Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine (original) (raw)
Main
Most AIDS vaccine strategies (reviewed in refs. 1, 2) have failed to protect against heterologous viruses because of the HIV-1 Envelope strain variability2,3,4,5. Vaccines with live attenuated viruses can protect against heterologous viruses1,6,7,8; however, delayed disease onset and the appearance of revertant viruses9,10,11 hamper their use in humans.
We chose to target the Tat protein of HIV because Tat is produced early after infection and is essential for virus replication and infectivity12,13,14. In addition, Tat is released extracellularly by infected cells15,16,17,18 and is taken up by neighbor cells where activates virus replication16,19,20. Extracellular Tat also favors transmission of both macrophage-tropic and T cell-tropic HIV-1 strains by inducing CCR5 and CXCR4 co-receptors21,22. Tat is also essential in the pathogenesis of AIDS pathogenesis and AIDS-associated Kaposi's sarcoma14,15,18,20,23,24,25,26,27,28,29,30.
Tat is also immunogenic, and antibodies against Tat may have protective effects in controlling disease progression31,32,33,34 by inhibiting both the effect of extracellular Tat on HIV replication16,33 and its immunosuppressive effects on T cells26. Furthermore, the presence of anti-Tat cytotoxic T lymphocytes (CTLs) in the initial phase of infection correlates inversely with progression to AIDS (refs. 35, 36, 37). Tat protein is efficiently taken up by cells16,17,19,38 and can induce CD8+ T cell-mediated CTLresponses by entering the major histocompatibility complex (MHC) class I pathway39. Finally, Tat is conserved in its immunogenic epitopes among the different subtypes, with the exception of the O subtype40.
Thus, although a Tat vaccine cannot block virus entry, the immune response to Tat may control virus replication and transmission. As a result, the infection could be confined and progression to AIDS could be blocked, as has been suggested41.
Vaccination of cynomolgus monkeys with Tat
We immunized seven cynomolgus monkeys (Macaca fascicularis) with a biologically active Tat protein17 (Table 1 ). Six monkeys were immunized subcutaneously with Tat (10 μg) and the adjuvant RIBI (RIBI; n = 3) or the adjuvant aluminum phosphate (alum; n = 3), and one monkey, with Tat (6 μg), intradermally, in the absence of adjuvants. Two control monkeys were injected subcutaneously with either RIBI or alum alone. A naive monkey was included in the protocol at the time of challenge as an additional control. Boosts were given at 2, 6, 11, 15, 21, 28, 32 and 36 weeks after the first immunization. The last boost was given intramuscularly with Tat in immune-stimulating complexes42. Monkey 54222, immunized intradermally, was vaccinated on a slightly different schedule (on weeks 0, 5, 12, 17, 22, 27, 32, 38, 42 and 48) and did not receive the boost of immune-stimulating complexes.
Table 1 Vaccination protocols
No signs of local or systemic toxicity were seen at the time of vaccination, and clinical laboratory tests (blood cell counts, blood chemistry and FACS analysis), done each time blood was drawn, were always in the normal range (data not shown).
Humoral and cellular responses to Tat after vaccination
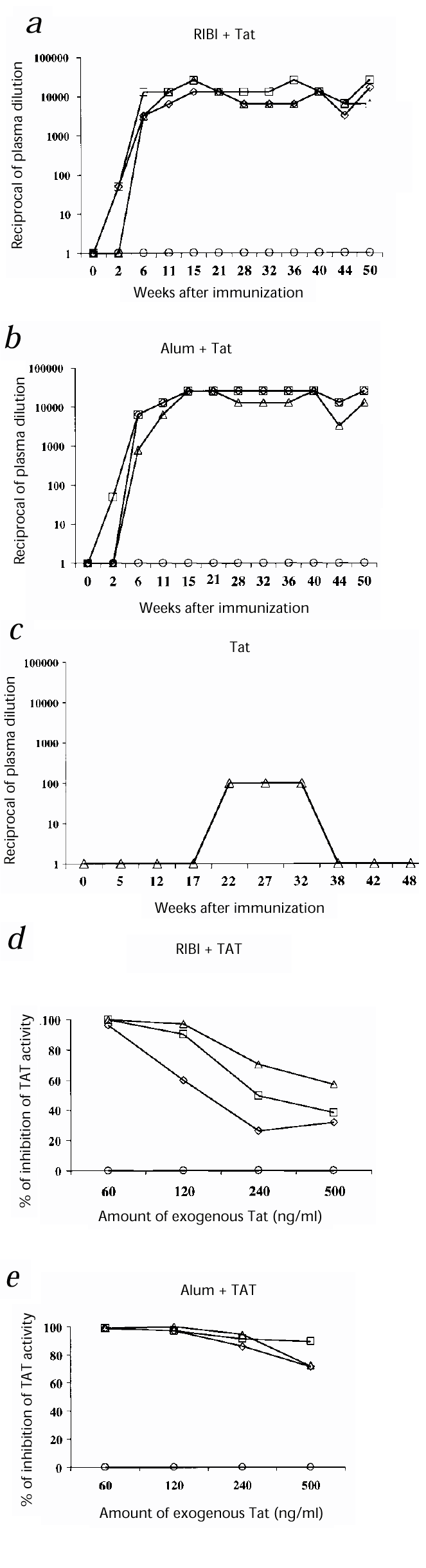
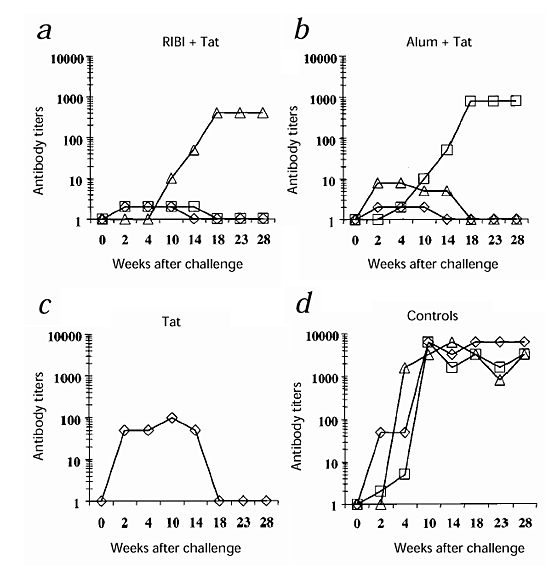
The six monkeys inoculated with Tat and RIBI or alum seroconverted by week 6 after the first immunization, and the antibody titers increased up to 1:25,600 in all monkeys immunized with Tat and alum, and up to 1:12,800 in the monkeys immunized with Tat and RIBI; these titers remained stable up to 50 weeks after the first immunization (Fig. 1_a_ and b ). Monkey 54222 (given Tat intradermally) developed low titers of antibodies against Tat (1:100), which were detected up to 32 weeks after immunization (Fig. 1_c_).
Figure 1: Humoral responses to Tat.
a_–_c, Titers of antibody against Tat after the first immunization (week 0). Monkeys were inoculated with: a, RIBI + Tat: 54844 (⋄) 54879 (□), 54963 (▵) or RIBI alone: 55123 (○); b, alum + Tat: 54899(⋄), 55396 (□), 54240 (▵) or alum alone: 55129 (○); or c, Tat alone: 54222 (▵). Titers represent the reciprocal of the last plasma dilution at which the test was still positive. d_–_e, Percentages of inhibition by plasma (1:2 dilution) from vaccinated monkeys of the rescue of Tat-defective proviruses induced by 60–500 ng/ml Tat. Plasma obtained at week 21 after immunization. Macaques were vaccinated with: d, RIBI + Tat: 54844 (⋄), 54879 (□), 54963 (▵); or e, alum + Tat: 54899(⋄), 55396 (□), 54240 (▵). Controls (○), pooled pre-immune plasma from the corresponding groups.
In addition, at week 21 after immunization, plasma from the six macaques inoculated with Tat and RIBI or alum were capable of neutralizing the activity of 120–500 ng/ml of Tat on HIV-1 replication, compared with pre-immune plasma (Fig. 1_d_–e), as shown by the inhibition of the rescue of Tat-defective proviruses induced after the addition to the cells of serial concentrations of Tat (refs. 16, 17,20). Furthermore, at week 44 after immunization, plasma from monkeys 54963 (given RIBI and Tat) and 54899 and 55396 (given alum and Tat) were capable of neutralizing the replication of the SHIV89.6P after in vitro acute infection of CEMx174 cells, compared with pre-immune plasma (data not shown). In both assays, neutralization correlated with the titers of antibody against Tat.
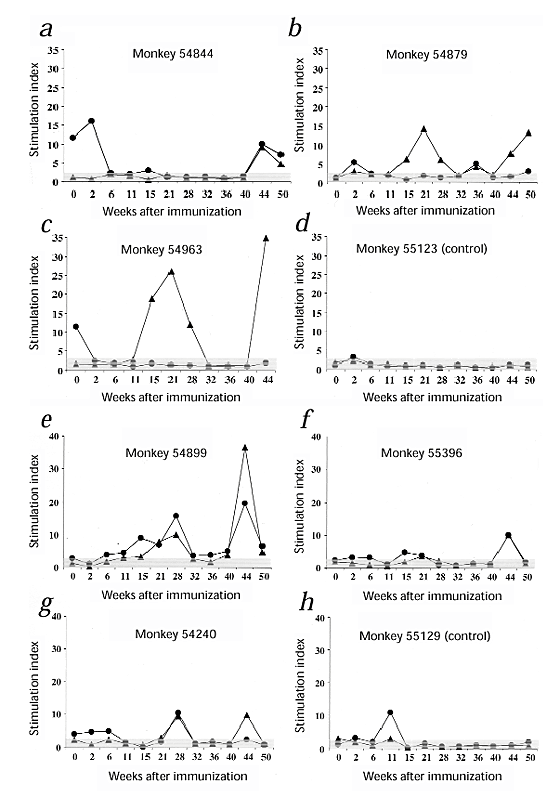
Tat-specific proliferation was seen in three of three monkeys vaccinated with Tat and RIBI (Fig. 2_a_–c) and in three of three vaccinated with Tat and alum (Fig. 2_e_ –g), whereas no response was detected in the macaque vaccinated intradermally with Tat alone (data not shown) and in the control monkeys (Fig. 2_d_ and 2_h_).
Figure 2: Lymphoproliferative responses.
▴, Tat; ●, tetanus toxoid. Monkeys were inoculated with: a_–_c, RIBI + Tat; d, RIBI alone (control); _e_– g, alum + Tat; or h, alum alone (control). Vertical axis, fold of proliferation (measured by 3H-thymidine incorporation) of antigen-stimulated cells compared with unstimulated cells (stimulation index); values greater than 3 (cut-off) were considered positive. Monkey 54222, inoculated with Tat alone, responded to tetanus toxoid (fivefold to tenfold) but not to Tat (data not shown). Response to PHA, positive control (stimulation index ≥ 4).
Specific anti-Tat CTL activity began to be detectable in the vaccinated monkeys at week 28 after immunization, but only reached levels above the cut-off (10%) at week 36 in one of two macaques vaccinated with Tat and RIBI, in one of three monkeys vaccinated with Tat and alum and in the monkey vaccinated with Tat alone (intradermally) (Table 2). Although at week 28 monkey 54240 (given alum and Tat) showed detectable but low (below 10%) CTL activity, at week 36 the test was inconclusive.
Table 2 Tat-specific CTLs and TNF-α production in vaccinated monkeys
To confirm and extend these data, at week 44 after immunization, we tested the production of tumor necrosis factor-α (TNF-α), a known mediator of CTL activity43,44,45,46, after stimulation of peripheral blood mononuclear cells (PBMCs) with Tat or phytohemagglutinin (PHA). Monkeys 54879 and 54240 (for which CTL activity could not be tested or was inconclusive, respectively) and the monkeys that showed anti-Tat CTL activity all produced TNF-α after Tat stimulation (Table 2). In contrast, monkeys lacking CTL activity, including the control monkeys, produced TNF-α after stimulation with PHA but not with Tat ( Table 2).
To determine the cell source of TNF-α after Tat stimulation, we separated PBMCs from three of the five responsive monkeys (54879, 54899 and 54240, for which cells were available) into CD8– and CD8+ subsets and evaluated Tat-induced TNF-α production separately in the two cell fractions, which we also analyzed by FACS. The main source of TNF-α (~90%) was the CD8+ cell fraction, which was mostly (range, 85–94.5%) T cells (CD3+/CD8+) with a minority (range, 5.5–15%) of NK cells (CD3–/CD8+)(Table 3).
Table 3 TNF-α production by CD8– and CD8+ cells after Tat stimulation, and correlation with cell phenotype
From week 11 or 15 after primary immunization, skin tests to Tat produced positive results in two of three monkeys inoculated with Tat and RIBI ( 54879 and 54963) and in three of three monkeys inoculated with Tat and alum (data not shown). Monkey 54222 (given Tat intradermally) did not show any reactivity to Tat. All monkeys responded to the recall antigen tetanus toxoid.
Challenge with SHIV89.6P
We used SHIV-89.6P for the virus challenge because it is highly pathogenic in macaques and because it contains the tat gene of HIV-1 (ref. 47). The virus stock used for challenge was derived from a cynomolgus macaque inoculated with the original SHIV89.6P from rhesus monkeys.
To determine virus pathogenicity in cynomolgus and the monkey infectious dose (MID50), we inoculated virus stocks obtained from rhesus and cynomolgus macaques into six and eight monkeys, respectively. There were high levels of virus replication and a profound and persistent decrease in CD4 T cells in all monkeys inoculated with each virus stock (from 2,852 to 2.8 MID50) (data not shown), as described47. Therefore, we challenged all vaccinated macaques and the two control monkeys intravenously with 10 MID50 of SHIV-89.6P. As an additional control, we inoculated a naive monkey (12) with 2.8 MID50 of the virus, a dose lower than the challenge dose.
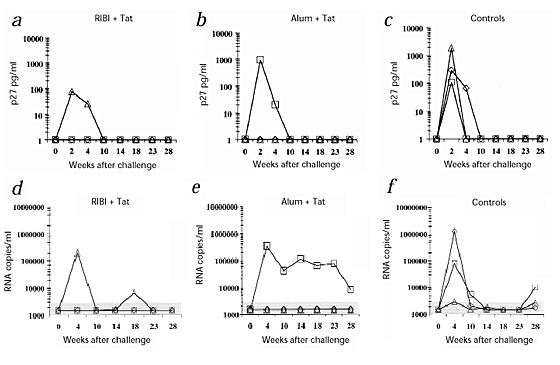
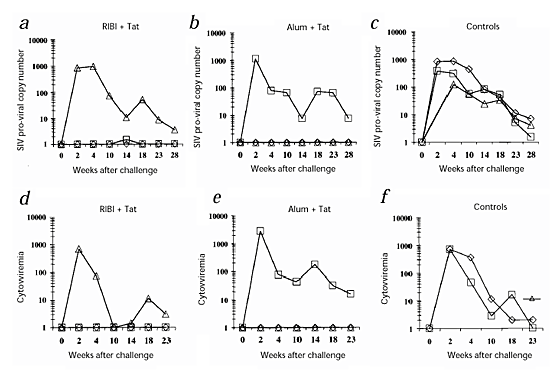
After challenge, all three control monkeys were infected, as indicated by the presence of p27 antigen (Fig. 3_a_–c) and viral RNA (Fig. 3_d_–f) in plasma. In contrast, only two vaccinated monkeys (54963, given Tat and RIBI, and 55396, given Tat and alum) were infected, as shown by both assays ( Fig. 3). For the other five vaccinees, including monkey 54222 (given Tat alone), p27 and viral RNA were always undetectable. Moreover, PBMCs from both the control macaques and the two vaccinated, infected monkeys had a high proviral copy number from week 2 after challenge (Fig. 4a_–_c). One other macaque (54879, given Tat and RIBI), had a very low proviral copy number (1.5 copies/μg DNA) only at week 14 after challenge and not later (Fig. 4a_–_c). In contrast, in all the other vaccinated monkeys, including monkey 54222 (given Tat alone), proviral DNA was always undetectable.
Figure 3: Detection of viral infection up to 28 weeks after challenge with SHIV-89.
6P. a_–_c, Detection of p27 'antigenemia'. _d_– f, Detection of plasma viremia (SHIV RNA copies/ml plasma). Monkeys were inoculated with: a and d, RIBI + Tat: 54844 (⋄), 54879 (□), 54963 (▵); b and e, alum + Tat: 54899 (⋄), 55396 (□), 54240 (▵); or c and f, RIBI alone: 55123 (⋄), alum alone: 55129 (□) or nothing: 12 (▵).
Figure 4: Detection of viral infection after challenge with SHIV-89.6P.
a_–_c, Quantitation of the SIV proviral copy number (copies/μg DNA) up to 28 weeks after challenge. d–f, Cytoviremia (infected cells/106 PBMCs) or virus isolation up to 23 weeks after challenge. From week 14 after challenge, all monkeys negative in cytoviremia were tested for virus isolation (instead of cytoviremia) after CD8+ T-cell depletion and stimulation with PHA and rhIL-2, and the results were always negative. Monkeys were inoculated with: a and d, RIBI + Tat: 54844 (⋄), 54879 (□), 54963 (▵); b and e, alum + Tat: 54899 (⋄), 55396 (□), 54240 (▵); or c and f, RIBI alone: 55123 (⋄), alum alone: 55129 (□) or nothing: 12 (▵). c, For monkey 12 (control monkey inoculated with 2.8 MID50 of SHIV), DNA PCR was also positive at week 2, however, the proviral copy number was not determined. f, Before week 23, monkey 12 was always tested by virus isolation and results were always positive (data not shown).
The control monkeys and the two vaccinated and infected monkeys (54963 and 55396) also had consistently high levels of cell associated viral load (cytoviremia) (Fig. 4_d_–f). In contrast, the other five vaccinated macaques were always negative, including monkey 54222 (given Tat alone). Moreover, each attempt to isolate virus (done since week 14 after challenge) from PBMCs depleted of CD8+ T cells and stimulated with PHA from these five monkeys failed (Fig. 4).
To verify that infection had occurred also in the 'protected' monkeys, we did serological assessments. Antibodies against SIV were detected in all the macaques from week 2 or 4 after challenge (Fig. 5). Control monkeys had the highest titers (at least 2 logs higher) and these increased rapidly compared with those of the vaccinated monkeys. The two infected, vaccinated monkeys, however, had higher titers than the five 'protected' monkeys, but these were delayed compared with those of the control monkeys. Moreover, in the five 'protected' monkeys, titers of antibody against SIV became undetectable from week 14 or 18 after challenge. Antibodies against the HIV-1 Env of SHIV89.6P were detected in plasma from three of three control monkeys and in the two vaccinated and infected monkeys, but not in the other monkeys (data not shown). However, they were detected by in vitro antibody production in the supernatants of cultured PBMCs stimulated with mitogen pokeweed (PWM) from all the monkeys, with the highest titers in the control monkeys (data not shown). Thus, infection probably had occurred in all monkeys after challenge; however, in five of seven vaccinated monkeys, it was kept at a very low or undetectable level.
Figure 5: Titers of antibody against HIV-2/SIV up to 28 weeks after challenge with SHIV-89.
6P. Monkeys were inoculated with: a, RIBI + Tat: 54844 (⋄), 54879 (□), 54963 (▵); b, alum + Tat: 54899 (⋄), 55396 (□), 54240 (▵); c, Tat alone: 54222 (⋄); or d, RIBI alone: 55123 (⋄), alum alone: 55129 (□) or nothing: 12 (▵). Titers represent the reciprocal of the last dilution at which plasma were still positive.
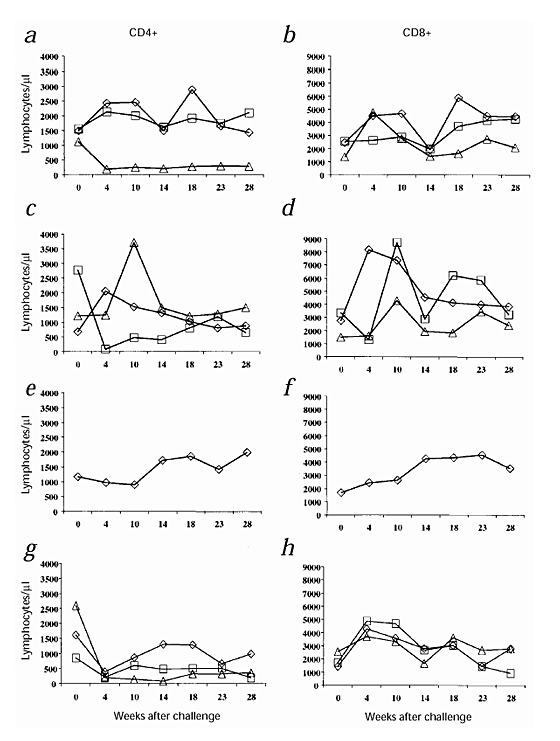
Consistent with these data, the CD4+ T-cell counts decreased considerably in both the control monkeys and the two vaccinated and infected monkeys, whereas they remained high and stable in the five 'protected' macaques (Fig. 6). An increase in CD8+ cells was detected in all monkeys after challenge. However, at week 23 or 28 after challenge, the number of CD8+ lymphocytes returned to values similar to values before challenge (Fig. 6).
Figure 6: CD4+ and CD8+ T-cell counts up to 28 weeks after challenge with SHIV-89.
6P. a, c, e and g. Numbers of CD4+ T cells/μl; b, d ,f and h. Numbers of CD8+ T cells/μl. Monkeys were inoculated with: a and b, RIBI + Tat, 54844 (⋄); 54879 (□), 54963 (▵); c and d, alum + Tat: 54899 (⋄), 55396 (□), 54240 (▵); e and f, Tat alone: 54222 (⋄); or g and h, RIBI aone: 55123 (⋄), alum alone: 55129 (□) or nothing: 12 (▵).
Discussion
These data indicate that immunization with a biologically active Tat protein is safe and induces a complete Tat-specific immune response. Vaccination with Tat controlled (up to 28 weeks after challenge) infection with the highly pathogenic virus SHIV-89.6P in five of seven vaccinated monkeys. In these monkeys, the CD4+ T-cell numbers did not decrease after challenge, confirming that the Tat vaccine blocked virus replication and, consequently, prevented the CD4+ T-cell decrease. All parameters of virus replication and a profound CD4 T-cell decrease were seen in all the naive monkeys (n = 16, including the controls of the vaccination protocol) inoculated in our lab with different MID50 (from 2,852 to 2.852) of SHIV89.6P (grown in Rhesus or in cynomolgus), except in five of seven of the Tat-vaccinated monkeys.
Although high titers of neutralizing antibodies against Tat were present in all vaccinated monkeys (except for monkey 54222, inoculated with Tat intradermally), two of three of the monkeys capable of neutralizing both Tat activity and in vitro SHIV replication were infected after challenge. No virus was detected in the monkey inoculated with Tat alone, which had low antibody titers. In contrast, anti-Tat CTLs and/or Tat-induced TNF-α production were found in all 'protected' monkeys, but they were undetectable in the two infected, vaccinated monkeys. Most of this TNF-α originated from the CD8+ T-cell subset, confirming that CTLs detected in the monkeys belong to this cell type39.
The low levels of the anti-Tat CTLs detected before challenge in our monkeys are not surprising, because although Tat-specific CTLs responses can interfere with disease progression36, the CTL levels are generally much lower and therefore are not comparable with those detected against other viral proteins such as Gag, Pol or Env (refs. 36,37,48–50).
These data indicate that a cellular response to Tat may be involved in controlling infection, as suggested by previous work36 and by data showing an inverse correlation between the presence of CTLs and HIV-1 viral load50. However, these data do not exclude the possibility of a role for antibodies against Tat in controlling infection and progression to AIDS. In particular, the two vaccinated, infected monkeys had slowly increasing and lower titers of antibodies against SIV or HIV, compared with those of the control monkeys, indicating that neutralizing antibodies against Tat may interfere with virus replication.
An AIDS vaccine based on Tat represents a strategy aimed at controlling HIV replication and at modifying the virus–host interaction that leads to progressive immunodeficiency and disease onset. However, the immune response against Tat cannot block virus entry. In fact, it is likely that a low level and/or abortive infection has occurred also in the five 'protected' monkeys, as indicated by the presence of transient and low titers of antibody against SIV.
Sera from HIV-1-infected Ugandan patients, infected with the A and D subtypes of HIV-1 (ref. 51), recognize our Tat protein (derived from an HIV-1, subtype B strain) (S.B. et al., unpublished data), which indicates that Tat is sufficiently conserved to be an optimal vaccine target against infection with all or most viral strains. Because Tat delivered as DNA or as a Tat toxoid is safe and immunogenic in animals and humans34,48,52,53,54, Tat, alone or combined with other viral products, may represent an optimal target for AIDS vaccine development for both preventive and therapeutic applications.
Methods
Animals.
Adult cynomolgus monkeys (Macaca fascicularis ) were housed in single cages within level 3 biosafety facilities according to the European guidelines for non-human primate care (ECC, Directive No. 86-609, Nov. 24, 1986). Monkeys were examined and their weights and rectal temperatures were measured while they were sedated by ketamine hydrochloride anaesthesia (10 mg/kg). Blood samples for hematological, immunological and virological analysis were obtained in the morning before food administration.
HIV-1 Tat protein expression, purification and inoculation.
HIV-1 Tat from the HTLV-IIIB isolate (subtype B) was expressed in Eschericia coli, purified to homogeneity by heparin-affinity chromatography and high-performance liquid chromatography and stored lyophilized at –80 °C as described17. Purified Tat had full biological activity in several assays15,16,17. As Tat is sticky, oxydizes easily and is photo- and thermo-sensitive16, it was resuspended in degassed buffer before use in vitro17 or in saline containing 20% of autologous serum for monkey injection. Plasticware, syringes and needles were rinsed in medium containing 10% fetal calf serum (FCS) or in saline containing 20% autologous serum, and all of these procedures were done in the dark with samples on ice.
Adjuvants.
RIBI containing monophosphoryl lipid A, trehalose dimycolate, cell wall skeleton in oil (squalene) and Tween 80 was obtained through the European Concerted Action on Macaque models for AIDS Research from the AIDS Reagent project (National Institute for Biological Standards and Control, Potters Bar, UK). Alum (aluminum phosphate) was a gift from P. Frezza (Hardis, Naples, Italy). Immune-stimulating complexes containing the Tat protein (80 μg/ml) were prepared with a described procedure42. ELISA and western blot analysis were done to confirm the co-localization of the immune-stimulating complexes with the Tat protein (data not shown).
Detection of antibodies against Tat.
Polyvinyl chloride microtiter plates were coated with Tat (100 ng/200 μl per well of 0.05 M carbonate buffer, pH 9.6) for 12 h (4 °C) and extensively washed with PBS without Ca2+ and Mg2+ containing 0.05 % Tween 20 (PBS/Tween). Plasma, 200 μl diluted in buffer, was then added to each well in duplicate. Plates were incubated for 90 min (37 °C), washed five times with PBS/Tween and 100 μl horseradish peroxidase-conjugated secondary antibody (Sigma) diluted 1:1,000 in PBS/Tween (containing 1% BSA) was added for 90 min (37 °C). After extensive washing of the plates, 100 μl peroxidase substrate (ABTS 1 mM, Amersham Pharmacia Biotech, Milan, Italy) was added and the absorbance at 405 nm was measured with a spectrophotometer. A rabbit polyclonal antiserum against Tat, used at serial twofold dilutions (1:200–1:6,400), was the positive control. Monkey preimmune plasma (diluted 1:50 and 1:100) was the negative control. The mean of the negative controls + 3 standard deviations represented the cut-off value. The minimal plasma dilution used was 1:50.
Neutralization of Tat activity on HIV replication by the rescue assay.
Tat activity was measured by the rescue assay in which the replication of Tat-defective HIV-1 proviruses is induced by serial concentrations of Tat added to HLM-1 cells (HeLa CD4+ cells containing a Tat-defective HIV-1 provirus) as described16,17,20. Growth medium (300 μl) containing either preimmune or immune plasma (1:2 dilution) were added to each well (in duplicate). Supernatants were collected 48 h later and p24 Gag content was determined by an antigen capture assay (NEN).
Lymphoproliferative responses.
PBMCs were purified from citrated peripheral blood on a Ficoll gradient with a standard procedure. PBMCs (2 × 105 in 100 μl of growth medium) were plated with 100 μl of medium, PHA ((2 μg/ml); Murex Biotech Limited, Datford, UK), tetanus toxoid (5 μg/ml) or Tat (5 μg/ml) each in triplicate wells. After 5 d, 1 μCi 3H-thymidine was added and the radioactivity in samples was measured 16 h later with a Betaplate (Wallac, Turku, Finland).
CTL assay.
PBMCs were seeded (5 × 106/well in 0.5 ml complete medium) in a 24-well plate with Tat (1 μg). One day later, 5 × 106 PBMCs were incubated for 3 h with Tat (1 μg), washed twice and added to the wells containing the PBMCs stimulated previously. On day 2, 2 IU of recombinant human IL-2 (rhIL-2) was added to each well. Half of the supernatant was replaced with medium containing rhIL-2 twice each week. On day 14, cells were collected, counted, resuspended in growth medium containing 1 mM sulfinpyrazone (Sigma) and seeded (96-well round-bottomed plates) at serial twofold dilutions (in duplicate) (effectors). The day before the assay, herpesvirus Papio-transformed autologous B lymphocytes55 were pulsed overnight with or without Tat (4 μg/106 cells; targets), labeled with the fluorescence-enhancing ligand bis (acetoxymethyl 2,2':6',2"-terpyridine-6,6"-dicarboxylate (BATDA) according to the manufacturer's instructions (Delfia; Wallac, Turku, Finland)(ref. 56), and 5 × 103 cells were added to the effectors. After 2 h, 20 μl of supernatants were mixed with 200 μl of Europium solution, and fluorescence was measured after 20 min with a time-resolved fluorescence reader (Victor; Wallac, Turku, Finland). The percent specific lysis was calculated for each effector:target ratio as follows: (test release–spontaneous release)/(maximum release–spontaneous release) × 100. The percent specific lysis against unpulsed autologous B lymphocytes was calculated and subtracted from the percent specific lysis against the Tat-pulsed targets. The assay was considered positive for values exceeding 10%.
TNF-α production in PBMCs, CD8+ or CD8– T cells after Tat stimulation.
PBMCs were seeded (in duplicate wells) in a 24-well plate in 1 ml complete medium (1 × 106 cells/ml) and stimulated with PHA (2 μg/ml) or Tat (5 μg/ml). After 2 days, 100 μl of the cell supernatants was collected, and TNF-α production was measured by ELISA as suggested by the manufacturer (Cytoscreen Monkey for TNF-α Biosource, Camarillo, California).
To determine the cell source of TNF-α production, unfractionated PBMCs were seeded in a 6-well plate in 4 ml complete medium (2 × 106 cells/ml) and stimulated with Tat (5 μg/ml). After 2 d, cells were washed, counted and separated into CD8+ and CD8– cells using Dynabeads (Dynal, Oslo, Norway) following the manufacturer's instructions. The purity of the cell populations was evaluated by three-color FACS analysis using monoclonal antibodies against CD3, CD4 and CD8 . CD8– and CD8+ cells were separately plated (5 × 104 cells/well) in a total volume of 200 μl in a U-bottomed 96-well plate in the presence of 2 U/ml of IL-2. Supernatants (100 μl) from the two different cell populations were collected after an additional 24 h, and TNF-α production was evaluated by ELISA.
Skin tests for tetanus toxoid and Tat.
PBS containing 0.1% BSA (150 μl) alone (negative control) or with tetanus toxoid (7 μg) or Tat (5 or 1 μg) was injected intradermally on a shaved area of the upper back. Monkeys were assessed at 24, 48 and 72 h after this injection. The test was considered strongly positive (++) or positive (+) in the presence of induration and erythema with a diameter of >=5 mm or 1–4 mm, respectively. Erythema without induration or erythema < 1 mm were considered weakly positive (±) or negative (–), respectively.
Generation of SHIV-89.6P virus stock, and in vivo and in vitro titration.
To prepare the virus stock, the parental SHIV-89.6P (ref. 47) (obtained from N. Letvin, Harvard Medical School, Boston, Massachusetts) was used to infect a cynomolgus macaque. At day 14 after infection, the monkey was killed and total or CD8+ T cell-depleted cells obtained from spleen and lymph nodes were stimulated in vitro with PHA (2 μg/ml) and cultured in RPMI containing 15% FCS and 50 IU/ml of rhIL-2. Supernatants showing reverse transcriptase activity > 50,000 cpm/ml were pooled, centrifuged at 912_g_ for 20 min (4 °C), clarified at 3,200_g_ for 30 min (4 °C). The supernatant was then complemented with 10% of human serum AB0 and frozen at –152 °C. Frozen vials (0.5 ml) were then tested to determine the tissue culture infectious dose (TCID50) using CEMx174 cells, C8166 cells and PBMCs from four naive macaques.
To determine the MID50, the viral stock was titered in eight monkeys by intravenous inoculum of serial virus dilutions (5 × 10–1 to 5 × 10–6). Infection was monitored by antibody response, plasma antigenemia and viremia, virus isolation, proviral DNA and CD4 T-cell counts. All these parameters were in the same range of those published47 and those from six monkeys inoculated in our lab with the original virus stock from rhesus macaques. The MID50 was then calculated according to Reed and Muench.
Quantitation of the SHIV RNA copies in plasma.
The quantitation of plasma SHIV-89.6P RNA copies was done by the Chiron Corporation in the Chiron Diagnostics Reference Testing Laboratory (Amsterdam, the Netherlands) with a branched DNA signal amplification assay recognizing the pol region of the SIVmac strains as described57. The cut-off value was 1,500 RNA copies/ml; however, values less than 3,000 RNA copies/ml were not always reproducible.
Quantitation of SIV proviral copies.
DNA was extracted from whole blood (QIAamp Blood Kit; Qiagen) and tested for amplification of the β-globin gene as described58. To determine the number of SIV proviral copies, semiquantitative DNA PCR was done to amplify a 496-bp region of the gag gene of SIVmac251 with primers and methodologies described8.
Virus isolation and cell associated viral load (cytoviremia).
For virus isolation, CD8+ T cell-depleted PBMCs (3 × 106) were co-cultured with 1 × 106 CEM × 174 cells in the presence of PHA (2 μg/ml) for 2–3 d and cultured for 30–40 d in RPMI 1640 containing 10% FCS, antibiotics and rhIL-2 (50 IU/ml). The titer of p27 SIV-Gag antigen was determined twice a week.
For cytoviremia, serial twofold dilutions of CD8-depleted PBMCs (1× 106 to 4.8 × 102 cells/well, in duplicate), were co-cultured with CEMx174 cells (1 × 104 cells/well) and assessed for the presence of p27 antigen production on day 12. The 50% endpoints were calculated with the method of Reed and Muench, and results are expressed as the number of infected cells per 1 × 106 PBMCs.
Determination of antibodies against HIV-2/SIV and HIV-1 Env in plasma and in vitro antibody production.
Antibody titers against SIV were determined by end-point dilution using an HIV-2 ELISA assay (Elavia, Ac- Ab-Ak II kit; Diagnostic Pasteur, Paris, France). Antibodies against HIV-1 Env in plasma were determined by HIV-1 Elisa assay (HIV-1/HIV-2 Third Generation Plus; Abbott, Chicago Illinois).
For the in vitro antibody production assay, 2 × 106 PBMCs/well were seeded onto a 24-well culture plate with 2 μg/ml of PWM (Sigma) (ref. 59) and incubated for 7 d. Supernatants were then collected, centrifuged (3,200_g_ for 10 min) and tested for HIV-1 Env antibodies.
Detection of p27 'antigenemia'.
Levels of p27 SIV-Gag protein were measured in plasma by using an antigen capture ELISA assay (Innotest, Innogenetics, Zwijndrecht, Belgium) with a limit of detection of 20 pg/ml.
CD4+ and CD8+ T-cell counts.
Citrated peripheral blood (80–100 μl) was stained with phycoerythrin (PE)-conjugated monoclonal antibodies against CD4 (Biosource, Camarillo, California) and with peridinin chlorophyll protein (PerCP)-conjugated monoclonal antibodies against CD8 (Becton-Dickinson, Mountain View, California) and analyzed with a FACScan cytometer and software (Becton-Dickinson, Mountain View, California) as described8. Isotype-matched PE- and PerCP-labeled antibodies were the negative controls. Absolute cell numbers were calculated from the blood cell counts.
References
- Almond, N.M., & Heeney, J.L. AIDS vaccine development in primate models. AIDS 12, 133–140 (1998).
Google Scholar - Burton, D.R. & Moore, J.P. Why do not have an HIV vaccine and how can we make one? Nature Med. 4, 495– 498 (1998).
Article CAS PubMed Google Scholar - Berman, P.W. et al. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature 345, 622–625 (1990).
Article CAS PubMed Google Scholar - Girard, M. et al. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 88, 542–546 (1991).
Article CAS PubMed PubMed Central Google Scholar - Stott, E.J. et al. Evaluation of a candidate human immunodeficiency virus type 1 (HIV-1) vaccine in macaques: effects of vaccination with HIV-1 gp120 on subsequent challenge with heterologous simian immunodeficiency virus-HIV-1 virus. J. Gen. Virol. 79, 423– 432 (1998).
Article CAS PubMed Google Scholar - Kestler, H.D. et al. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65, 651–662 (1991).
Article CAS PubMed Google Scholar - Daniel, M.D., Kirchhoff, F., Czajak, S.C., Sehgal, P.K., & Desrosiers, R.C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258, 1938–1941 ( 1992).
Article CAS PubMed Google Scholar - Titti, F. et al. Live-attenuated simian immunodeficiency virus prevents super-infection by cloned SIVmac251 in Cynomolgus monkeys. J. Gen. Virol. 78, 2529–2539 (1997).
Article CAS PubMed Google Scholar - Whatmore, A.M. et al. Repair and evolution of nef in vivo modulates Simian Immunodeficiency Virus virulence. J. Virol. 69, 5117–5123 (1995).
CAS PubMed PubMed Central Google Scholar - Baba, T.W. et al., Pathogenicity of live attenuated SIV after mucosal infection of neonatal macaques. Science 267, 1820– 1825 (1995).
Article CAS PubMed Google Scholar - Ezzell, C. The monkey's got AIDS: what now for live AIDS vaccine? J. NIH Res. 9, 21–22 (1997 ).
Google Scholar - Arya, S.K., Guo, C., Josephs, S.F. & Wong-Staal, F. The trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science 229, 69–73 ( 1985).
Article CAS PubMed Google Scholar - Fisher, A.G. et al. The trans-activator gene of HTLV-III is essential for virus replication. Nature 320, 367– 371 (1986).
Article CAS PubMed Google Scholar - Chang, H.-K., Gallo, R.C. & Ensoli, B. Regulation of cellular gene expression and function by the human immunodeficiency virus type 1 Tat protein. J. Biomed. Sci. 2, 189–202 ( 1995).
Article CAS PubMed Google Scholar - Ensoli, B. et al. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature 345 , 84–87 (1990).
Article CAS PubMed Google Scholar - Ensoli, B. et al. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 67, 277–287 ( 1993).
CAS PubMed PubMed Central Google Scholar - Chang, H.C., Samaniego, F., Nair, B.C., Buonaguro, L. & Ensoli, B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 11, 1421–1431 (1997).
Article CAS PubMed Google Scholar - Westendorp, M.O. et al. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp 120. Nature 275, 497– 500 (1995).
Article Google Scholar - Frankel, A.D. & Pabo, C.O. Cellular uptake of the Tat protein from human immunodeficiency virus. Cell 55, 1189–1193 (1988).
Article CAS PubMed Google Scholar - Barillari, G. et al. Effect of cytokines from activated immune cells on vascular cell growth and HIV-1 gene expression. Implications for AIDS-Kaposi's sarcoma pathogenesis. J. Immunol. 149, 3727– 3734 (1992).
CAS PubMed Google Scholar - Huang, L., Bosh, I., Hofmann, W., Sodroski, J. & Pardee, A.B. Tat protein induces human immunodeficiency virus type 1 (HIV-1) coreceptors and promotes infection with both macrophage-tropic and T-lymphotropic HIV-1 strains. J. Virol. 72, 8952–8960 (1998).
CAS PubMed PubMed Central Google Scholar - Li, C.J. et al. Tat protein induces self-perpetuating permissivity for productive HIV-1 infection. Proc. Natl. Acad. Sci. USA 94, 8116–8120 (1997).
Article CAS PubMed PubMed Central Google Scholar - Viscidi, R.P., Mayur, K., Lederman, H.M. & Frankel, A.D. Inhibition of antigen-induced lymphocyte proliferation by Tat protein from HIV-1. Science 246, 1606– 1608 (1989).
Article CAS PubMed Google Scholar - Barillari, G., Gendelman, R., Gallo, R.C. & Ensoli, B. The Tat protein of human immunodeficiency virus type-1, a growth factor for AIDS Kaposi's sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc. Natl. Acad. Sci. USA 90, 7941–7945 (1993).
Article CAS PubMed PubMed Central Google Scholar - Subramanyam, M., Gutheil, W.G., Bachovchin, W.W. & Huber, B.T. Mechanism of HIV-1 Tat induced inhibition of antigen-specific T cell responsiveness. J. Immunol. 150, 2544– 2553 (1993).
CAS PubMed Google Scholar - Zagury, J.F. et al. Interferon alpha and Tat involvement in the immunosuppression of uninfected T cells and C-C chemokine decline in AIDS. Proc. Natl. Acad. Sci. USA 95, 3851–3856 (1998).
Article CAS PubMed PubMed Central Google Scholar - Ensoli, B. et al. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi's sarcoma. Nature 371 , 674–680 (1994).
Article CAS PubMed Google Scholar - Li C.J., Friedman D.J., Wang C., Metelev V. & Pardee A.B. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268, 429– 431 (1995).
Article CAS PubMed Google Scholar - Zauli, G. et al. Pleiotropic effects of immobilized versus soluble recombinant HIV-1 Tat protein on CD3-mediated activation, induction of apoptosis, and HIV-1 long terminal repeat transactivation in purified CD4+ T lymphocytes. J. Immunol. 157, 2216– 2224 (1996).
CAS PubMed Google Scholar - Zagury, J.F. et al. Mode of AIDS immunopathogenesis based on the HIV-1 gp120 and Tat-induced dysregulation of uninfected immune cells. Cell Pharmacol . 3, 123–128 ( 1996).
Google Scholar - Reiss, P. et al. Speed of progression to AIDS and degree of antibody response to accessory gene products of HIV-1. J. Med. Virol. 30, 163–168 (1990).
Article CAS PubMed Google Scholar - Rodman, T.C., To, S.E., Hashish, H. & Manchester, K. Epitopes for natural antibodies of human immunodeficiency virus (HIV)-negative (normal) and HIV-positive sera are coincident with two key functional sequences of HIV Tat protein. Proc Natl Acad Sci. USA 90, 7719–7723 (1993).
Article CAS PubMed PubMed Central Google Scholar - Re, C. et al. Effect of antibody to HIV-1 Tat protein on viral replication in vitro and progression of HIV-1 disease in vivo. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10, 408–416 (1995).
Article CAS PubMed Google Scholar - Zagury, J.F. et al. Antibodies to the HIV-1 Tat protein correlated with nonprogression to AIDS: a rationale for the use of Tat toxoid as an HIV-1 vaccine. J. Hum. Virol. 4, 282–292 (1998).
Google Scholar - Venet, A., Bourgault, I., Aubertin, A.M., Kiény, M.P. & Levy, J.P. Cytotoxic T lymphocyte response against multiple simian immunodeficiency virus (SIV) proteins in SIV-infected macaques. J. Immunol. 148, 2899–2908 (1992).
CAS PubMed Google Scholar - Van Baalen, C.A. et al. HIV-1 Rev and Tat specific cytotoxic T lymphocyte frequencies inversely correlate with rapid progression to AIDS. J. Gen. Virol . 78, 1913–1918 ( 1997).
Article CAS PubMed Google Scholar - Froebel, K.S. et al. Cytotoxic T lymphocyte activity in children infected with HIV. AIDS Res. Hum. Retrovir. 2, 83- 88 (1994).
Google Scholar - Fawell, S. et al. Tat-mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. USA 91, 664– 668 (1994).
Article CAS PubMed PubMed Central Google Scholar - Kim, D.T. et al. Introduction of soluble proteins into the MHC class I pathway by conjugation to an HIV tat peptide. J. Immunol. 159 , 1666–1668 (1997).
CAS PubMed Google Scholar - Human Retroviruses and AIDS 1995. A Compilation and Analysis of Nucleic Acids and Amino Acid Sequences (eds. Korber, B. et al.) II-A-55, 56 (Theoretical Biology and Biophysics, Los Alamos National Laboratory, Los Alamos, New Mexico, 1995).
- Goldstein, G. HIV-1 Tat protein as a potential AIDS vaccine. Nature Med. 1, 960–964 (1996).
Article Google Scholar - Davis, D. et al. A recombinant prime, peptide boost vaccination strategy can focus the immune response on to more than one epitope even though these may not be immunodominant in the complex immunogen. Vaccine 15, 1661–1669 (1997).
Article CAS PubMed Google Scholar - Perez, C. et al. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell 63, 251–258 (1990).
Article CAS PubMed Google Scholar - Jassoy, C. et al. Human immunodeficiency virus type 1-specific cytotoxic T lymphocytes release gamma interferon, tumor necrosis factor alpha (TNFα), and TNF-β when they encounter their target antigens. J. Virol. 67, 2844–2852 (1993).
CAS PubMed PubMed Central Google Scholar - Ratner, A. & Clark, W.R. Role of TNF-α in CD8+ cytotoxic T lymphocyte-mediated lysis. J. Immunol. 150, 4303–4314 (1993).
CAS PubMed Google Scholar - Ando, K. et al. Perforin, Fas/Fas ligand, and TNF-α pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J. Immunol. 158, 5283– 5291 (1997).
CAS PubMed Google Scholar - Reimann, K.A. et al. A chimeric simian/human immunodeficiency virus expressing a primary patients human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70, 6922–6928 (1996).
CAS PubMed PubMed Central Google Scholar - Lamhamedi-Cherradi, S. _et al._Qualitative and quantitative analysis of human cytotoxic T-lymphocyte responses to HIV-1 proteins. AIDS 6, 1249 –1258 (1992).
Article CAS PubMed Google Scholar - Rinaldo, C.R. et al. Anti-HIV type 1 cytotoxic T lymphocyte effector activity and disease progression in the first 8 years of HIV type 1 infection of homosexual men. AIDS Res. Hum. Retrovir. 11, 481– 489 (1995).
Article PubMed Google Scholar - Ogg, G.S. et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279, 2103– 2106 (1998).
Article CAS PubMed Google Scholar - Buonaguro, L. et al. Heteroduplex mobility assay and phylogenetic analysis of V3 region sequences of human immunodeficiency virus type 1 isolates from Gulu, northern Uganda. The Italian-Ugandan Cooperation AIDS Program. J. Virol. 69, 7971–7981 (1995).
CAS PubMed PubMed Central Google Scholar - Calarota, S. et al. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet 351, 1320 –1325 (1998).
Article CAS PubMed Google Scholar - Gringeri, A. et al. Safety and Immunogenicity of HIV-1 Tat Toxoid in Immunocompromised HIV-1 Infected Patients. J. Hum. Virol. 1, 293–298 (1998).
CAS PubMed Google Scholar - Caselli, E. et al. DNA Immunization with HIV-1 tat Mutated in the Transactivation Domain Induces Humoral and Cellular Immune Responses against Wild-Type TAT. J. Immunol. (in the press).
- Chen, Z.W. et al. Cytotoxic T lymphocytes do not appear to select for mutations in an immunodominant epitope of simian immunodeficiency J. Immunol. 149, 4060–4066 ( 1992).
CAS PubMed Google Scholar - Lövgren, J. & Blomberg, K. Simultaneous measurement of NK cell cytotoxicity against two target cell lines labelled with fluorescent lanthanide chelates J. Immunol. Methods 173, 119–125 (1994).
Article PubMed Google Scholar - Sodora, D.L., Lee, F., Dailey, P.J. & Marx, P.A. A genetic and viral load analysis of the simian immunodeficiency virus during the acute phase in macaques inoculated by the vaginal route. AIDS Res. Hum. Retroviruses 14, 171–181 ( 1998).
Article CAS PubMed Google Scholar - Saiki, R.K. et al. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230, 1350–1354 ( 1985).
Article CAS PubMed Google Scholar - Fiore, J.R. et al. Pokeweed mitogen-stimulated peripheral blood mononuclear cells from at-risk seronegative subjects produce in vitro HIV-1-specific antibodies. AIDS 5, 1034– 1036 (1991).
CAS PubMed Google Scholar
Acknowledgements
We thank all personnel responsible of the animal facility; C. Sgadari, D. Negri, I. Macchia, Z. Michelini, M. Barbisin, E. Salvi and C. Rovetto (Laboratory of Virology, Istituto Superiore di Sanità, Rome, Italy) for technical help; P. Markham and B.C. Nair (Advanced BioScience laboratories, Kensington, Maryland) for Tat protein production; A. Lippa and F. M. Regini for editorial assistance; and G. Benagiano (Director General, ISS, Rome, Italy) for his support to the study. This work was supported by Italian grants from the ISS, Rome, Italy, IX AIDS Project and from the Associazione Nazionale per la Lotta contro l'AIDS (ANLAIDS).
Author information
Authors and Affiliations
- Laboratory of Virology, Istituto Superiore di Sanità , 00161, Rome, Italy
Aurelio Cafaro, Claudio Fracasso, Maria T. Maggiorella, Delia Goletti, Silvia Baroncelli, Monica Pace, Leonardo Sernicola, Martin L. Koanga-Mogtomo, Alessandra Borsetti, Roberto Belli, Franco Corrias, Stefano Buttò, Paola Verani, Fausto Titti & Barbara Ensoli - Department of Experimental and Diagnostic Medicine University of Ferrara, 44100, Ferrara, Italy
Antonella Caputo & Monica Betti - Department of Virology, The National Veterinary Institute Biomedical Centre, S-751 83, Uppsala, Sweden
Lennart Åkerblom - Department of Virology, Biomedical Primate Research Centre, Rijswijk ZH 2288, GJ, The Netherlands
Jonathan Heeney
Authors
- Aurelio Cafaro
You can also search for this author inPubMed Google Scholar - Antonella Caputo
You can also search for this author inPubMed Google Scholar - Claudio Fracasso
You can also search for this author inPubMed Google Scholar - Maria T. Maggiorella
You can also search for this author inPubMed Google Scholar - Delia Goletti
You can also search for this author inPubMed Google Scholar - Silvia Baroncelli
You can also search for this author inPubMed Google Scholar - Monica Pace
You can also search for this author inPubMed Google Scholar - Leonardo Sernicola
You can also search for this author inPubMed Google Scholar - Martin L. Koanga-Mogtomo
You can also search for this author inPubMed Google Scholar - Monica Betti
You can also search for this author inPubMed Google Scholar - Alessandra Borsetti
You can also search for this author inPubMed Google Scholar - Roberto Belli
You can also search for this author inPubMed Google Scholar - Lennart Åkerblom
You can also search for this author inPubMed Google Scholar - Franco Corrias
You can also search for this author inPubMed Google Scholar - Stefano Buttò
You can also search for this author inPubMed Google Scholar - Jonathan Heeney
You can also search for this author inPubMed Google Scholar - Paola Verani
You can also search for this author inPubMed Google Scholar - Fausto Titti
You can also search for this author inPubMed Google Scholar - Barbara Ensoli
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toBarbara Ensoli.
Rights and permissions
About this article
Cite this article
Cafaro, A., Caputo, A., Fracasso, C. et al. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine.Nat Med 5, 643–650 (1999). https://doi.org/10.1038/9488
- Received: 04 March 1999
- Accepted: 25 March 1999
- Issue Date: June 1999
- DOI: https://doi.org/10.1038/9488