Bid-induced release of AIF from mitochondria causes immediate neuronal cell death (original) (raw)
Main
Progressive degeneration and death of neurons are the major features of several acute and chronic neurodegenerative diseases such as ischemic stroke, Alzheimer's disease, or Parkinson's disease.1 The main mechanisms of neuronal cell death are, for example, disturbed calcium homeostasis, oxidative stress, breakdown of the mitochondrial membrane potential, and release of mitochondrial factors that initiate downstream apoptotic cell death programs.2 In particular, mitochondrial membrane permeabilization is considered as a critical step for the release of pro-apoptotic proteins such as cytochrome c, Smac/Diablo (second mitochondria-derived activator of caspase/direct IAP binding protein with low p_I_), HtrA2/Omi, apoptosis-inducing factor (AIF), or endonuclease G, which trigger caspase-dependent or caspase-independent mechanisms of DNA degradation and cell death.3, 4
An increasing number of recent studies provide evidence that AIF is a major factor for an alternative post-mitochondrial cell death pathway, for example, following hypoxia,5, 6 ischemia,7, 8 or excitotoxic lesions.9, 10 AIF is a 63 kDa flavoprotein located at the inner mitochondrial membrane that is released early after oxygen–glucose deprivation in vitro or cerebral ischemia in vivo.7 Using harlequin (Hq) mutant mice expressing low AIF levels and small interfering RNA (siRNA) approaches, we recently demonstrated a causal role of AIF in neuronal cell death in models of ischemic stroke in adult rodents8 and neonatal hypoxia.6 In these studies, we found that nuclear translocation of AIF correlated well with DNA damage and progression of infarct development. Furthermore, AIF downregulation in mutant mice and by AIF siRNA significantly reduced neuronal damage in models of ischemic stroke in vivo and in vitro, respectively.
Despite this prominent role of AIF in neuronal cell death signaling, upstream mechanisms of AIF release from mitochondria are poorly defined. Although previous studies from our and other laboratories had suggested a particular role of poly-(ADP-ribose)-polymerase-1 (PARP)8, 11, 12 and calpain-113 in the release of AIF from mitochondria, the immediate upstream mechanisms of mitochondrial AIF release and, in particular, the role of pro-apoptotic bcl-2 family members are not well understood. Our previous studies demonstrated a significant involvement of Bid (BH3-interacting domain death agonist), a pro-apoptotic bcl-2 family protein, in ischemic brain injury14 and a pronounced protective effect of pharmacological Bid inhibitors in models of neuronal cell death in vitro. Very similar neuroprotective effects were observed in mutant Hq mice or by AIF siRNA,8 suggesting a link between pro-apoptotic Bid activities and AIF release from mitochondria. In the present study, we therefore investigated the potential role of Bid in mitochondrial release of AIF during excitotoxic neuronal cell death in more detail.
Results
Bid gene silencing and Bid inhibition attenuate glutamate neurotoxicity
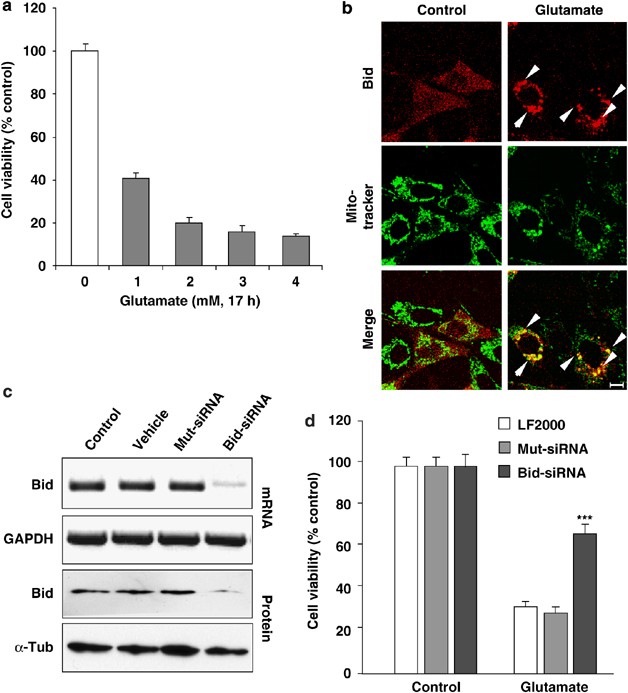
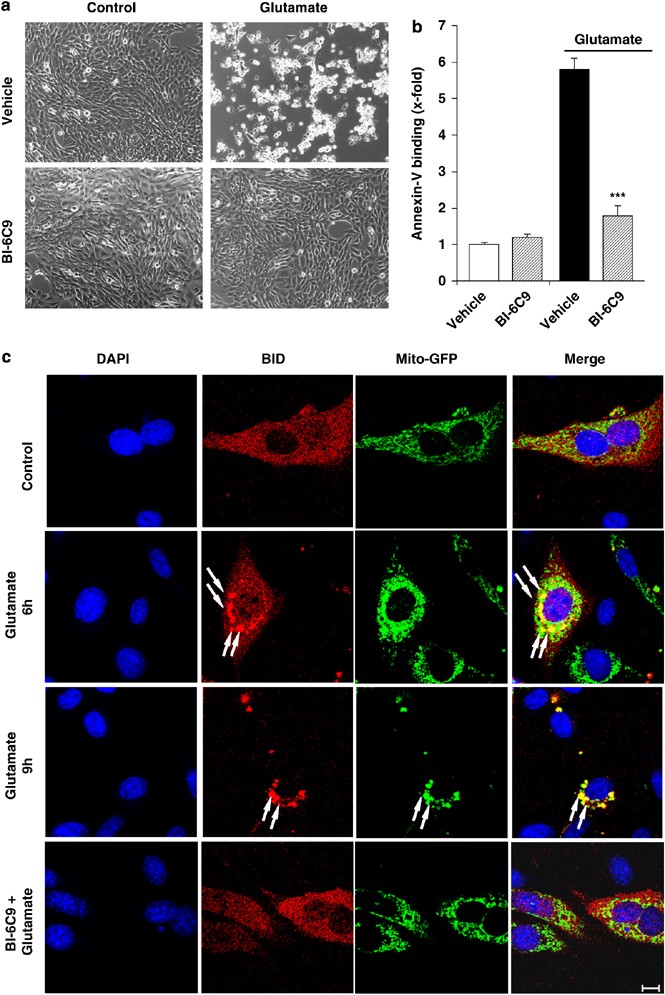
Significant (60–80%) glutamate-induced cell death occurs in immortalized mouse hippocampal neurons (HT-22) in a concentration-dependent manner (Figure 1a). Bid translocated to mitochondria within 5 h after the onset of glutamate challenge (Figure 1b). Bid siRNA specifically reduced Bid expression by >80% (Figure 1c) and significantly attenuated glutamate-induced cell death (Figure 1d). In addition to the siRNA approach, the specific Bid inhibitor BI-6C9 (Sigma, Taufkirchen, Germany)15, 16 was applied to confirm the essential role of Bid in glutamate-induced neuronal death. HT-22 neurons exposed to glutamate for 17 h show typical morphology of dying cells: the neuronal cells appear shrunken and rounded and detached from the culture dish (Figure 2a). HT-22 cells pretreated with the Bid inhibitor BI-6C9 preserved their normal spindle-shaped morphology and were completely rescued from glutamate-induced cell death (Figure 2a). Fluorescent-activated cell sorting (FACS) analysis of fluorescein isothiocyanate (FITC)-annexin-V-stained HT-22 neurons confirmed the pronounced protective effect of the Bid inhibitor against glutamate-induced cell death (Figure 2b). Bid inhibition-related neuroprotection was associated with a lack of Bid translocation to the mitochondria as shown in experiments using the pDsRed2-Bid vector and Mito-green fluorescent protein (GFP) co-transfections (Figure 2c) or Mitotracker Green staining (not shown), indicating that Bid translocation is essential for neuronal cell death. In addition, the Bid inhibitor prevented the perinuclear accumulation of mitochondria in glutamate-treated cells (Figure 2c).
Figure 1
Bid knockdown rescued HT-22 neurons from glutamate-induced apoptosis. (a) Glutamate reduces the viability of HT-22 neurons in a concentration-dependent manner as evaluated by the MTT assay 17 h after the onset of treatment. (b) Fluorescence photomicrographs of HT-22 neurons expressing a Bid-DsRed fusion protein show colocalization of MitoTracker Green and DsRed signal in glutamate-damaged (5 mM, 5 h) cells. Similar results were obtained in cells exposed to 3 mM glutamate. Note that the homogeneous distribution of Bid in control cells significantly changes after glutamate damage where Bid accumulates at mitochondria (yellow in merged panels). (c) RT-PCR analysis of Bid mRNA (upper panels) and western blot analysis of Bid protein (lower panels) in HT-22 cells pretreated with 20 nM Bid siRNA for 48 h. RT-PCR with primers specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and anti-_β_-actin antibodies used as controls for respective analyses is shown. (d) Bid siRNA significantly attenuated glutamate-induced cell death as determined by MTT assay. ***P<0.001 compared with glutamate-exposed (3 mM) HT-22 cells pretreated with nonfunctional Mut siRNA or vehicle (Lipofectamine 2000) (analysis of variance (ANOVA), Scheffé's)
Figure 2
The Bid inhibitor BI-6C9 protects HT-22 cells against glutamate-induced apoptosis. (a) Photomicrographs (× 10 objective) show morphological evidence for severe damage of HT-22 cells 17 h after glutamate (3 mM) exposure. Glutamate-treated cells lose their spindle-like morphology, shrink, and detach from the culture well bottom; in contrast, cells pretreated with the Bid inhibitor BI-6C9 (10 _μ_M) are fully protected against glutamate-induced death and are not different from the controls. (b) FACS analysis of HT-22 cells after FITC-annexin-V labeling to detect apoptotic cells. Exposure to glutamate (3 mM, 17 h) resulted in enhanced annexin-V binding of apoptotic HT-22 cells compared with controls. BI-6C9 (10 _μ_M, 1 h before damage) significantly reduced glutamate-induced apoptosis. ***P<0.001 compared with glutamate-treated cells (Student's _t_-test). (c) The Bid inhibitor prevents mitochondrial translocation of Bid after glutamate exposure. Fluorescence photomicrographs of HT-22 neurons expressing a Bid-DsRed fusion protein and Mito-GFP show colocalization of mitochondrial staining and DsRed signal in glutamate-damaged (5 mM, 6–9 h) cells. The Bid inhibitor BI-6C9 (10 _μ_M) prevented the accumulation of Bid at the mitochondria and retained the homogeneous distribution of Bid in the cytosol
Concomitant detection of propidium iodide (PI) revealed that less than 9.5±1.7% of the glutamate-treated cells were PI positive versus 5.4±0.6% in the controls, suggesting that most HT-22 cells exposed apoptotic and not necrotic features after glutamate exposure (data not shown). Strikingly, morphological analysis and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay revealed that the Bid inhibitor BI-6C9 significantly rescued HT-22 neurons even when applied up to 8 h after glutamate exposure, suggesting that Bid-mediated cell death depends on sustained Bid activation with a remarkable time window (Supplementary Figure 1).
The specificity of BI-6C9 in the presently used culture system was confirmed in HT-22 neurons transfected with a truncated Bid (tBid) expression vector. Expression of tBid in HT-22 neurons reduced cell viability by approximately 40%, whereas cells pretreated with the Bid inhibitor were significantly protected from tBid-induced cell death (Supplementary Figure 2). It is important to note that the percentage of cell death detected in tBid-transfected HT-22 neurons corresponds well with the transfection efficiency achieved with the current protocol for DNA vector transfection (45±3–51±1%), indicating that pharmacological inhibition of tBid translocation to the mitochondria rescued all transfected neurons.
Glutamate induces Bid-dependent mitochondrial depolarization
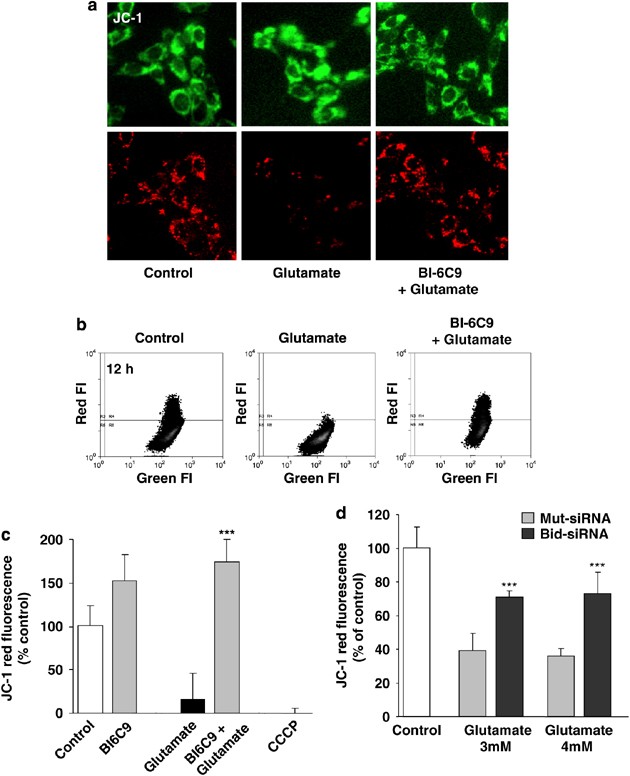
Glutamate treatment caused a significant loss of 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1) red fluorescence, that is, loss of mitochondrial membrane potential after glutamate treatment within 6–12 h (Figure 3). In parallel experiments, carbonyl cyanide _m_-chlorophenylhydrazone (CCCP) was used as a positive control to induce mitochondrial membrane depolarization (Figure 3c). The Bid inhibitor BI-6C9 significantly prevented glutamate-induced breakdown of the mitochondrial membrane potential, suggesting that the Bid inhibitor significantly prevented Bid translocation to the mitochondria and consequently prevented glutamate-induced breakdown of the mitochondrial membrane potential in HT-22 neurons (Figures 2c and 3). In addition, protection of the mitochondrial membrane potential was confirmed by Bid siRNA, which significantly attenuated the loss of JC-1 red fluorescence compared with nonfunctional Mut siRNA after the glutamate challenge (Figure 3d).
Figure 3
The Bid inhibitor BI-6C9 prevents glutamate-induced mitochondrial depolarization. (a) Mitochondrial membrane potential was analyzed by JC-1 fluorescence: the upper panels show epifluorescence photomicrographs indicating equal cellular uptake of JC-1 by green fluorescence, and the lower panels depict intact mitochondria exposing red fluorescence. Glutamate-treated (3 mM, 12 h) HT-22 cells show significantly reduced red fluorescence compared with controls, whereas BI-6C9 (10 _μ_M) prevents the breakdown of mitochondrial membrane potential as indicated by preservation of the red JC-1 fluorescence. (b, c) FACS analyses of _n_=4 independent experiments per group reveal a decrease of the red JC-1 fluorescence to 20% of control levels 12 h after glutamate treatment (3 mM), which is prevented by BI-6C9. CCCP was used as a positive control to induce a fast breakdown of the mitochondrial membrane potential. ***P<0.001 compared with controls and BI-6C9-treated cells (ANOVA, Scheffé's). (d) FACS analyses of _n_=3 independent experiments per group show a rescue of the red JC-1 fluorescence by Bid siRNA compared with nonfunctional Mut siRNA-treated HT-22 cells determined 6 h after the onset of glutamate damage. ***P<0.001 compared with Mut siRNA-treated cells (ANOVA, Scheffé's)
Caspase activation is not required for glutamate neurotoxicity
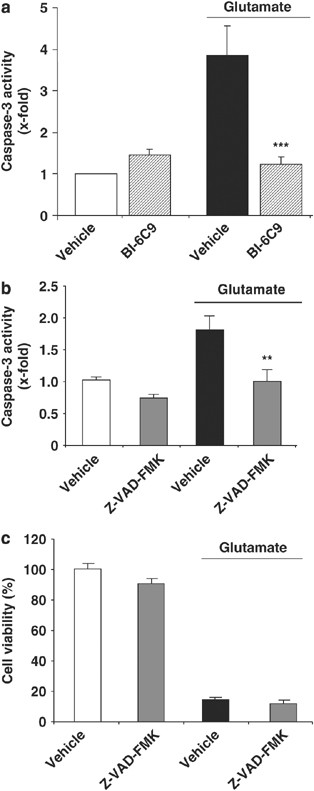
After Bid activation, subsequent mitochondrial membrane permeabilization may trigger both caspase-dependent and caspase-independent execution of cell death through the release of cytochrome c and apoptosome formation or AIF, respectively.2 Measurements of caspase-3 activity indeed revealed moderate caspase-3 activation after exposure of HT-22 neurons to glutamate and this activation was completely blocked by Bid inhibition (Figure 4a). Similar inhibition of caspase-3 activity was also achieved with the membrane-permeable general caspase inhibitor Z-VAD-FMK (Figure 4b); however, this treatment did not affect glutamate-induced cell death (Figure 4c). These data therefore suggest that caspase-3 activation occurs after glutamate exposure in HT-22 neurons but is not required for execution of the cell death program. It is interesting to note that respective inhibitors of caspase-8 (IETD-fmk, 10–50 _μ_M; Supplementary Figure 3), caspase-2 (Z-VDVAD, 5–50 _μ_M), calpain (calpastatin, 0.5–5 _μ_M after preincubation with equimolar amounts of activated penetratin-1), or cathepsins (E-64-d, 10–50 _μ_M) also did not prevent glutamate-induced cell death in HT-22 neurons. In addition, caspase-8 activity was not altered after glutamate challenge, suggesting that this prominent pathway of Bid activation was not involved in the present model system (not shown).
Figure 4
Caspase activation is not required in glutamate neurotoxicity. (a) Caspase-3 activity assay showed an increased caspase-3 activity after glutamate treatment (2 mM, 17 h) that was prevented by BI-6C9 (10 _μ_M). (b) Caspase-3 activation was prevented by the pan-caspase inhibitor Z-VAD-FMK (50 _μ_M) applied 1 h before glutamate exposure. (c) MTT assay revealed that Z-VAD-FMK (50 _μ_M) could not protect HT-22 neurons from glutamate-induced cell death. **P<0.01 and ***P<0.001 compared to vehicle-treated cells exposed to glutamate (ANOVA, Scheffé's test)
Bid-induced neurotoxicity is mediated by AIF
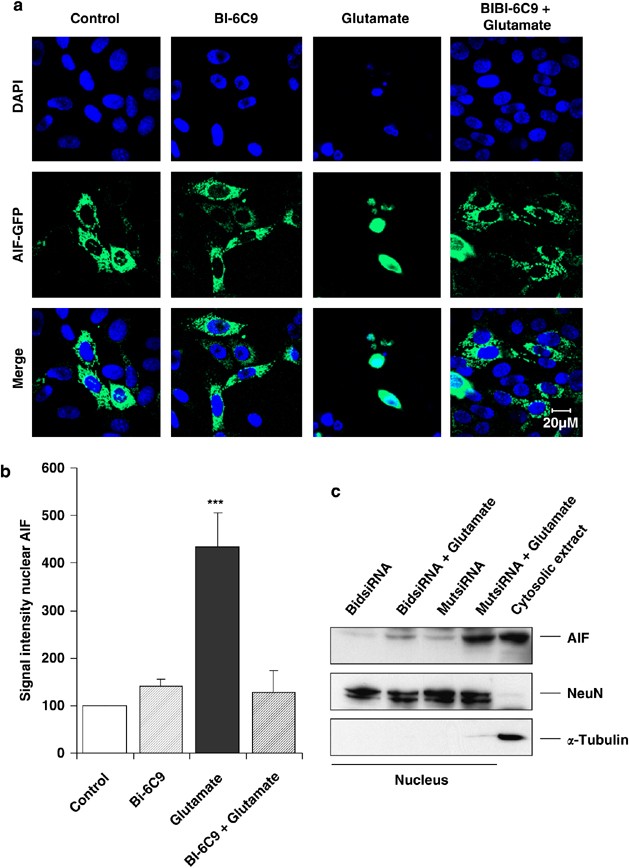
The current failure of caspase inhibitors to prevent HT-22 cell death further supports our recent data demonstrating a major role for AIF in glutamate-induced neuronal cell death.8, 16 To evaluate the specific role of Bid in mitochondrial AIF release and subsequent nuclear translocation, we exposed HT-22 neurons expressing AIF-GFP fusion protein to glutamate and analyzed AIF translocation to the nucleus in the presence or absence of the Bid inhibitor. Confocal microscopy time-lapse recordings taken every 5 min over a period of 18 h after glutamate exposure revealed that mitochondria containing AIF-GFP fusion protein accumulated around the nucleus within 8–10 h after the onset of apoptotic challenge. Accumulation of mitochondria around the nucleus was followed by a rapid release of AIF into the nucleus within less than 15 min (Supplementary Movie). This very close temporal relationship between nuclear AIF translocation and cell death points to a crucial role of AIF in the cell death process and suggests that AIF is one of the final steps in caspase-independent cell death signaling. The Bid inhibitor BI-6C9 blocked glutamate-induced translocation of endogenous and GFP-linked AIF as detected by confocal microscopy (Figure 5a and Supplementary Figure 4) and western blot analysis of nuclear protein extracts (Figure 5b). Furthermore, the involvement of Bid in endogenous AIF translocation to the nucleus was verified by using Bid siRNA. Western blot analysis confirmed that Bid siRNA prevented AIF translocation to the nucleus after glutamate challenge (Figure 5c).
Figure 5
Bid-mediated cell death involves AIF translocation to the nucleus. (a) Fluorescence photomicrographs of HT-22 neurons expressing an AIF-GFP fusion protein show colocalization of GFP and 4′,6-diamidino-2′-phenylindole-dihydrochloride (DAPI) signal in glutamate-damaged (2 mM, 17 h) cells. Bid inhibitor BI-6C9 (10 _μ_M) prevented AIF translocation to the nucleus. (b) Quantification of western blot analysis of nuclear protein extracts (_n_=3 per group) confirmed the inhibition of glutamate-induced AIF translocation to the nucleus by the Bid inhibitor BI-6C9. (c) Immunoblot analyses demonstrate inhibition of AIF translocation in glutamate-exposed cells by Bid siRNA, whereas AIF translocation after glutamate damage was not affected by nonfunctional Mut siRNA-treated cells. Blots were counterstained with anti-NeuN antibodies to control for equal protein loading and with anti-_α_-tubulin antibodies to check the purity of the extracts. The additional control lane on the right shows the corresponding signals from a cytosolic fraction. ***P<0.001 compared to controls and BI-6C9-treated cells (ANOVA, Scheffé's test)
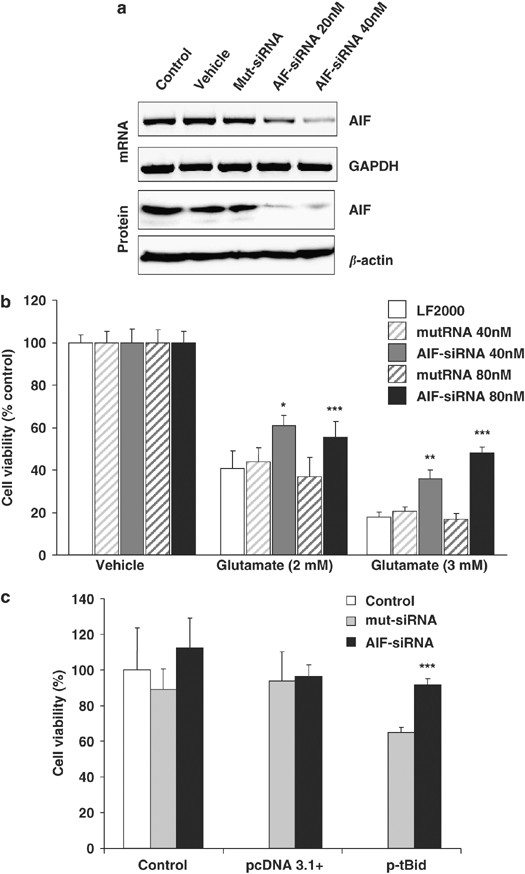
The potential role of AIF in the execution of cell death downstream of Bid-mediated mitochondrial demise was further addressed by a gene silencing approach. AIF siRNA reduced AIF mRNA and protein levels (Figure 6a) and significantly attenuated glutamate-induced cell death in HT-22 cells (Figure 6b). Most intriguingly, we found that AIF siRNA prevented tBid-induced cell death in HT-22 neurons (Figure 6c), demonstrating that tBid-induced neurotoxicity is mediated by AIF. Taken together, these findings demonstrate that Bid is responsible for mitochondrial AIF release and induces caspase-independent execution of neuronal cell death.
Figure 6
AIF siRNA attenuates glutamate- and tBid-induced toxicity in HT-22 neurons. (a) AIF siRNA (20–40 nM) was applied to HT-22 cells 48 h before analysis of mRNA levels by RT-PCR (upper panels) or protein analysis by western blot (lower panels). Nonfunctional Mut siRNA or Lipofectamine 2000 (vehicle) was applied in control experiments, and GAPDH or _β_-actin was analyzed as a respective control for RT-PCR or western blot. (b) AIF siRNA (40–80 nM) was applied to HT-22 cells 48 h before glutamate (2–3 mM). Cell viability was assessed by the MTT assay 18 h later. Mean values and S.D. of _n_=6 experiments per group are shown; *P<0.05, **P<0.01, and ***P<0.001 compared with respective mut siRNA treatments (ANOVA, Scheffé's). (c) HT-22 cells were transfected with AIF siRNA (50 nM) 24 h before transfection with a tBid-expressing plasmid (1 _μ_g). Analysis of cell viability by the MTT assay 24 h later revealed protection of HT-22 neurons against tBid-induced cell death by AIF siRNA. ***P<0.001 compared with p-tBid-transfected cells (Student's _t_-test). Metabolic activity of HT-22 was neither affected by control plasmid 3.1+ nor by nonfunctional mut siRNA
Discussion
The present study demonstrates a key role for the pro-apoptotic bcl-2 family protein Bid in mitochondrial membrane permeabilization and the subsequent nuclear translocation of AIF in HT-22 neurons exposed to lethal glutamate concentrations. The glutamate challenge induced translocation of Bid to mitochondria, followed by mitochondrial membrane depolarization and AIF release to the nucleus rapidly followed by nuclear pyknosis and disintegration of the cell membrane, that is, cell death. The pharmacological Bid inhibitor BI-6C9 and siRNA-mediated gene silencing both significantly prevented mitochondrial Bid translocation, migration of AIF to the nucleus, and, hence, glutamate-induced cell death.
These findings are corroborated by findings in primary neurons, as mitochondrial cell death pathways are key mechanisms in various models of neuronal cell death, including related models of glutamate-mediated excitotoxicity. In particular, recent data established a pivotal role for Bid upstream of mitochondrial demise in neuronal cell death.17 In addition to pro-apoptotic Bid activation by caspase-8-mediated cleavage to tBid and subsequent translocation from the cytosol to mitochondria in death receptor-mediated cell death, more recent data suggest a role for full-length Bid in caspase-independent apoptosis.18, 19, 20 In these studies in primary cultured neurons, translocation of full-length Bid to mitochondria following exposure to glutamate coincided with a collapse of the mitochondrial membrane potential.18, 19, 20 How full-length Bid is activated to mediate mitochondrial demise is currently unknown. Beyond potential interactions with Bax (Bcl-2-associated X protein) or Bak (Bcl-2 antagonist killer) to form mitochondrial outer membrane pores that allow the unspecific release of a whole series of mitochondrial proteins, for example cytochrome c, Smac/Diablo, or AIF, Bid may affect mitochondrial morphology by increasing mitochondrial fission or alterations in mitochondrial cristae organization.21, 22 In addition, PARP, another pro-apoptotic factor that has been linked to mitochondrial demise and AIF translocation to the nucleus in neuronal cell death,8, 11, 12 may complement the presently investigated Bid-dependent mechanisms as PARP inhibition also prevents AIF-dependent neuronal death.8, 11 To the best of our knowledge, however, an interaction between Bid and PARP in mitochondrial cell death pathways has not been elucidated yet. These and other so far not defined activities of mitochondrial Bid may explain the apparent delay between Bid accumulation in mitochondria and the observed mitochondrial morphological changes, perinuclear accumulation, and AIF release after the glutamate challenge in HT-22 cells.
Here, we demonstrate that pharmacological Bid inhibition prevents the translocation of Bid to mitochondria and the pro-apoptotic activity of tBid as well as the subsequent loss of mitochondrial membrane potential and AIF-induced nuclear fragmentation. In line with earlier studies in HeLa cells and isolated mitochondria15, 16 the specificity of the Bid inhibitor was confirmed in tBid-transfected HT-22 cells (Supplementary Figure 2). The causal role of Bid in the current paradigm was further confirmed in parallel experiments using Bid siRNA, which also prevented AIF translocation and glutamate-induced cell death, findings corroborated by pharmacological inhibition of Bid (Supplementary Figure 4). In addition, our parallel experiments in primary cultured neurons demonstrated pronounced neuroprotective effects of different Bid inhibitors in models of glutamate-induced apoptosis16 or oxygen–glucose deprivation.8 In both models of neuronal cell death, activation of glutamate receptors mediates a rapid increase in intracellular calcium levels and an excitotoxic death program that involves the formation of reactive oxygen species, permeabilization of the mitochondrial membrane, and mitochondrial release of AIF.8, 16 It is therefore concluded that Bid plays a key role in neuronal cell death induced by excitotoxic stimuli and oxidative stress, which have both been associated with a wide variety of neurodegenerative conditions following acute brain injury by cerebral ischemia or brain trauma as well as chronic neurodegenerative diseases, such as Alzheimer's disease.2 It is interesting to note that similar to glutamate toxicity, pharmacological inhibition of Bid also prevented AIF translocation and cell death following exposure to amyloid-beta (Supplementary Figure 6). These findings suggest that Bid-induced AIF-mediated cell death signaling is not confined to glutamate toxicity alone but also present in other cell death paradigms.
The relevance of the current data on the key role for Bid in neuronal cell death in vitro is further underlined by recent data obtained in in vivo models of cerebral ischemia14, 23 and brain trauma.24 In both models of acute brain injury, Bid cleavage has been detected after the respective insults, and knockout of the Bid gene resulted in significant cerebroprotective effects compared with wild-type animals. Therefore, the understanding of Bid-dependent cell death mechanisms is of utmost importance, and the presently used Bid inhibitors may emerge as promising lead structures for neuroprotective drugs in the treatment of neurodegenerative diseases.
The second important aspect of the present study addresses the role of AIF in mediating caspase-independent cell death downstream of Bid activation. It has been well established that mitochondrial membrane permeabilization results in the release of pro-apoptotic factors such as cytochrome c, Smac/Diablo, Omi/HtrA2, and AIF, which may execute cell death through caspase-dependent or caspase-independent mechanisms. For example, formation of the apoptosome by cytochrome c, Apaf-1, and procaspase-9 and downstream catalytic activation of executor caspases (caspase-3, -6, or -7) are widely established mechanisms of caspase-dependent cell death execution downstream of mitochondrial damage in many cells, including neurons. Here, we detected caspase-3 activation downstream of Bid, suggesting that this pathway may also play a role in neuronal cell death in the present model. Caspase-3 inhibition alone, however, did not prevent glutamate-induced cell death (Figure 4). Because other signs of executor caspase activation, for example, cleavage of _α_-fodrin or lamin, were also not detected (data not shown), our data strongly suggest a major role for caspase-independent cell death downstream of mitochondrial membrane permeabilization. Using an AIF-GFP construct, we found mitochondrial release of AIF and translocation to the nucleus, which occurred around 8–10 h after the onset of glutamate exposure. Time-lapse video recordings revealed that the time point of massive AIF translocation to the nucleus may vary from cell to cell around 8–12 h after the insult, but once started, the AIF translocation occurs within 15 min and is followed by rapid nuclear condensation and cellular fragmentation (Supplementary Movie). This is, to our knowledge, the first time that the kinetics of AIF-mediated cell death has been recorded in living cells. After hours of increasing Bid translocation to the mitochondria and perinuclear accumulation of Bid-loaded mitochondria, the final execution of cell death by the released AIF takes only a few minutes. The markedly fast kinetics of AIF translocation into the nucleus once released from the mitochondria further exposes the unique role of AIF downstream of mitochondrial demise to mediate cell death independent of other established execution mechanisms of intrinsic cell death pathways, such as apoptosome formation and activation of caspase-3. Furthermore, AIF siRNA significantly attenuated glutamate-induced neurotoxicity in HT-22 cells (Figure 6a and b) and in primary neurons;16 most intriguingly, AIF siRNA also prevented tBid-induced cell death in HT-22 neurons (Figure 6c), clearly demonstrating that Bid neurotoxicity is predominantly mediated by mitochondrial AIF release and hence caspase-independent cell death pathways.
The present findings are in accordance with recent data where we and others demonstrated a key role for AIF in delayed neuronal cell death after excitotoxic or hypoxia/ischemia insults in vitro and in vivo.6, 8, 9 Furthermore, these results propose that the release of pro-apoptotic factors is a coordinated process where the release of a particular apoptotic factor and its role in the execution of apoptosis also depends on the stress challenge and the pathway to mitochondrial damage. The present model of glutamate toxicity in HT-22 neurons features Bid-mediated mitochondrial membrane permeabilization and release of AIF, whereas activation of Bid and AIF release were not required for staurosporine (STS)-induced apoptosis (Supplementary Figure 5), which is consistent with previous reports.25, 26 Similar differences between mitochondrial proteins regarding their release and involvement in neuronal death were detected after cerebral ischemia in vivo, where early AIF release from the mitochondria correlated well with the progression of DNA damage and nuclear pyknosis in neurons whereas cytochrome c release and activation of caspases were detected as a later event, less frequently and in different cells than the AIF translocation.7
In summary, our data link enhanced Bid activation to mitochondrial AIF release and caspase-independent execution of neuronal cell death, which are both key features in delayed neuronal cell death after excitotoxic or ischemic insults in models of stroke, epilepsy, and brain trauma. Therefore, Bid emerges as a promising target for therapeutic approaches that prevent mitochondrial damage after neuronal injury.
Materials and Methods
Cell culture and induction of neuronal cell death
HT-22 cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Karlsruhe, Germany) supplemented with 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, 100 _μ_g/ml streptomycin, and 2 mM glutamine. Glutamate (1–4 mM) or STS (300 nM) was added to the serum-containing medium, and cell viability was evaluated 17–20 h later.
Plasmids and gene transfer
Plasmid pCDNA 3.1+ was obtained from Invitrogen. The mouse AIF-GFP fusion protein vector (mAIF_pd2EGFP-N1) was derived from a pd2pEGFP-N1 vector (Clontech, Palo Alto, CA, USA) and an AIF-expressing vector (pcDNA3.1_mAIF, a kind gift of S Susin, CNRS, Paris, France). The tBid vector and control vectors were generated as described previously.27 The plasmid pDsRed2-Bid for expression of DsRed fused to the C-terminus of Bid to allow translocation analyses of full-length Bid and tBid was derived from Clontech. Plasmid transfections of 8 × 104 HT-22 cells seeded in 24-well plates were performed in antibiotic-free growth medium using Lipofectamine 2000 (Invitrogen). Controls were treated with 100 _μ_l/ml Optimem only and vehicle controls with 1.5_μ_l/ml Lipofectamine 2000.
Immunostaining and confocal laser scanning microscopy
For (immuno)fluorescence analyses, HT-22 cells were seeded in collagen A-coated Ibitreat _μ_-slide eight-well plates (Ibidi, Munich, Germany) at a density of 1 × 104 cells/well. Mitochondria were stained with MitoTracker Green according to the manufacturer's protocol (Invitrogen) or visualized by co-transfection with a mitochondria-targeted GFP construct as described previously.28 End-point pictures were taken after fixation with 4% paraformaldehyde and 4′,6-diamidino-2′-phenylindole-dihydrochloride counterstaining of the nuclei between 5 and 17 h after the onset of treatment. Images were acquired using a confocal laser scanning microscope (LSM 510, Carl Zeiss, Jena, Germany) equipped with a UV, an argon, and a helium/neon laser delivering light at 364, 488, and 543 nm, respectively. For real-time confocal microscopy, the CO2 chamber was adjusted to 37°C, 5% CO2 and a humidified atmosphere. Images were acquired every 5 min up to 17 h after the onset of treatments.
Evaluation cell viability and apoptosis
For morphological analysis of cells, transmission light microscopy of living HT-22 neurons growing as monolayers was performed using an Axiovert 200 microscope (Carl Zeiss) equipped with a Sony DSC-S75 digital camera (Sony Corporation, Tokyo, Japan) and image analysis was performed with the Axiovision 3.1.0.18 software (Carl Zeiss). Quantification of cell viability in HT-22 cells was performed in 96-well plates by MTT reduction at 0.5 mg/ml for 2 h.29 Apoptotic cell death was detected by annexin-V/propidium iodide staining (Sigma-Aldrich, Taufkirchen, Germany) and subsequent flow cytometry analysis using a CyanTM MLE flow cytometer (DakoCytomation, Copenhagen, Denmark). Cells were appropriately gated by forward versus side scatter and pulse width, and 1 × 104 gated events per sample were collected. Surviving cells did not show any staining whereas annexin-V staining indicated apoptosis and cells positive for both annexin V and propidium iodide were regarded necrotic.
Analysis of mitochondrial membrane potential
Mitochondrial membrane potential of HT-22 neurons was determined by JC-1 reduction according to the manufacturer's protocol (Mitoprobe, Invitrogen) and analyzed by subsequent flow cytometry or epifluorescence microscopy. Additional controls were treated with CCCP (50 _μ_M) 5 min before staining to induce mitochondrial membrane depolarization.
Gene silencing
AIF siRNA (AAGAGAAACAGAGAAGAGCCA) and nonfunctional control siRNA (mut siRNA, 5′-AAGAGAAAAAGCGAAGAGCCA-3′) were purchased at MWG Biotech (Munich, Germany) or AIF and Bid siRNA mixtures were generated using recombinant dicer enzyme as previously described.8 The Bid cDNA template (409 bp) for T7-RNA polymerase in vitro transcription was generated from mouse mRNA by reverse transcription-PCR (RT-PCR) using the following primers: forward, 5′-GCGTAATACGACTCACTATAGGGAGATGGGCTTCTGTCTAAGGAGA-3′, and reverse, 5′-GCGTAATACGACTCACTATAGGGAGAAGTGAGGCCTTGTCTCTGAA-3. For the generation of AIF cDNA template, the following primers were used: forward, 5′-GCGTAATACGACTCACTATAGGGAGATCCAGGCAACTTGTTCCAGC-3′, and reverse, 5′-GCGTAATACGACTCACTATAGGGAGACCTCTGCTCCAGCCCTATCG-3′. For siRNA transfections, Lipofectamine 2000 (Invitrogen) and AIF siRNA or nonfunctional mut siRNA were dissolved separately in Optimem I (Invitrogen) and the respective transfection mixtures were added to the antibiotic-free cell culture medium to a final concentration of 20 nM siRNA for dicer products and up to 80 nM siRNA for the single siRNA sequences. Controls were treated with 100 _μ_l/ml medium only and vehicle controls with 1.5–2 _μ_l/ml Lipofectamine.
Caspase activity measurements
HT-22 neurons cultivated in six-well plates with a density of 4 × 105 cells/well were lysed in 150 _μ_l lysis buffer (MgCl2 406.8 mg, EGTA 152 mg, Triton X-100 400 _μ_l, HEPES solution 50 mM 200 ml, Aqua dest. Ad 400 ml). Cell membranes were removed by centrifugation at 15 000 × g (15 min, 4°C) and supernatants (20 _μ_l) were incubated with 90 _μ_l of freshly prepared substrate solution. Fluorescence (excitation 360 nm, emission 465 nm) was measured with a SpectraFluor Plus (SpectraFluorPlus, Tecan, Crailsheim, Germany) multiplate reader. Caspase activity was inhibited by using the potent general caspase inhibitor Z-VAD-FMK (R&D Systems, Wiesbaden, Germany).
RT-PCR
Total RNA was extracted (Nucleospin RNA II kit, Macherey-Nagel, Düren, Germany) and RT-PCR was performed as described.30 Primers and PCR conditions for AIF, for example Bid, were the same as given before. Primers for GAPDH were as follows: forward, 5′-CGTCTTCACCACCATGGAGAAGGC-3′, and reverse, 5′-AAGGCCATGCCAGTGAGCTTCCC-3′. PCR for GAPDH was performed as follows: initial denaturation at 95°C for 2 min; amplification by 26 cycles of 30 s at 95°C, 1 min at 57°C, and 2 min at 70°C. The final extension was performed at 70°C for 10 min. RT-PCR products were visualized under UV illumination after electrophoresis on a 1.5% agarose gel containing ethidium bromide.
Protein extracts and immunoblots
For western blot analysis, HT-22 neurons were lysed with 50–150 _μ_l 1 : 5 diluted cell lysis reagent 5 × (Promega, Mannheim, Germany), supplemented with 1 tablet per 10 ml Complete Mini Protease Inhibitor Cocktail (Roche, Mannheim, Germany). After centrifugation at 15 000 × g for 15 min at 4°C, the supernatants were stored at −80°C until further use. Nuclear extracts were separated from cytosolic fractions using the nuclear extract kit according to the manufacturer's instructions (Active Motif, Rixensart, Belgium). Protein amounts were determined with the Pierce BCA kit (Perbio Science, Bonn, Germany).
Western blot analysis was performed as previously described.7 Briefly, the blot was probed with an anti-AIF goat polyclonal antibody (sc-9416, 1 : 1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-Bid monoclonal antibody (611867, 1 : 500, BD Biosciences Pharmingen, Heidelberg, Germany) at 4°C overnight. Membranes were then exposed to the appropriate HRP-conjugated rabbit anti-goat or horse anti-mouse secondary antibody (1 : 5000, Vector Laboratories, Burlingame, CA, USA) followed by a chemiluminescence detection of antibody binding (Spray & Glow, Millipore, Schwalbach, Germany). Equal protein loading was controlled by re-probing the membrane with a monoclonal anti-_α_-tubulin antibody (T9026, 1 : 20 000, Sigma) or an anti-NeuN antibody (mouse monoclonal, 1 : 100, MAB377, Chemicon/Millipore, Billerica, MA, USA). Scion Image for Windows software (Scion Corporation, USA) was used for quantification of western blot signals.
Statistical analysis
All data are given as means±S.D. For statistical comparisons between two groups, Student's _t_-test was used; multiple comparisons were performed by ANOVA followed by Scheffé's post hoc test. Calculations were performed with the Winstat standard statistical software package.
Abbreviations
AIF:
apoptosis-inducing factor
ANOVA:
analysis of variance
CCCP:
carbonyl cyanide _m_-chlorophenylhydrazone
FACS:
fluorescent-activated cell sorting
FITC:
fluorescein isothiocyanate
GFP:
green fluorescent protein
Hq:
harlequin
JC-1:
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide
MTT:
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
PARP:
poly-(ADP-ribose)-polymerase-1
RT-PCR:
reverse transcription-PCR
siRNA:
small interfering RNA
Smac/Diablo:
second mitochondria-derived activator of caspase/direct IAP binding protein with low p_I_
STS:
staurosporine
tBid:
truncated Bid
References
- Mattson MP, Duan W, Pedersen WA, Culmsee C . Neurodegenerative disorders and ischemic brain diseases. Apoptosis 2001; 6: 69–81.
Article CAS Google Scholar - Culmsee C, Landshamer S . Molecular insights into mechanisms of the cell death program: role in the progression of neurodegenerative disorders. Curr Alzheimer Res 2006; 3: 269–283.
Article CAS Google Scholar - Polster BM, Fiskum G . Mitochondrial mechanisms of neural cell apoptosis. J Neurochem 2004; 90: 1281–1289.
Article CAS Google Scholar - Yuan J, Yankner BA . Apoptosis in the nervous system. Nature 2000; 407: 802–809.
Article CAS Google Scholar - Zhu C, Qiu L, Wang X, Hallin U, Cande C, Kroemer G et al. Involvement of apoptosis-inducing factor in neuronal death after hypoxia–ischemia in the neonatal rat brain. J Neurochem 2003; 86: 306–317.
Article CAS Google Scholar - Zhu C, Wang X, Huang Z, Qiu L, Xu F, Vahsen N et al. Apoptosis-inducing factor is a major contributor to neuronal loss induced by neonatal cerebral hypoxia–ischemia. Cell Death Differ 2007; 14: 775–784.
Article CAS Google Scholar - Plesnila N, Zhu C, Culmsee C, Groger M, Moskowitz MA, Blomgren K . Nuclear translocation of apoptosis-inducing factor after focal cerebral ischemia. J Cereb Blood Flow Metab 2004; 24: 458–466.
Article Google Scholar - Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M et al. Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen–glucose deprivation and focal cerebral ischemia. J Neurosci 2005; 25: 10262–10272.
Article CAS Google Scholar - Cheung EC, Melanson-Drapeau L, Cregan SP, Vanderluit JL, Ferguson KL, McIntosh WC et al. Apoptosis-inducing factor is a key factor in neuronal cell death propagated by BAX-dependent and BAX-independent mechanisms. J Neurosci 2005; 25: 1324–1334.
Article CAS Google Scholar - Heo K, Cho YJ, Cho KJ, Kim HW, Kim HJ, Shin HY et al. Minocycline inhibits caspase-dependent and -independent cell death pathways and is neuroprotective against hippocampal damage after treatment with kainic acid in mice. Neurosci Lett 2006; 398: 195–200.
Article CAS Google Scholar - Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ et al. Mediation of poly(ADP-ribose)polymerase-1-dependent cell death by apoptosis-inducing factor. Science 2002; 297: 259–263.
Article CAS Google Scholar - Dawson VL, Dawson TM . Deadly conversations: nuclear–mitochondrial cross-talk. J Bioenerg Biomembr 2004; 36: 287–294.
Article CAS Google Scholar - Polster BM, Basanez G, Etxebarria A, Hardwick JM, Nicholls DG . Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem 2005; 280: 6447–6454.
Article CAS Google Scholar - Plesnila N, Zinkel S, Le DA, Amin-Hanjani S, Wu Y, Qiu J et al. BID mediates neuronal cell death after oxygen/glucose deprivation and focal cerebral ischemia. Proc Natl Acad Sci USA 2001; 98: 15318–15323.
Article CAS Google Scholar - Becattini B, Sareth S, Zhai D, Crowell KJ, Leone M, Reed JC et al. Targeting apoptosis via chemical design: inhibition of bid-induced cell death by small organic molecules. Chem Biol 2004; 11: 1107–1117.
Article CAS Google Scholar - Becattini B, Culmsee C, Leone M, Zhai D, Zhang X, Crowell KJ et al. Structure–activity relationships by interligand NOE-based design and synthesis of antiapoptotic compounds targeting Bid. Proc Natl Acad Sci USA 2006; 103: 12602–12606.
Article CAS Google Scholar - Culmsee C, Plesnila N . Targeting Bid to prevent programmed cell death in neurons. Biochem Soc Trans 2006; 34: 1334–1340.
Article CAS Google Scholar - Ward MW, Rehm M, Duessmann H, Kacmar S, Concannon CG, Prehn JH . Real time single cell analysis of Bid cleavage and Bid translocation during caspase-dependent and neuronal caspase-independent apoptosis. J Biol Chem 2006; 281: 5837–5844.
Article CAS Google Scholar - Konig HG, Rehm M, Gudorf D, Krajewski S, Gross A, Ward MW et al. Full length Bid is sufficient to induce apoptosis of cultured rat hippocampal neurons. BMC Cell Biol 2007; 8: 7.
Article Google Scholar - Pei Y, Xing D, Gao X, Liu L, Chen T . Real-time monitoring full length bid interacting with Bax during TNF-alpha-induced apoptosis. Apoptosis 2007; 12: 1681–1690.
Article CAS Google Scholar - Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 2006; 126: 177–189.
Article CAS Google Scholar - Kim TH, Zhao Y, Ding WX, Shin JN, He X, Seo YW et al. Bid-cardiolipin interaction at mitochondrial contact site contributes to mitochondrial cristae reorganization and cytochrome C release. Mol Biol Cell 2004; 15: 3061–3072.
Article CAS Google Scholar - Yin XM, Luo Y, Cao G, Bai L, Pei W, Kuharsky DK et al. Bid-mediated mitochondrial pathway is critical to ischemic neuronal apoptosis and focal cerebral ischemia. J Biol Chem 2002; 277: 42074–42081.
Article CAS Google Scholar - Bermpohl D, You Z, Korsmeyer SJ, Moskowitz MA, Whalen MJ . Traumatic brain injury in mice deficient in Bid: effects on histopathology and functional outcome. J Cereb Blood Flow Metab 2006; 26: 625–633.
Article CAS Google Scholar - Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler M et al. Two distinct pathways leading to nuclear apoptosis. J Exp Med 2000; 192: 571–580.
Article CAS Google Scholar - Cande C, Vahsen N, Garrido C, Kroemer G . Apoptosis-inducing factor (AIF): caspase-independent after all. Cell Death Differ 2004; 11: 591–595.
Article CAS Google Scholar - Kazhdan I, Long L, Montellano R, Cavazos DA, Marciniak RA . Targeted gene therapy for breast cancer with truncated Bid. Cancer Gene Ther 2006; 13: 141–149.
Article CAS Google Scholar - Duvezin-Caubet S, Jagasia R, Wagener J, Hofmann S, Trifunovic A, Hansson A et al. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem 2006; 281: 37972–37979.
Article CAS Google Scholar - Liu Y, Peterson DA, Kimura H, Schubert D . Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) reduction. J Neurochem 1997; 69: 581–593.
Article CAS Google Scholar - Culmsee C, Gerling N, Lehmann M, Nikolova-Karakashian M, Prehn JH, Mattson M et al. Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75. Neuroscience 2002; 115: 1089–1108.
Article CAS Google Scholar
Acknowledgements
The authors gratefully acknowledge outstanding technical support and generation of vectors for transfection experiments by Melinda Kiss as well as additional control experiments by Markus Hofer. This work was supported in part by NIH grant HL082574 to MP.
Author information
Author notes
- N Plesnila
Present address: Current address: Department of Neurodegeneration, Royal College of Surgeons in Ireland (RCSI), 2 St. Steven's Green, Dublin 2, Ireland, - C Culmsee
Present address: Current address: Clinical Pharmacy – Pharmacology and Toxicology, Faculty of Pharmacy, Philipps-University of Marburg, Karl-von-Frisch Strasse 1, 35043 Marburg, Germany,
Authors and Affiliations
- Pharmaceutical Biology-Biotechnology, Ludwig-Maximilians-University, Munich, Germany
S Landshamer, M Hoehn, E Wagner & C Culmsee - Department of Pharmacy, Pharmaceutical Biology, Ludwig-Maximilians-University, Munich, Germany
N Barth, S Zahler & A Vollmar - Adolf-Butenandt-Institute for Physiological Chemistry, Ludwig-Maximilians-University, Munich, Germany
S Duvezin-Caubet & A Reichert - Department of Physics, Ludwig-Maximilians-University, Munich, Germany
G Schwake - Clinical Pharmacy – Pharmacology and Toxicology, Faculty of Pharmacy, Philipps-University of Marburg, Germany
S Tobaben - Department of Medicine, Division of Medical Oncology, University of Texas Health Science Center, San Antonio, TX, USA
I Kazhdan - The Burnham Institute, La Jolla, CA, USA
B Becattini & M Pellecchia - Institute for Surgical Research, University of Munich Medical Center-Großhadern, Munich, Germany
N Plesnila - Department of Neurosurgery, University of Munich Medical Center-Großhadern, Munich, Germany
N Plesnila
Authors
- S Landshamer
- M Hoehn
- N Barth
- S Duvezin-Caubet
- G Schwake
- S Tobaben
- I Kazhdan
- B Becattini
- S Zahler
- A Vollmar
- M Pellecchia
- A Reichert
- N Plesnila
- E Wagner
- C Culmsee
Corresponding authors
Correspondence toN Plesnila or C Culmsee.
Additional information
Edited by G Kroemer
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Supplementary information
Rights and permissions
About this article
Cite this article
Landshamer, S., Hoehn, M., Barth, N. et al. Bid-induced release of AIF from mitochondria causes immediate neuronal cell death.Cell Death Differ 15, 1553–1563 (2008). https://doi.org/10.1038/cdd.2008.78
- Received: 30 November 2007
- Revised: 14 April 2008
- Accepted: 05 May 2008
- Published: 06 June 2008
- Issue Date: October 2008
- DOI: https://doi.org/10.1038/cdd.2008.78