Prediction of vascular invasion in hepatocellular carcinoma by next-generation des-r-carboxy prothrombin (original) (raw)
Main
Des-r-carboxy prothrombin (DCP), also known as protein-induced vitamin K absence or antagonist-II (PIVKA-II), is known to be a biomarker for hepatocellular carcinoma (HCC), with a sensitivity of 40–56% and a specificity of 81–98% (Suehiro et al, 1994; Imamura et al, 1999; Koike et al, 2001; Marrero et al, 2003). Several studies have demonstrated that the DCP value better reflects the malignant potential of HCC, associated with conditions such as vascular invasion or intrahepatic metastasis than alpha-fetoprotein (AFP) (Adachi et al, 1996; Imamura et al, 1999; Koike et al, 2001; Shirabe et al, 2007; Hirokawa et al, 2014). However, conventional DCP has not become a popular marker, because it is difficult to accurately quantify in the presence of vitamin K deficiency, the use of anticoagulants, or poor nutritional status associated with alcoholic abuse or jaundice (Toyoda et al, 2012; Nanashima et al, 2013; Tameda et al, 2013; Tanaka et al, 2013). The diagnostic accuracy of AFP alone is also unsatisfactory and ranges from 41% to 51%. The AFP value reflects the degree of chronic hepatitis and cirrhosis (Hirokawa et al, 2014). These two markers have different implications in the diagnosis of HCC, and the sensitivity increases to 67–89% when both markers are used (Suehiro et al, 1994; Adachi et al, 1996; Imamura et al, 1999; Koike et al, 2001; Marrero et al, 2003; Shirabe et al, 2007).
The local recurrence of HCC depends on tumour diameter, vascular invasion, and intrahepatic metastasis via the portal vein (Adachi et al, 1996; Koike et al, 2001; Shirabe et al, 2007). Thus identifying which patients have vascular invasion before determining treatment would be ideal. However, pathological vascular invasion can rarely be diagnosed on imaging studies alone.
Next-generation DCP (NX-DCP) was created to improve the disadvantage of having to use two different antibodies (P-11 and P-16) (Toyoda et al, 2012; Tameda et al, 2013; Tanaka et al, 2013). The diagnostic accuracy of NX-DCP for HCC associated with various conditions has been reported recently (Marrero et al, 2003; Toyoda et al, 2012; Hirano et al, 2013; Miyahara et al, 2013; Nanashima et al, 2013; Takeji et al, 2013; Tameda et al, 2013; Tanaka et al, 2013). To date, however, no study has focussed on the relation between vascular invasion and NX-DCP. The present study was designed to assess the predictive value of NX-DCP for vascular invasion in patients with HCC.
Patients and methods
Patients
Between May 2012 and May 2013, data were collected for 102 consecutive patients who underwent liver resection for HCC in Nihon University Itabashi Hospital. To objectively assess the predictive powers of conventional DCP and NX-DCP, we excluded six patients in whom liver resection was contraindicated by poor functional reserve (_n_=4) or poor nutritional status (_n_=2), three patients with intrahepatic cholestasis (_n_=3), and one patient with a recent history of receiving intravenous cephem antibiotics (_n_=1) before operation. Ten patients were excluded because they had a pathologically confirmed diagnosis of cholangiocellular carcinoma (_n_=2), obstructive jaundice (_n_=2), were receiving warfarin (_n_=1) or had liver adenoma (_n_=1), liver yolk sac tumour (_n_=1), or unresectable tumour at operation (_n_=3). The remaining 82 patients were studied. Vascular invasion was pathologically confirmed in 21 patients (positive group) and absent in 61 patients (negative group).
Blood samples (6 ml) were obtained from the patients under general anaesthesia before operation. NX-DCP in the patients’ concentrated sera was quantified with the use of a Sandwich Electrochemiluminescence Immunoassay Kit (EIDIA Co., Ltd., Tokyo, Japan), employing two independent, novel anti-human DCP monoclonal antibodies, P11 and P16 (Toyoda et al, 2012; Nanashima et al, 2013). Informed consent was obtained from all patients, and the scientific committee in our hospital approved this study.
Surgical procedures
The indications and procedures for liver resection were selected in accordance with Makuuchi’s criteria for hepatic functional reserve (Makuuchi et al, 1993). Nearly all liver transections were performed with intermittent clamping of the hepatoduodenal pedicle (Pringle’s manoeuvre) for 15 min, followed by release for 5 min. Before liver transection, intraoperative ultrasonography was performed in all patients to detect vascular invasion around HCC. The perioperative management procedures in our hospital have been described previously (Yamazaki et al, 2011, 2012a, b).
Histopathological study
All resected specimens were cut into 10-mm-thick slices. If visible vessels were found in the specimens, they were sliced longitudinally. The sliced specimens were fixed in 10% formalin. A pathologist examined the fixed specimens. All possible metastases and sites of vascular invasion were trimmed for paraffin blocks. Then 5-_μ_m-thick slices of microscopic sections were stained with haematoxylin and eosin. Glisson’s sheath was found near the tumour or was difficult to discriminate because of vascular invasion, and all specimens were additionally stained with elastica van Gieson stain. Vascular invasion was defined by the presence of clusters of cancer cells linked by endothelial cells in the vascular space. If the tumour invaded Glisson’s sheath and the structure was partially destroyed by cancer cells, some of the serial microscopic sections were stained.
Statistical analysis
Continuous variables were compared using Student’s _t_-test or the Mann–Whitney _U_-test. Multiple comparisons were made by repeated-measure analysis of variance. The cutoff values and correlation coefficients of each variable were obtained from a receiver operating characteristic (ROC) curve. _P_-values of <0.05 were considered to indicate statistical significance. All analyses were performed using the JMP 9.0 software (SAS, Chicago, IL, USA).
Results
Patients
There was no significant difference in gender (_P_=0.72), age (_P_=0.31), or the number of tumours (_P_=0.50) between the positive group and the negative group (Table 1). The tumour diameter was significantly larger in the positive group than in the negative group (45 mm (11–165) vs 20 mm (9–65), respectively; P<0.0001). The liver functional reserve before operation did not differ significantly between the groups; however, the platelet count differed significantly (16.4 mm4 dl−1 (5.8–33.9) vs 11.9 mm4 dl−1 (4.2–23.9), respectively; P<0.0001). Pathological liver cirrhosis was seen in a higher proportion of patients in the negative group than in the positive group (25 patients (41.0%) vs 4 patients (19.1%), respectively; _P_=0.07), although the difference did not reach statistical significance. The proportions of patients with different histological types of HCC did not differ significantly between the groups.
Table 1 Patients’ characteristics
The NX-DCP level (510 mAU ml−1 (10–98 450) vs 34.0 mAU ml−1 (12–541), respectively; P<0.0001) and conventional DCP level (250 mAU ml−1 (17–18 790) vs 31.0 mAU ml−1 (16–813), respectively; P<0.0001) were significantly higher in the positive group than in the negative group (Table 2). In contrast, the AFP value did not differ significantly between the two groups and did not reflect vascular invasion (9.7 ng ml−1 (1.6–43,960.0) vs 11.0 ng ml−1 (1.6–1650), respectively; _P_=0.49). There was also no significant difference in the vascular endothelial growth factor (VEGF) value (20.3 (7.81–91.1) vs 24.5 (7.8–150.8), respectively; _P_=0.63) or the VEGF receptor value (135.0 (5.8–474.2) vs 138.8 (79.6–540.4), respectively; _P_=0.92).
Table 2 Biomarkers for detecting pathological vascular invasion
Relation between tumour diameter and vascular invasion
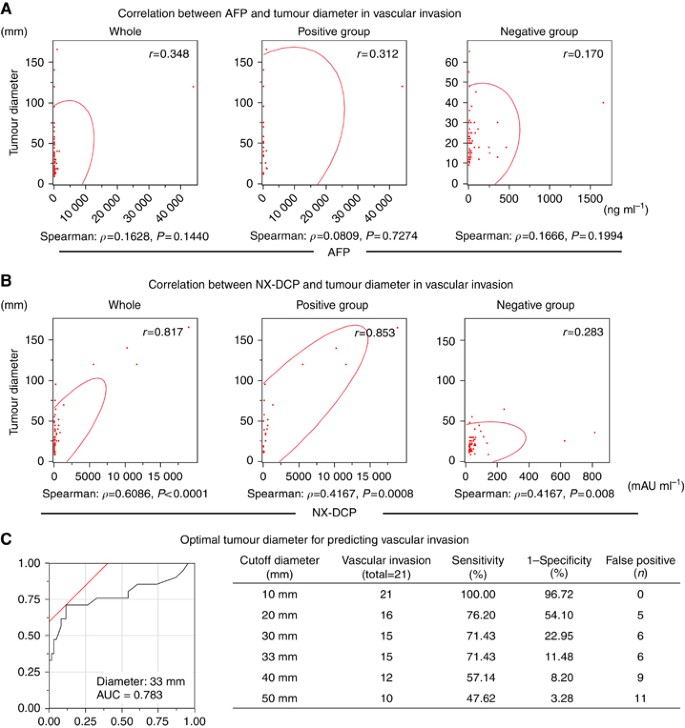
The AFP value was unrelated to tumour diameter in the study group as a whole (_r_=0.348). There was also no relation between the AFP value and tumour diameter in the positive (_r_=0.312) or negative group (_r_=0.170) (Figure 1A). In contrast, the NX-DCP value strongly correlated with tumour diameter in the study group as a whole (_r_=0.817), as well as in the positive group (Figure 1B). The correlation was stronger in the vascular invasion positive group (_r_=0.853) than in the negative group (_r_=0.283). In the positive group, the ROC curve analysis revealed that the optimal tumour cutoff diameter for predicting pathological vascular invasion was 33 mm (AUC 0.783, sensitivity=71.43%, 1−specificity (false-positive rate)=11.48%) (Figure 1C).
Figure 1
Correlation between tumour markers and tumour diameter. (A) The AFP value poorly correlated with tumour diameter (_r_=0.348) in the study group as a whole. Moreover, this trend was unchanged in patients positive (_r_=0.312) or negative (_r_=0.170) for vascular invasion. (B) A strong correlation was observed between tumour diameter and the NX-DCP value (_r_=0.817). This trend was more evident in the vascular invasion positive group (_r_=0.853) while the correlation was weak in the negative group (_r_=0.283). (C) The ROC curve analysis revealed that the optimal tumour cutoff diameter for predicting pathological vascular invasion was 33 mm (AUC 0.783, sensitivity=71.43%, 1−specificity=11.48%).
Correlation between DCP and AFP in vascular invasion
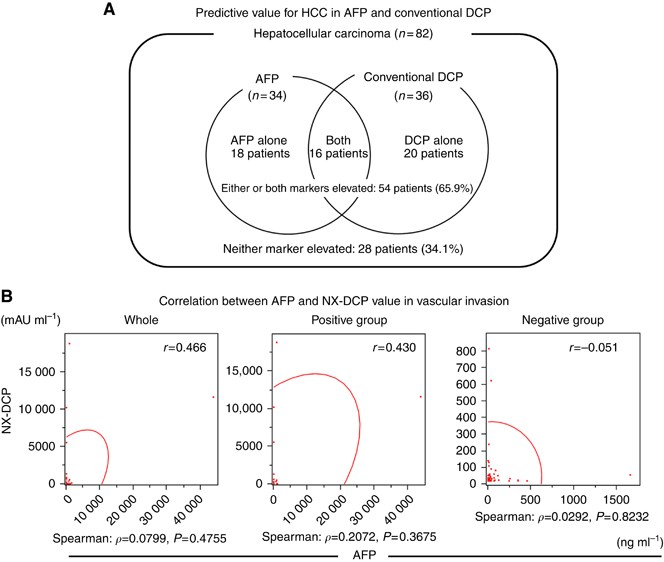
Among the 82 patients, the serum conventional DCP level was above the upper limit of normal in 36 patients (43.9%), the AFP value was above the upper limit of normal in 34 patients (41.4%), and both markers were elevated in 16 patients (19.5%). The predictive value for HCC when either or both markers were elevated was 65.9% (54 out of 82 patients; Figure 2A).
Figure 2
Independence between AFP and DCP. (A) Among a total 82 patients, the serum AFP level was above the normal limit in 34 patients (41.5%), conventional DCP was above the normal limit in 36 patients (43.9%), and either or both of these markers were elevated in 54 patients (65.9%). Neither marker was elevated in 28 patients (34.1%). (B) The correlation coefficient between AFP and NX-DCP was _r_=0.466 in the study group as a whole. The correlation coefficient was _r_=0.430 in the vascular invasion positive group and _r_=−0.051 in the negative group.
Next, as for vascular invasion, the correlation coefficient between AFP and NX-DCP was _r_=0.466 in the study group as a whole and _r_=0.430 in the vascular invasion positive group. (Figure 2B) In contrast, the correlation was weaker in the negative group (_r_=−0.051).
Diagnostic power of each biomarker for vascular invasion
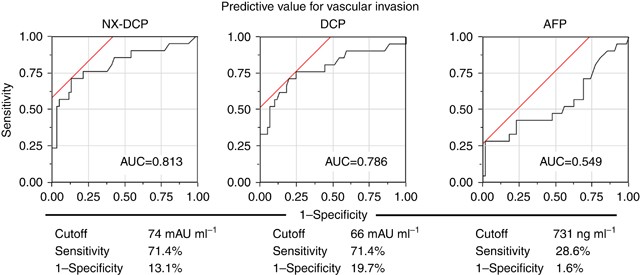
The results of ROC analysis performed to compare the predictive powers of NX-DCP, conventional DCP, and AFP for vascular invasion are shown in Figure 3. The AUC of NX-DCP was the largest (AUC=0.813), with a sensitivity of 71.4% and a 1−specificity of 13.1% at the cutoff value of 74 mAU ml−1. Conventional DCP had an AUC of 0.786, a sensitivity of 71.4%, and a 1−specificity of 19.7% at the cutoff value of 66 mAU ml−1 (normal limit: 40 mAU ml−1). In contrast, the AUC of AFP was the smallest (AUC=0.550, sensitivity=28.6%, 1−specificity=1.60%), and the cutoff value was very high (731 ng ml−1).
Figure 3
Predictive value of each biomarker for vascular invasion. The area under the curve (AUC) of NX-DCP was 0.813, with a sensitivity of 71.4% and a 1−specificity of 13.1% at the cutoff value of 74 mAU ml−1. The AUC of conventional DCP was 0.786 (sensitivity=71.4%, 1−specificity=19.7%, cutoff value: 66 mAU ml−1). The AUC of AFP was 0.550 (sensitivity=28.6%, 1−specificity=1.60%, cutoff value: 731 ng ml−1).
When the cutoff value for NX-DCP obtained on ROC analysis was used to predict the presence of vascular invasion, vascular invasion was diagnosed in 15 patients (71.43%) in the positive group (_n_=21). The diagnostic powers of conventional DCP (12 patients, 57.14%) and of AFP (9 patients, 42.86%), using the respective upper limits of normal as the cutoff values, were lower than that of NX-DCP (Table 3).
Table 3 Diagnostic power of each biomarker in the vascular invasion group (_n_=22)
Discussion
Our study showed that the NX-DCP level might be an excellent tumour marker for HCC. The NX-DCP level predicted pathological vascular invasion with strong accuracy, associated with an AUC of 0.813, a sensitivity of 71.4%, and a 1−specificity of 13.1%.
Small HCC can now be detected owing to recent advancements in imaging modalities. However, it is often difficult to preoperatively detect vascular invasion on radiological studies alone (Trevisani et al, 2004; Takayama et al, 2008). Vascular invasion, along with tumour diameter, is known to be an independent predictor of early local recurrence (Adachi et al, 1996; Imamura et al, 1999; Shirabe et al, 2007). The conventional DCP level has been demonstrated to be predictive of vascular invasion. A recent gene-expression profiling study revealed that HCC with microvascular invasion could be divided into invasive and highly invasive phenotypes associated with two distinct gene-expression profiles (Tanaka et al, 2010). Clinically, a high DCP level is closely related to histological vascular invasion and is therefore an independent predictor of outcomes in liver transplantation (Shimada et al, 2005; Iguchi et al, 2015). Moreover, patients with high DCP levels should be alerted to the high risk of vascular invasion and are not suitable candidates for radiofrequency ablation (RFA; Asaoka et al, 2014). However, conventional DCP levels often cannot be accurately measured in patients with impaired activity of vitamin K and are thus unreliable (Marrero et al, 2003; Takeji et al, 2013). These studies showed that the treatment strategy for HCC associated with high biological malignancy owing to the presence of factors such as vascular invasion should be carefully considered before initiating therapy. Therefore, we believe that the NX-DCP level will most likely have an important role in treatment planning for HCC in future.
The AFP level has also been shown to reflect tumour differentiation and the degrees of hepatitis and cirrhosis. In contrast, the conventional DCP level is considered to reflect tumour invasiveness, intrahepatic metastasis, and vascular invasion (Adachi et al, 1996; Koike et al, 2001; Marrero et al, 2003; Shirabe et al, 2007; Hirokawa et al, 2014). Our study found no relation between AFP and DCP. The sensitivity of NX-DCP for the detection of vascular invasion was higher than that of AFP. This trend tended to be stronger in patients who had vascular invasion. The antibodies used to measure NX-DCP (P-11 and P-16) are closely related to vascular invasion, similar to the MU-3 antibodies used to measure conventional DCP. Available evidence suggests that some cases of HCC with malignant potential can be detected by NX-DCP before operation. Such screening power might contribute to the planning of treatment strategies for HCC in future.
A positive surgical margin, vascular invasion, and a large tumour size have been previously demonstrated to be poor prognostic factors. Vascular invasion directly reflects the high invasiveness of HCC. Thus anatomic resection or wide RFA was performed when vascular invasion was anticipated (Machi et al, 2001; Groeschl et al, 2013). However, the detection rate of vascular invasion on imaging studies performed before liver resection was only 10.5% as compared with a pathological vascular invasion rate of 25.6% (21 out of 82 patients) in the present study. Moreover, our study also showed that the relation between the tumour diameter and tumour marker value was more specific for NX-DCP than for AFP. Therefore, assessment of AFP alone is insufficient for the diagnosis and treatment of HCC.
There have been six preliminary reports on NX-DCP (Makuuchi et al, 1993; Yamazaki et al, 2011; Toyoda et al, 2012; Nanashima et al, 2013; Tameda et al, 2013; Tanaka et al, 2013). Our study confirmed the sensitivity and prognostic value of HCC and the reliability in any condition, such as in patients receiving warfarin therapy. Some groups have advocated that the DCP/NX-DCP ratio (i.e., conventional DCP divided by NX-DCP) is more specific for HCC (Toyoda et al, 2012; Nanashima et al, 2013; Tameda et al, 2013; Tanaka et al, 2013). However, the results of our study indicate that the NX-DCP value itself is satisfactory for the diagnosis of HCC and vascular invasion. Moreover, measuring two DCP markers to determine the ratio was too complex for routine clinical use. Therefore, the NX-DCP value alone combined with AFP may be adequate in patients with HCC.
In conclusion, the diagnostic power of NX-DCP combined with AFP is mandatory in HCC. Our study revealed that NX-DCP is a more specific tumour marker than DCP for predicting the presence of vascular invasion associated with HCC. The NX-DCP value facilitates the planning of adequate treatment strategies for HCC, including liver resection and RFA. We therefore recommend NX-DCP with AFP as the clinical standard for the diagnosis of HCC.
Change history
12 January 2016
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
- Adachi E, Maeda T, Kajiyama K, Kinukawa N, Matsumata T, Sugimachi K, Tsuneyoshi M (1996) Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivariate analyses of 232 resected cases without preoperative treatments. Cancer 77: 2022–2031.
Article CAS Google Scholar - Asaoka Y, Tateishi R, Nakagomi R, Kondo M, Fujiwara N, Minami T, Sato M, Uchino K, Enooku K, Nakagawa H, Kondo Y, Shiina S, Yoshida H, Koike K (2014) Frequency of and predictive factors for vascular invasion after radiofrequency ablation for hepatocellular carcinoma. PLoS One 9: e111662.
Article Google Scholar - Groeschl RT, Gamblin TC, Turaga KK (2013) Ablation for hepatocellular carcinoma: validating the 3-cm breakpoint. Ann Surg Oncol 20: 3591–3595.
Article Google Scholar - Hirano H, Saito M, Seo Y, Yano Y, Azuma T (2013) NX-DCP as a novel biomarker would be related to liver function in cirrhotic patients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol 25: 748–749.
Article Google Scholar - Hirokawa F, Hayashi M, Miyamoto Y, Asakuma M, Shimizu T, Komeda K, Inoue Y, Uchiyama K (2014) Outcomes and predictors of microvascular invasion of solitary hepatocellular carcinoma. Hepatol Res 44: 846–853.
Article CAS Google Scholar - Iguchi T, Shirabe K, Aishima S, Wang H, Fujita N, Ninomiya M, Yamashita Y, Ikegami T, Uchiyama H, Yoshizumi T, Oda Y, Maehara Y (2015) New pathologic stratification of microvascular invasion in hepatocellular carcinoma: predicting prognosis after living-donor liver transplantation. Transplantation 99: 1236–1242.
Article CAS Google Scholar - Imamura H, Matsuyama Y, Miyagawa Y, Ishida K, Shimada R, Miyagawa S, Makuuchi M, Kawasaki S (1999) Prognostic significance of anatomical resection and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma. Br J Surg 86: 1032–1038.
Article CAS Google Scholar - Koike Y, Shiratori Y, Sato S, Obi S, Teratani T, Imamura M, Yoshida H, Shiina S, Omata M (2001) Des-gamma-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: a prospective analysis of 227 patients. Cancer 91: 561–569.
Article CAS Google Scholar - Machi J, Uchida S, Sumida K, Limm WM, Hundahl SA, Oishi AJ, Furumoto NL, Oishi RH (2001) Utrasound-guided radiofrequency thermal ablation of liver tumors: percutaneous, laparoscopic, and open surgical approaches. J Gastrointest Surg 5: 477–489.
Article CAS Google Scholar - Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S (1993) Surgery for small liver cancers. Semin Surg Oncol 9: 298–304.
Article CAS Google Scholar - Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, Lok AS (2003) Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients. Hepatology 37: 1114–1121.
Article CAS Google Scholar - Miyahara K, Nouso K, Morimoto Y, Tomoda T, Kobayashi S, Takeuchi Y, Hagihara H, Kuwaki K, Ohnishi H, Ikeda F, Miyake Y, Nakamura S, Shiraha H, Takaki A, Yamamoto K Okayama Liver Cancer Group (2013) Evaluation of the effect of sorafenib using serum NX-des-γ-carboxyprothrombin in patients with hepatocellular carcinoma. Hepatol Res 43: 1064–1070.
CAS PubMed Google Scholar - Nanashima A, Abo T, Taura N, Shibata H, Ichikawa T, Takagi K, Arai J, Oyama S, Nagayasu T (2013) NX-PVKA levels before and after hepatectomy of hepatocellular carcinoma as predictors of patient survival: a preliminary evaluation of an improved assay for PIVKA-II. Anticancer Res 33: 2689–2698.
CAS PubMed Google Scholar - Shimada M, Yonemura Y, Ijichi H, Harada N, Shiotani S, Ninomiya M, Terashi T, Yoshizumi T, Soejima Y, Maehara Y (2005) Living donor liver transplantation for hepatocellular carcinoma: a special reference to a preoperative des-gamma-carboxy prothrombin value. Transplant Proc 37: 1177–1179.
Article CAS Google Scholar - Shirabe K, Itoh S, Yoshizumi T, Soejima Y, Taketomi A, Aishima S, Maehara Y (2007) The predictors of microvascular invasion in candidates for liver transplantation with hepatocellular carcinoma-with special reference to the serum levels of des-gamma-carboxy prothrombin. J Surg Oncol 95: 235–240.
Article CAS Google Scholar - Suehiro T, Sugimachi K, Matsumata T, Itasaka H, Taketomi A, Maeda T (1994) Protein induced by vitamin K absence or antagonist II as a prognostic marker in hepatocellular carcinoma. Comparison with alpha-fetoprotein. Cancer 73: 2464–2471.
Article CAS Google Scholar - Takayama T, Makuuchi M, Kojiro M, Lauwers GY, Adams RB, Wilson SR, Jang HJ, Charnsangavej C, Taouli B (2008) Early hepatocellular carcinoma: pathology, imaging, and therapy. Ann Surg Oncol 15: 972–978.
Article Google Scholar - Takeji S, Hirooka M, Koizumi Y, Tokumoto Y, Abe M, Ikeda Y, Nadano S, Hiasa Y, Onji M (2013) Des-gamma-carboxy prothrombin identified by P-11 and P-16 antibodies reflects prognosis for patients with hepatocellular carcinoma. J Gastroenterol Hepatol 28: 671–677.
Article CAS Google Scholar - Tameda M, Shiraki K, Sugimoto K, Ogura S, Inagaki Y, Yamamoto N, Ikejiri M, Takei Y, Ito M, Nobori T (2013) Des-γ-carboxy prothrombin ratio measured by P-11 and P-16 antibodies is a novel biomarker for hepatocellular carcinoma. Cancer Sci 104: 725–731.
Article CAS Google Scholar - Tanaka S, Mogushi K, Yasen M, Noguchi N, Kudo A, Nakamura N, Ito K, Miki Y, Inazawa J, Tanaka H, Arii S (2010) Gene-expression phenotypes for vascular invasiveness of hepatocellular carcinomas. Surgery 147: 405–414.
Article Google Scholar - Tanaka T, Taniguchi T, Sannomiya K, Takenaka H, Tomonari T, Okamoto K, Kitamura S, Okahisa T, Tamaki K, Mikasa H, Suzuki S, Takayama T (2013) Novel des-γ-carboxy prothrombin in serum for the diagnosis of hepatocellular carcinoma. J Gastroenterol Hepatol 28: 1348–1355.
Article CAS Google Scholar - Toyoda H, Kumada T, Osaki Y, Tada T, Kaneoka Y, Maeda A (2012) Novel method to measure serum levels of des-gamma-carboxy prothrombin for hepatocellular carcinoma in patients taking warfarin: a preliminary report. Cancer Sci 103: 921–925.
Article CAS Google Scholar - Trevisani F, Cantarini MC, Labate AM, De Notariis S, Rapaccini G, Farinati F, Del Poggio P, Di Nolfo MA, Benvegnù L, Zoli M, Borzio F, Bernardi M Italian Liver Cancer (ITALICA) group (2004) Surveillance for hepatocellular carcinoma in elderly Italian patients with cirrhosis: effects on cancer staging and patient survival. Am J Gastroenterol 99: 1470–1476.
Article Google Scholar - Yamazaki S, Takayama T, Kimura Y, Moriguchi M, Higaki T, Nakayama H, Fujii M, Makuuchi M (2011) Transfusion criteria for fresh frozen plasma in liver resection: a 3+3 cohort expansion study. Arch Surg 146: 1293–1299.
Article CAS Google Scholar - Yamazaki S, Takayama T, Moriguchi M, Mitsuka Y, Okada S, Midorikawa Y, Nakayama H, Higaki T (2012a) Criteria for drain removal following liver resection. Br J Surg 99: 1584–1590.
Article CAS Google Scholar - Yamazaki S, Takayama T, Moriguchi M, Okada S, Hayashi Y, Nakayama H, Higaki T, Sugitani M (2012b) Validation of biological and clinical outcome between with and without thoracotomy in liver resection: a matched cohort study. World J Surg 36: 144–150.
Article Google Scholar
Acknowledgements
Author contributions
TK and YM collected blood samples, SY conducted the study design and wrote the manuscript, MM supported statistical analysis, MS conducted pathological evaluation, and TT organised the study.
Author information
Authors and Affiliations
- Department of Digestive Surgery, Nihon University School of Medicine, 30-1 Ohyaguchikami-machi, Itabashi-ku, 173-8610, Tokyo, Japan
Tomoharu Kurokawa, Shintaro Yamazaki, Yusuke Mitsuka, Masamichi Moriguchi & Tadatoshi Takayama - Department of Pathology, Nihon University School of Medicine, 30-1 Ohyaguchikami-machi, Itabashi-ku, 173-8610, Tokyo, Japan
Masahiko Sugitani
Authors
- Tomoharu Kurokawa
You can also search for this author inPubMed Google Scholar - Shintaro Yamazaki
You can also search for this author inPubMed Google Scholar - Yusuke Mitsuka
You can also search for this author inPubMed Google Scholar - Masamichi Moriguchi
You can also search for this author inPubMed Google Scholar - Masahiko Sugitani
You can also search for this author inPubMed Google Scholar - Tadatoshi Takayama
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toShintaro Yamazaki.
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Kurokawa, T., Yamazaki, S., Mitsuka, Y. et al. Prediction of vascular invasion in hepatocellular carcinoma by next-generation des-r-carboxy prothrombin.Br J Cancer 114, 53–58 (2016). https://doi.org/10.1038/bjc.2015.423
- Revised: 27 August 2015
- Accepted: 13 October 2015
- Published: 17 December 2015
- Issue Date: 12 January 2016
- DOI: https://doi.org/10.1038/bjc.2015.423