Plasma antioxidants from chocolate (original) (raw)
To determine the antioxidant content of different chocolate varieties, we took dark chocolate and milk chocolate prepared from the same batch of cocoa beans and defatted them twice with _n_-hexane before extracting them with a mixture of water, acetone and acetic acid (70.0:29.8:0.2 by volume). We measured their in vitro total antioxidant capacities using the ferric-reducing antioxidant potential (FRAP) assay4; FRAP values were 147.4 ± 4.5 and 78.3 ± 3.4 μmol reduced iron per 100 g for dark and milk chocolate, respectively. Volunteers must therefore consume twice as much milk chocolate as dark chocolate to receive a similar intake of antioxidants.
We recruited 12 healthy volunteers (7 women and 5 men with an average age of 32.2 ± 1.0 years (range, 25–35 years). Subjects were non-smokers, had normal blood lipid levels, were taking no drugs or vitamin supplements, and had an average weight of 65.8 ± 3.1 kg (range, 46.0–86.0 kg) and body-mass index of 21.9 ± 0.4 kg m−2 (range, 18.6–23.6 kg m−2). On different days, following a crossover experimental design, subjects consumed 100 g dark chocolate, 100 g dark chocolate with 200 ml full-fat milk, or 200 g milk chocolate (containing the equivalent of up to 40 ml milk).
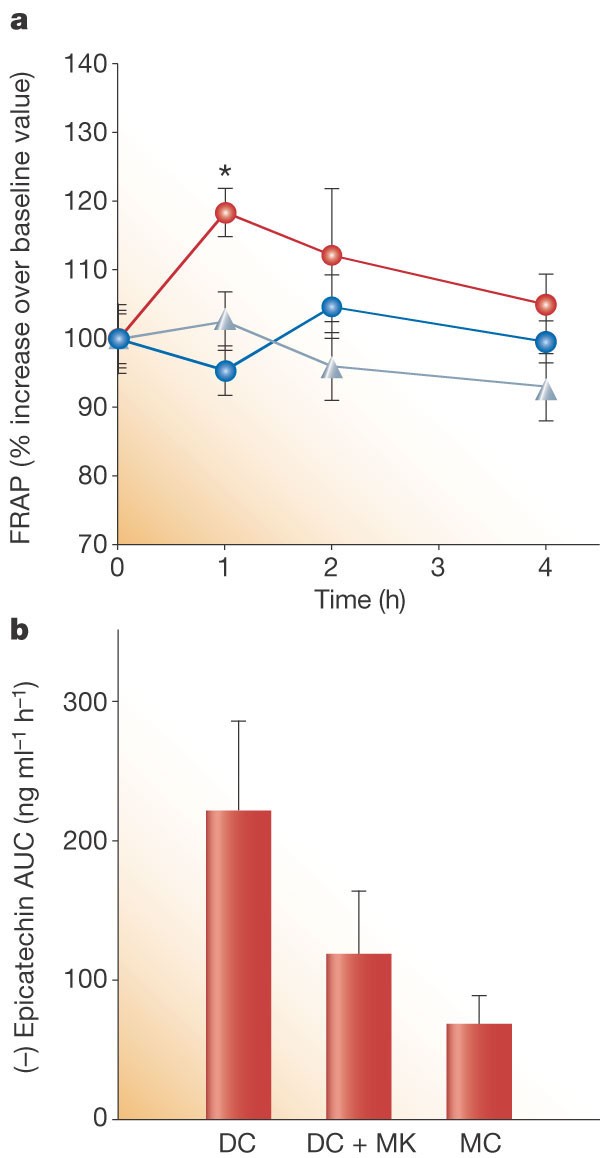
One hour after subjects had ingested the chocolate, or chocolate and milk, we measured the total antioxidant capacity of their plasma by FRAP assay. Plasma antioxidant levels increased significantly after consumption of dark chocolate alone, from 100 ± 3.5% to 118.4 ± 3.5% (_t_-test, P < 0.001), returning to baseline values (95.4 ± 3.6%) after 4 h (Fig. 2a). There was no significant change in plasma FRAP values over the same period after ingestion of milk chocolate alone or of dark chocolate with milk (Fig. 2a).
Figure 2: Effects of acute ingestion of 100 g dark chocolate (DC), 100 g dark chocolate with 200 ml milk (DC + MK) or 200 g milk chocolate (MC) on the total antioxidant capacity (TAC) and (−)epicatechin content of human plasma.
a, Mean TAC of plasma samples at the indicated times after chocolate consumption, expressed as ferric-reducing antioxidant potential (FRAP)4. Values are mean percentage increases (±s.e.m.) relative to baseline values (n = 12). Red circles, DC; blue circles, DC + MK; grey triangles, MC. Asterisk denotes P < 0.001. b, Mean (−)epicatechin levels in plasma, expressed as the area under the curve in a (AUC, in ng ml−1 h−1) for the 4-h period after chocolate consumption. Values are significantly different from one another (see text).
The areas under the curves of (−)epicatechin plasma levels plotted against time5 were measured over the same 4-h period after ingestion for the three different conditions. Absorption of (−)epicatechin into the bloodstream after ingestion of chocolate was significantly less when the chocolate was accompanied by milk (− 46.4 ± 4.1%; analysis of variance (ANOVA), P < 0.001) or if the chocolate itself contained milk (− 69.1 ± 3.9%; ANOVA, P < 0.001; Fig. 2b).
Addition of milk, either during ingestion or in the manufacturing process, therefore inhibits the in vivo antioxidant activity of chocolate and the absorption into the bloodstream of (−)epicatechin. This inhibition could be due to the formation of secondary bonds between chocolate flavonoids and milk proteins6,7, which would reduce the biological accessibility of the flavonoids and therefore the chocolate's potential antioxidant properties in vivo.
Our findings highlight the possibility that the in vivo antioxidant activity of flavonoids could be impaired by other dietary constituents. Other food combinations may also counteract the absorption and protective effects of flavonoids. There is therefore a need to take into account dietary habits when designing studies to assess the association between flavonoid-rich foods, antioxidant activity and degenerative diseases.