB/c mice (Fig. 3c).
Our data indicate that the obligate intracellular T. gondii parasite may have evolved to have a strict reliance on its own de novo pyrimidine biosynthesis pathway in vivo. The parasite's pyrimidine salvage pathway cannot apparently salvage significant amounts of pyrimidines from its host cell. This adaptation of the protozoan parasite may have arisen as a consequence of the apparent low availability of pyrimidines in animal tissues13,14. Notably, the pyrimidine starvation phenotype of the cps1-1 and cps2-1 mutants results in a complete block of parasite replication in vitro (Fig. 2) and in vivo (Fig. 3). Identifying compounds that limit the de novo biosynthesis of pyrimidines in T. gondii might be a route for antiparasite drug design. The auxotrophic mutants described here are remarkably avirulent and can also induce long-term protective immunity to toxoplasmosis. To our knowledge, these are the first reported mutants of T. gondii that do not kill gko mice15. T. gondii elicits a strong cell-mediated immune response that controls its own growth and that can stimulate nonspecific resistance to unrelated pathogens and tumours16,17. Consequently, these auxotrophic mutants may offer even greater promise as a strategy for vaccine development. Developing pyrimidine auxotrophs in other protozoan parasites might verify drug targets in de novo pyrimidine biosynthesis and might also provide a broad-ranging approach to obtaining protozoan parasite mutants that are severely attenuated in their virulence.
Methods
Parasite culture and phenotypic analysis
Human foreskin fibroblasts (HFFs) were cultured in EMEM medium in 10% fetal bovine serum (FBS) at 37 °C in 95% air/5% CO2. We cultured parasites in EMEM in 1% FBS at 37 °C in 95% air/5% CO2. Plaques were visualized by fixing HFF monolayers in 50% (v/v) methanol and 7% (v/v) glacial acetic acid, and staining with saturated Coomassie blue dissolved in fixative. The CPSII enzyme assays were done as described8. We isolated parasite DNA and carried out Southern analysis as described18.
To determine whether added uracil could rescue intracellular mutants, replicate sets of flasks of confluent HFF cells were inoculated with 2 × 105, 2 × 104, 2 × 103 or 2 × 102 plaque forming units (p.f.u.) of uracil auxotroph tachyzoites. After a 3-h incubation at 37 °C in medium lacking uracil for attachment and invasion, the remaining extracellular parasites were removed (t = 0) by washing the monolayer with PBS, and cultures were incubated in medium lacking uracil. At various times, medium was removed from duplicate sets of the inoculated flasks and was replaced with medium containing 0.2 mM uracil. We stained monolayers and scored plaques by visual inspection 8 d after uracil addition.
Mutant construction and analysis
A genomic DNA fragment of the T. gondii CPSII gene (strain RH) was cloned using a polymerase chain reaction (PCR) signature homology strategy. A CPSII PCR DNA fragment of the expected size of 450 base pairs (bp) was isolated and subcloned into pBluescript. We examined 30 individual clones, each of which had an identical sequence with a high amino-acid similarity to other CPSII species. We used the 450-bp CPSII fragment to identify a single-copy 6.6-kb _Hin_dIII fragment of genomic DNA by Southern blot analysis and to screen a T. gondii _Hin_dIII plasmid library constructed in pBluescript to obtain the 6.6-kb CPSII _Hin_dIII clone. The Δ6.6-kb CPSII _Hin_dIII plasmid was constructed by creating a 1.1-kb _Bam_HI deletion (deleting bp 2,261 to 3,347) in the central part of the 6.6-kb CPSII _Hin_dIII clone.
We made the targeting plasmid by inserting a dihydrofolate reductase/herpes simplex virus thymidine kinase/thymidylate synthase positive/negative selection marker18 adjacent to the 3′ CPSII sequences in the Δ6.6-kb CPSII _Hin_dIII plasmid. This plasmid was integrated into the CPSII locus by transfection18 of strain RH and selection in pyrimethamine with uracil supplementation. We screened clones derived from this selection for ones that grew normally in medium with 0.2 mM uracil, but had disrupted CPSII activity and could not plaque in medium lacking uracil. These cps1 and cps2 mutants were then subjected to negative selection in ganciclovir in the presence of uracil. This strategy selected parasites that lost expression of the integrated thymidine kinase marker18. We subcloned ganciclovir-resistant and pyrimethamine-sensitive parasites from this selection to produce T. gondii strains, cps1-1 and cps2-1. Uracil auxotrophs cps1-1 and cps2-1 have two tandem copies of the targeting plasmid integrated into the CPSII locus. Only the CPSII locus was disrupted, and integration was achieved by homologous recombination in CPSII sequences on the 5′ side of the _Bam_HI sites of the 6.6-kb CPSII _Hin_dIII fragment (data not shown). We maintained the uracil auxotrophs in culture in medium supplemented with 0.2 mM uracil.
Murine virulence assay
We obtained tachyzoites by allowing infected HFF monolayers to lyse completely. Tachyzoites were purified by filtration through sterile 3-µm nuclepore membranes, washed in PBS and collected by centrifugation. We resuspended tachyzoites pellets in PBS and counted them under the microscope. Tachyzoites were injected intraperitoneally (i.p.) in 0.2 ml into mice aged 6–8 weeks. The actual p.f.u. in the inoculum was determined by plaque assay. In all of the mouse injection experiments and for all of the parasite strains tested, the p.f.u. to parasite ratio was between 0.4 and 0.6. We used four mice per parasite dose with each strain. Experiments with groups of mice were repeated twice. Mice were monitored for 40 d, and in some experiments for more than 1 year. We cared for mice according to NIH guidelines.
References
- Luft, B. J. & Remington, J. S. Toxoplasmic encephalitis in AIDS. Clin. Inf. Dis. 15, 211–222 (1992).
Article CAS Google Scholar - Pfefferkorn, E. R. Toxoplasma gondii: the enzymic defect of a mutant resistant to 5-fluorodeoxyuridine. Exp. Parasitol. 44, 26–35 (1978).
Article CAS Google Scholar - Iltzsch, M. H. Pyrimidine salvage pathways in Toxoplasma gondii. J. Euk. Micro. 40, 24–28 (1993).
Article CAS Google Scholar - Donald, R. G. & Roos, D. S. Insertional mutagenesis and marker rescue in a protozoan parasite: cloning of the uracil phosphoribosyltransferase locus from Toxoplasma gondii. Proc. Natl Acad. Sci. USA 92, 5749–5753 (1995).
Article ADS CAS Google Scholar - Soldati, D. & Boothroyd, J. C. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science 260, 349–352 (1993).
Article ADS CAS Google Scholar - Donald, R. G. & Roos, D. S. Stable molecular transformation of Toxoplasma gondii: a selectable dihydrofolate reductase-thymidylate synthase marker based on drug-resistance mutations in malaria. Proc. Natl Acad. Sci. USA 90, 11703–11707 (1993).
Article ADS CAS Google Scholar - Sibley, L. D., Messina, M. & Niesman, I. R. Stable DNA transformation in the obligate intracellular parasite Toxoplasma gondii by complementation of tryptophan auxotrophy. Proc. Natl Acad. Sci. USA 91, 5508–5512 (1994).
Article ADS CAS Google Scholar - Asai, T. et al. Enzymes of the de novo pyrimidine biosynthetic pathway in Toxoplasma gondii. Mol. Biochem. Parasitol. 7, 89–100 (1983).
Article MathSciNet CAS Google Scholar - Flores, M. V., O'Sullivan, W. J. & Stewart, T. S. Characterisation of the carbamoyl phosphate synthetase gene from Plasmodium falciparum. Mol. Biochem. Parasitol. 68, 315–318 (1994).
Article CAS Google Scholar - Chansiri, K. & Bagnara, A. S. The structural gene for carbamoyl phosphate synthetase from the protozoan parasite Babesia bovis. Mol. Biochem. Parasitol. 74, 239–243 (1995).
Article CAS Google Scholar - Pfefferkorn, E. R. & Pfefferkorn, L. C. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp. Parasitol. 39, 365–376 (1976).
Article CAS Google Scholar - Suzuki, Y., Orellana, M. A., Schreiber, R. D. & Remington, J. S. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science 240, 516–518 (1988).
Article ADS CAS Google Scholar - Fields, P. I., Swanson, R. V., Haidaris, C. G. & Heffron, F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl Acad. Sci. USA 83, 5189–5193 (1986).
Article ADS CAS Google Scholar - Mahan, M. J., Slauch, J. M. & Mekalanos, J. J. Selection of bacterial virulence genes that are specifically induced in host tissues. Science 259, 686–688 (1993).
Article ADS CAS Google Scholar - Scharton-Kersten, T. M. et al. In the absence of endogenous IFN-γ, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 157, 4045–4054 (1996).
CAS PubMed Google Scholar - Mahmoud, A. A., Warren, K. S. & Strickland, G. T. Acquired resistance to infection with Schistosoma mansoni induced by Toxoplasma gondii. Nature 263, 56–57 (1976).
Article ADS CAS Google Scholar - Charest, H. et al. Recombinant attenuated Toxoplasma gondii expressing the Plasmodium yoelii circumsporozoite protein provides highly effective priming for CD8+ T cell-dependent protective immunity against malaria. J. Immunol. 165, 2084–2092 (2000).
Article CAS Google Scholar - Fox, B. A., Belperron, A. A. & Bzik, D. J. Negative selection of herpes simplex virus thymidine kinase in Toxoplasma gondii. Mol. Biochem. Parasitol. 116, 85–88 (2001).
Article CAS Google Scholar - Bzik, D. J., Li, W. B., Horii, T. & Inselburg, J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene. Proc. Natl Acad. Sci. USA 84, 8360–8364 (1987).
Article ADS CAS Google Scholar
Acknowledgements
We thank members of the Bzik lab, and D. S. Roos, S. N. Fiering and E. R. Pfefferkorn for discussions. This work was supported by grants from the National Institutes of Health and the US Department of Defense.
Author information
Authors and Affiliations
- Department of Microbiology and Immunology, Dartmouth Medical School, Lebanon, 03756, New Hampshire, USA
Barbara A. Fox & David J. Bzik
Authors
- Barbara A. Fox
You can also search for this author inPubMed Google Scholar - David J. Bzik
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toDavid J. Bzik.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Fox, B., Bzik, D. De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii.Nature 415, 926–929 (2002). https://doi.org/10.1038/415926a
- Received: 15 August 2001
- Accepted: 22 November 2001
- Issue Date: 21 February 2002
- DOI: https://doi.org/10.1038/415926a