Mutations in a signalling pathway (original) (raw)
Although genetic alterations in tyrosine kinases have previously been firmly implicated in tumorigenesis, only a few serine/threonine kinases (STKs) are known to be mutated in human cancers1,2,3,4. We selected 340 genes encoding STKs from the human genome5 and analysed them for mutations in tumours from colorectal cancer patients (for details, see supplementary information). As the catalytic domains of these genes are most likely to harbour mutations that activate the gene product1, we focused on stretches (exons) containing the kinase domains.
These exons were amplified by using polymerase chain reaction (PCR) on template DNA derived from 24 colorectal cancers and were then sequenced directly. Any observed changes were evaluated against DNA from patient-matched normal tissue to identify somatic (tumour-specific) mutations. The entire coding regions of those genes found to contain mutations were then further evaluated in a larger panel of 180 colorectal tumours. A total of 23 changes, including 20 non-synonymous point mutations, one insertion and one splice-site alteration, were identified (see supplementary information).
The gene mutations affected eight different proteins: six were in mitogen-activated protein-kinase kinase-4 (MKK4/JNKK1), six in myosin light-chain kinase-2 (MYLK2), three in phosphoinositide-dependent protein kinase-1 (PDK1, of which two mutations affect the same residue in the kinase domain), two in p21-activated kinase 4 (PAK4), two in v-akt murine thymoma viral oncogene homologue-2 kinase (AKT2), and two in MAP/microtubule affinity-regulating kinase-3 (MARK3); there was one alteration in cell-division cycle-7 kinase (CDC7) and another in a hypothetical casein kinase (PDIK1L). Eighteen of the 23 somatic mutations occurred at evolutionarily conserved residues.
MKK4/JNKK1 is altered in a variety of tumour types6, but no mutations in any of the other genes have previously been found in colorectal cancers. Three of the altered genes, PDK1, AKT2 and PAK4, encode proteins involved in the PI(3)K signalling pathway7,8 (Fig. 1), and two of these (AKT2 and PAK4) are overexpressed in human cancers1.
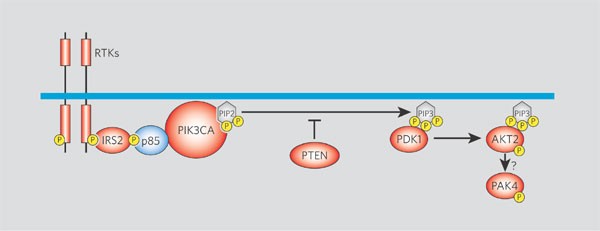
Figure 1: The phosphatidylinositol-3-OH kinase (PI(3)K) signalling pathway and mutations in its components found in colorectal cancers.
In the pathway (reviewed in ref. 7), receptor tyrosine kinases (RTKs) recruit IRS adaptor proteins that induce proper assembly of the p85/PIK3CA complex; the PIK3CA enzyme then phosphorylates phosphatidylinositol-4,5- bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3). (The enzyme PTEN normally reverses this process under appropriate circumstances.) PDK1 is then recruited to the cell surface by PIP3 and phosphorylates and activates AKT2; the activation of PAK4, a downstream step in the pathway, is dependent on PI(3)K signalling, presumably through AKT2. Members of the pathway highlighted in red were found to be genetically altered in the colorectal cancers examined here. Phosphate groups are indicated in yellow. Intermediates: IRS2, insulin-receptor substrate-2; PIK3CA, the phosphoinositide-3-kinase p110α catalytic subunit; PTEN, phosphatase-and-tensin homologue; PDK1, phosphoinositide-dependent protein kinase-1; AKT2, v-akt murine thymoma viral oncogene homologue-2 kinase; PAK4, p21-activated kinase 4.
We tested whether any of the three kinases could have been altered by amplification, another mechanism for kinase activation. Quantitative PCR analysis of 146 colorectal tumours showed co-amplification of AKT2 and PAK4 on chromosome 19q13.2 in two samples, which we confirmed by digital karyotyping9 and fluorescent in situ hybridization (see supplementary information).
We also evaluated other non-STK members of the PI(3)K pathway in the same 146 samples (Fig. 1, and see supplementary information)7 and found one mutation in the insulin-related receptor INSRR, one in the v-Erb-B erythroblastic leukaemia viral oncogene homologue ERBB4, seven in the phosphatase-and-tensin homologue PTEN, and three cases of amplification of the insulin-receptor substrate IRS2 (see supplementary information). When these alterations are compared with those previously discovered in the phosphoinositide-3-kinase p110α catalytic subunit PIK3CA (ref. 10), their distribution is striking: all but two of the 58 alterations were in different tumours (_P_=0.02, ξ2 test), indicating that the mutated genes probably have equivalent tumorigenic effects and are operating through the same pathway.
Overall, nearly 40% of colorectal tumours had alterations in one of eight PI(3)K-pathway genes: as most of these encode protein kinases, they could serve as sites for therapeutic intervention. Also, targeting the downstream genes PDK1 or AKT2 could be effective against the much larger fraction of tumours that contain mutations in PIK3CA or PTEN.