The Arabidopsis PARAQUAT RESISTANT2 gene encodes an S-nitrosoglutathione reductase that is a key regulator of cell death (original) (raw)
Introduction
In higher plants, programmed cell death (PCD) is an important mechanism to regulate multiple aspects of growth and development, as well as to remove damaged or infected cells during responses to environmental stresses and pathogen attacks 1, 2, 3. PCD in plant cells shares many morphological similarities with that of animal cells, including organelle degeneration, nuclear condensation, nuclear DNA fragmentation and eventually cell shrinkage. These common features suggest that a similar mechanism may be adopted by animal and plant cells to control PCD. However, no homologs corresponding to the major components of the animal PCD pathway have been found in any genomes of higher plants. Yet, little is known about the biochemical mechanism of PCD in plant cells. Most of our current knowledge on plant PCD has been obtained from studies of pathogen-plant interactions, in which the host often responds by eliciting a hypersensitive response (HR) around the initial infection site. HR has been characterized by a burst of reactive oxygen species (ROS), the accumulation of signaling molecules, such as nitric oxide (NO) and salicylic acid (SA), and the induction of pathogenesis-related genes, eventually followed by rapid cell death. HR acts to confine the pathogen and protect the plant, and is regulated by various signaling molecules, including ROS, NO and SA 4, 5.
Genetic studies on the Arabidopsis lesion mimic mutants, which show an HR-syndrome in the absence of pathogen infection, have shed significant light on the molecular mechanism of plant PCD. Because of their spontaneous cell death phenotype and constitutive activation of the defense response, the lesion mimic mutants, represented by accelerated cell death 6 and lesion stimulating disease resistance mutants 7, could be affected in key regulatory loci controlling HR-type cell death. Using a nonpathogen approach by screening for mutants resistant to the PCD-inducing compound, fumonisin B1 (FB1), several FB 1 -resistant (fbr) mutants have been identified 8, 9. In contrast to that of the lesion mimic mutants, these fbr mutants show an anti-apoptotic phenotype 9, 10, 11, 12. Whereas FBR6 and FBR12 encode a plant-specific transcription factor, AtSPL14, and a eukaryotic translation initiation factor 5A, respectively 10, 12, FBR11 encodes a subunit of serine palmitoyltransferase that catalyzes the first rate-limiting reaction of sphingolipid de novo synthesis 11, 13. Although sphingolipid-regulated cell death has been attributed to ROS production 11, 14, the molecular link between these two classes of signaling molecules remains unclear.
In addition to FB1, paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride), a nonselective herbicide, has also been shown as an efficient inducer of cell death in both animal and plant cells 15, 16. In plant cells, paraquat mainly targets chloroplasts by accepting electrons from photosystem I and then reacting with oxygen to produce superoxide and hydrogen peroxide, which cause photooxidative stress 17, 18. Consistently, chloroplast-targeted overexpression of superoxide dismutase (SOD) genes confers paraquat resistance in a number of transgenic species 19, 20, 21. Interestingly, the Arabidopsis radical-induced cell death1 (rcd1) mutant displays resistance to paraquat, and this phenotype is associated with the increased expression of plastidic Cu/Zn SOD and ascorbate peroxidase (APX) genes 22. These results suggest that paraquat-induced cell death is mediated by a ROS-based signaling pathway.
NO has been considered as an important signaling molecule involved in both biotic and abiotic stress responses, as well as in many aspects of plant growth and development 23, 24, 25, 26. The balance between intracellular NO and ROS levels has been shown to be a key determinant for HR 26, 27, 28, 29, 30. The ROS and NO levels appear to be reciprocally controlled through, at least partly, related scavenging enzymes, and NO has also been proposed to function as an antioxidant under certain conditions 28, 29, 31, 32, 33. Moreover, _S_-nitrosylation, the covalent addition of an NO molecule to a cysteine thiol of a protein or a peptide, is a key destination of the gaseous hormone and a redox-based posttranslational modification mechanism. _S_-nitrosylation appears to be an important mechanism to regulate the activity and stability of many proteins and enzymes 24, 34, 35, 36, 37, 38. In this complicated, yet not well-understood process, _S_-nitrosoglutathione (GSNO), derived by _S_-nitrosylation of the antioxidant tripeptide glutathione, is a major reservoir of biologically active NO. The evolutionally conserved GSNO reductase (GSNOR) is a key enzyme catalyzing the reduction of GSNO, thus controlling intracellular levels of GSNO and _S_-nitrosothiols (SNOs) 39, 40.
In Arabidopsis, the single-copied GSNOR1 gene has been genetically and biochemically characterized 41, 42, 43, 44, 45, 46, 47. Recombinant Arabidopsis GSNOR1 protein shows enzymatic activity of reducing GSNO in vitro 45. Moreover, mutations in GSNOR1 cause developmental defects and altered responses to both biotic and abiotic stresses 42, 46. A loss-of-function mutation in the GSNOR1 gene (gsnor1-3) causes an increased SNO level, accompanied with compromised defense responses mediated by distinctive resistance genes, as well as both basal and nonhost resistances 42. Conversely, gain-of-function mutations in GSNOR1 result in reduced SNO formation 42. These results indicate that GSNOR1 is a positive regulator of SA signaling. More recently, the Arabidopsis sensitive to hot temperatures5 (HOT5) gene was characterized to be identical to GSNOR1 46. Several allelic gsnor1/hot5 mutants show a similar phenotype characteristic of compromised heat acclimation and elevated levels of NO species, illustrating an important role of SNO homeostasis in the regulation of abiotic stress response as well 46. Apart from its role in SA signaling and the abiotic stress response, it however remains unknown if the intracellular NO level modulated by GSNOR1/HOT5 is involved in any other cellular activities. In this study, we report the identification and characterization of an anti-cell death mutant paraquat resistant2-1 (par2-1) that is allelic to gsnor1/hot5. Our results suggest that GSNOR1/HOT5/PAR2 plays an important role in the regulation of cell death.
Results
Genetic screen and analysis of par mutants
When germinated in the presence of paraquat, growth and development of wild-type Arabidopsis plants were strongly inhibited. Cotyledons remained small and brown-yellowish and occasionally turned green-yellowish. Root growth and true leaf initiation were arrested, and the plants died eventually (Supplementary information, Figure S1A and S1B). Accompanying cell death, paraquat induced the production of superoxide and hydrogen peroxide in wild-type leaves (Supplementary information, Figure S1D and S1E). When treated with paraquat, detached leaves rapidly turned bleached under light, but remained green when cultured in the dark (Supplementary information, Figure S1C), consistent with the photooxidative stress effect of the herbicide. We reasoned that mutations that render plants resistant to paraquat may represent important genetic loci that are involved in either detoxification or the regulation of cell death in plant cells. Therefore, we carried out a genetic screen for Arabidopsis par mutants by surveying ethyl- methanesulphonate (EMS)-mutagenized seeds (in Columbia-0 or Col-0 and Wassilewskija or WS background) on MS medium 48 containing 2 μM paraquat. From this screen, we have recovered 12 par mutants, which were in the Col-0 background (2 mutants) and the WS background (10 mutants). These par mutants showed resistance to paraquat at varying degrees. Here, we present a detailed study on a representative mutant, par2-1.
The par2-1 mutant was in the Col-0 background. We crossed par2-1 with wild-type Col-0 plants, and the paraquat-resistant phenotype was scored in F1 and F2 populations. All tested F1 plants (25 plants) were sensitive to paraquat. Among the analyzed F2 plants (derived from self-pollinated F1 plants), the paraquat-resistant phenotype was segregated in a 1:3 ratio (resistant:sensitive = 54:164; χ2= 0.90), indicating that the mutation is recessive in a single nuclear gene. F1 plants obtained from crosses between par2-1 and other par mutants showed a wild-type phenotype, indicating that par2-1 was not allelic to any other par mutants identified in this study. The original par2-1 mutant was backcrossed with Col-0 twice, and homozygous progenies of F3 or subsequent generations were used in all experiments described below.
The par2-1 mutant phenotype
When germinated and grown in the presence of paraquat, par2-1 showed resistance to paraquat, whereas growth and development of wild-type plants were completely inhibited (Figure 1A). In contrast to that of wild-type leaves, paraquat-induced cell death was barely detected in par2-1 leaves (Figure 1B), suggesting that paraquat-induced cell death program is impaired in the mutant. Because paraquat has been known to induce the generation of ROS, we examined the accumulation of superoxide and hydrogen peroxide in par2-1 leaves treated with paraquat. When treated with paraquat, the production of superoxide and hydrogen peroxide in par2-1 was comparable to that in wild-type leaves (Figure 1C and data not shown). This result indicates that the paraquat-induced production of superoxide does not require PAR2, which likely acts downstream of ROS to regulate cell death. In agreement with this notion, par2-1 also showed resistance to FB1 (Figure 1D), a fungal toxin that can efficiently induce PCD in plants, likely via regulating ROS generation 8, 9, 11, 14.
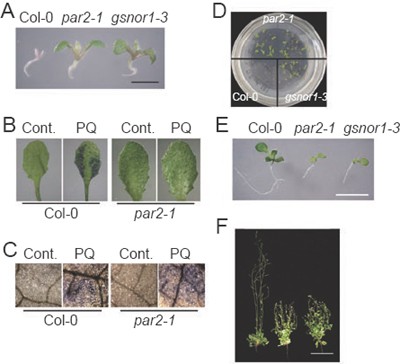
Figure 1
The par2-1 mutant phenotype. (A) Wild type (WT; Col-0), par2-1 and gsnor1-3 seedlings (7-day-old) germinated and grown in the presence of 2 μM paraquat. Bar, 1 mm. (B) Paraquat-induced cell death. Col-0 and par2-1 seedlings (4-week-old) were treated with (PQ) or without (Cont.) 5 μM paraquat for 24 h by spraying. Leaves were then detached from treated plants and stained with Evans blue. Under the assay conditions, less than 10% of untreated wild-type leaves and par2-1 leaves (treated or untreated) were stained as positive for cell death, whereas more than 90% paraquat-treated wild type leaves were stained as positive. (C) Accumulation of superoxide induced by paraquat. Wild type and par2-1 seedlings (4-week-old) treated as described in (B) for 6 h, and leaves were collected and then stained with nitroblue tetrazolium (NBT). Superoxide accumulation was shown as blue precipitates. Approximately 10%-20% of untreated leaves could be stained by NBT at varying degrees, and 80%-90% of paraquat-treated leaves were stained as positive by NBT. (D) Col-0, par2- 1 and gsnor1-3 seedlings (2-week-old) germinated and grown in the presence of 0.8 μM FB1. (E) Wild type, par2-1 and gsnor1-3 seedlings (10-day-old) germinated and grown on MS medium. Bar, 1 mm. (F) Wild type, gsnor1-3 and par2-1 (from left to right) plants (6-week-old) grown in soil. Bar, 5 cm.
To ask if the paraquat-induced cell death involves nuclear DNA fragmentation, a hallmark of PCD, we analyzed paraquat-treated wild type and mutant protoplasts by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). Positive TUNEL signals were easily detected in paraquat-treated protoplasts, but rarely observed in water-treated wild-type protoplasts. However, substantially reduced TUNEL signals were detected in paraquat-treated protoplasts derived from par2-1 leaves compared to that of wild-type plants (Figure 2). Consistent with its light-dependent killing effect, paraquat treatment in the dark did not induce apparent TUNLE-positive signals in protoplasts derived from either wild type or par2- 1 leaves (data not shown). Taken together, these results suggest that the par2-1 mutant phenotype involves an anti-cell-death mechanism related to the inhibition of PCD.
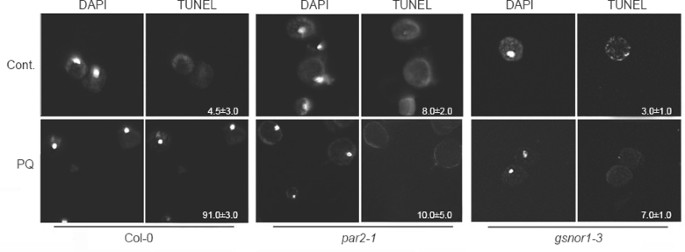
Figure 2
Nuclear DNA fragmentation induced by paraquat. Protoplasts prepared from leaves derived from 4-week-old wild-type seedlings were treated with (PQ) or without (Cont.) 0.5 μM paraquat for 6 h, and then subjected to terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analysis of free 3′-OH group of DNA. Nuclear DNA was stained with 4′,6-diamino-2-phenylindole (DAPI). Numbers inside the TUNEL panels represent percentage of TUNEL-positive protoplasts and standard derivations. The data were mean values obtained from three independent experiments.
Under normal growth conditions, par2-1 displayed a semi-dwarf and bushy phenotype with reduced fertility (Figure 1E and 1F), suggesting that PAR2 is also required for normal growth and development.
Molecular cloning of PAR2
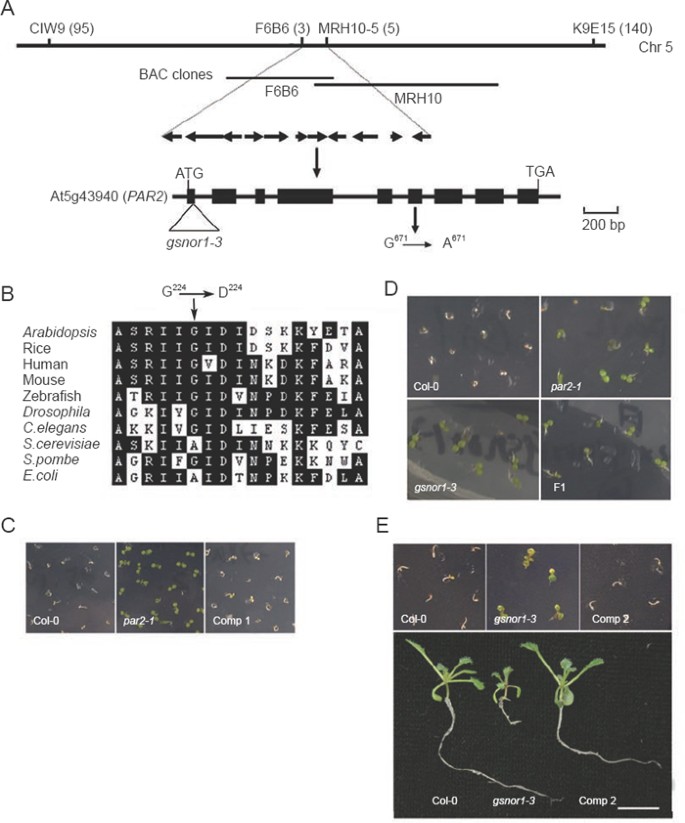
Using an F2 population obtained from crosses of par2- 1 × Landsberg erecta (L_er_), we mapped the mutation onto chromosomes V, between an SSLP marker CIW9 and an InDel marker K9E15. By monitoring genetic recombination in a population of 3363 F2 mutant plants, we located PAR2 between markers F6B6 and MRH10-5, an ∼47-kb region containing 11 open reading frames (Figure 3A). DNA sequencing analysis of all 11 genes revealed a mutation in At5g43940, characterized as a G-to-A transition in exon 6, which converts a glycine into an aspartic acid at residue 224 (Figure 3A). This glycine residue is highly conserved in related proteins across different kingdoms (Figure 3B).
Figure 3
Molecular characterization of the PAR2 gene. (A) A schematic map of positional cloning of PAR2. Top shows region with major markers used in genetic mapping of PAR2. Numbers in parentheses indicate recombinants found in the mapped population (3 363 F2 mutant plants) of a given marker. Middle: annotated genes between two markers F6B6 and MRH10–5. Arrows denote the transcribed direction of each gene. Bottom: structure of PAR2. Exons and introns are represented by filled boxes and solid lines, respectively. The position and nature of the mutated nucleotide are shown. The position of the T-DNA insertion in gsnor1-3 42 is also shown. (B) Amino acid residue sequences of GSNOR1/HOT5/PAR2 and its homologs from different species. The alignment is around the mutated residue (Gly224). Conserved residues are shaded. Accession numbers: Arabidopsis: NP_199207; Rice: BAD21999; Human: NP_000662; Mouse: NP_031436; Zebrafish: NP_571924; Drosophila: NP_524310; Caenorhabditis elegans: NP_741507; Saccharomyces cerevisiae: NP_010113; Saccharomyces pombe: NP_588247; Escherichia coli: NP_414890. (C) Molecular complementation of the par2-1 mutant. Seven-day-old seedlings were germinated and grown on MS medium containing 1 μM paraquat. Comp 1: par2-1 plants carrying a pER10-PAR2 transgene (see text). (D) Allelism test of par2-1 and gsnor1-3 mutants. Five-day-old seedlings were germinated and grown on MS medium containing 1 μM paraquat. F1: F1 progenies obtained from a cross between par2-1 and gsnor1-3. (E) Molecular complementation of the gsnor1-3 mutant. Top: 10-day-old seedlings germinated and grown on MS medium containing 1 μM paraquat. Bottom: 2-week-old seedlings germinated and grown on MS medium. Comp 2: gsnor1-3 plants carrying a pER10-PAR2 transgene. Bar, 1 cm.
To verify the identity of the candidate PAR2 gene, we performed a genetic complementation experiment. A 3.3-kb wild type genomic DNA fragment, which contains the putative promoter region, 5′-untranslated region (UTR), coding sequence and 3′-UTR of At5g43940, was cloned into a binary vector. The resulting construct was transformed into par2-1 mutant plants by floral dipping. Among the analyzed eight independent transgenic lines, all plants showed a phenotype similar to that of wild type, including the restored sensitivity to paraquat and normal growth and development (Figure 3C and data not shown), suggesting that the observed par2-1 mutant phenotype was caused by a mutation in At5g43940.
In previous studies, At5g43940 has been characterized to encode a GSNOR 41, 42, 44, 45. A loss-of-function mutation in GSNOR1 (gsnor1-3) caused no GSNOR1 transcript accumulation 42, thus likely representing a null mutation (see Figure 3A). The gsnor1-3 mutant showed a phenotype similar to par2-1, including the paraquat resistance and abnormal growth and development (Figures 3D and 1E–1F). To test possible allelism between par2-1 and gsnor1-3, we crossed these two mutants and analyzed the resulting F1 plants. When germinated in the presence of paraquat, all tested F1 plants were resistant to the herbicide and showed a dwarf and bushy phenotype similar to their parents (Figure 3D and data not shown). Moreover, the At5g43940 transgene also fully restored the sensitivity of gsnor1-3 to paraquat and rescued the developmental defects (Figure 3E and data not shown). Therefore, par2-1 and gsnor1-3 are allelic, and At5g43940 represents the PAR2 gene. Five independent mutant alleles (hot5 alleles) of GSNOR1 have been recently characterized, which show compromised acclimation to heat stress as well as various developmental abnormalities similar to those observed in par2-1 46.
We obtained a GSNOR1/HOT5/PAR2 cDNA clone by reverse transcription (RT)-PCR, which was identical to an EST clone (accession number: AK226412). Comparison of the genomic and cDNA sequences revealed that GSNOR1/HOT5/PAR2 contained 9 exons, encoding a protein of 379 amino acid residues (Figure 3A). We noticed that GSNOR1/HOT5/PAR2 was previously reported to contain 8 exons, although the same size of the protein (379 amino acid residues) was reported 46. This discrepancy might be due to variants of alternative splicing or incorrect annotation.
PAR2 encodes an GSNOR
GSNOR is a highly conserved protein found in all living organisms from bacteria to higher eukaryotes, and catalyzes the metabolism of GSNO to form GSSG and NH3 as main products 40, 49. GSNOR1 is a single-copied gene in Arabidopsis, and has been characterized in some detail 41, 42, 43, 44, 45, 46. Loss- or gain-of-function mutations in GSNOR1 caused altered innate immunity against a variety of pathogens and altered intracellular SNO levels 42, 43. Moreover, GSNOR1/HOT5 also plays a critical role in thermotolerance 46.
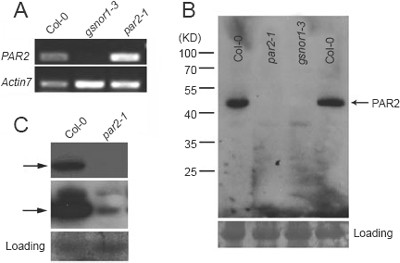
Similar to the results obtained from a previous study 42, expression of GSNOR1/HOT5/PAR2 was not detectable in gsnor1-3 by either RT-PCR or northern blot analysis (Figure 4A and data not shown). In par2-1, expression of GSNOR1/HOT5/PAR2 was comparable to that in wild type (Figure 4A), indicating that the mutation did not affect transcription or stability of the mutant mRNA. However, the mutant protein was remarkably reduced as revealed by an anti-PAR2 polyclonal antibody (Figure 4B and 4C), suggesting that the par2-1 mutation renders the mutant protein unstable and that the highly conserved Gly224 plays an important role in regulating the stability of PAR2 protein.
Figure 4
Expression of GSNOR1/HOT5/PAR2 in par2-1 and gsnor1-3. (A) Expression of GSNOR1/HOT5/PAR2 in wild type, par2-1 and gsnor1-3 plants (4-week-old seedlings) analyzed by reverse transcription (RT)-PCR. Actin7 was used as an internal control. (B) Western blot analysis of GSNOR1/HOT5/PAR2 protein in wild type, par2-1 and gsnor1-3 plants (4-week-old seedlings). Positions of molecular weight markers (in Kilo Daltons or KD) are shown at the left of the panel. Each lane contains 30 μg total proteins. The loading control was determined by staining the blot with Ponceau S. (C) Western blot analysis of GSNOR1/HOT5/PAR2 protein in wild type and par2-1 plants (4-week-old seedlings). GSNOR1/HOT5/PAR2 protein could be occasionally detected in par2-1. Wild type and mutant PAR2 proteins are denoted by arrows. Short- (top) and long-exposure (middle) of the same blot are shown.
Paraquat resistance is enhanced by NO donor SNP
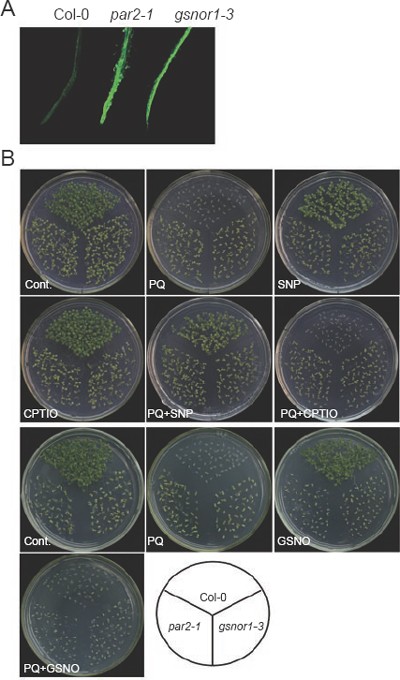
Previous studies indicated that gsnor1-3 and hot5 mutants had higher levels of SNO and NO species than wild-type plants 42, 43, 46. To analyze the intracellular NO level in par2-1, we stained wild type and mutant seedlings with 4,5-diaminofluorescein diacetate (DAF-2DA), a fluorescent dye for in vivo imaging of NO 50. In wild-type roots, specific fluorescent signals were slightly detectable under the assay condition. However, more intense signals were observed in par2-1 and gsnor1-3 mutant roots (Figure 5A), consistent with the observation made with hot5 mutant alleles 46.
Figure 5
Increased NO levels are correlated to the paraquat resistance. (A) Increased NO contents in par2-1 and gsnor1-3 roots. Two-week-old seedlings germinated and grown on MS medium were stained with DAF-2DA. Roots were observed and photographed under a fluorescent microscope. (B) Wild type (Col-0), par2-1 and gsnor1-3 seedlings germinated and grown under different conditions as indicated in the panel. Top two rows: 10-day-old seedlings; Bottom two rows: 12-day-old seedlings. Genotypes of the tested samples are indicated at the bottom. Cont.: control (MS medium); PQ: paraquat. Concentrations of reagents used in the experiment: paraquat, 1 μM; SNP, 100 μM; CPTIO, 150 μM; GSNO, 500 μM.
Because NO has been reported to act as an antioxidant 28, 32, 33, we reasoned that the paraquat-resistance phenotype of par2-1 may be related to the increased intracellular NO level in the mutant. To test this possibility, wild type, par2-1 and gsnor1-3 were germinated and grown in the presence of NO donors, sodium nitroprusside (SNP) and GSNO, or the NO scavenger, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidadazoline-1-oxy-3-oxide (CPTIO). When germinated and grown in the presence of SNP, whereas par2-1 and gsnor1-3 showed stronger resistance to paraquat, wild-type plants also displayed resistance to the herbicide (Figure 5B). Treatment with GSNO or CPTIO did not apparently alter sensitivity to paraquat (Figure 5B). A previous study found that CPTIO, applied alone or combined with SNP, caused apparent phenotype in hot5-1 seedlings in a heat-inhibited hypocotyl elongation assay. However, CPTIO did not have effects on wild-type seedlings, although SNP caused remarkable heat-sensitivity phenotype in the same assay 46. Presumably, heat-inhibition of hypocotyl elongation is a more sensitive assay than paraquat resistance. These results suggest that SNP is capable of inducing paraquat resistance in both wild-type and mutant plants.
Accumulation of GSNOR1/HOT5/PAR2 protein is regulated by paraquat and NO donors
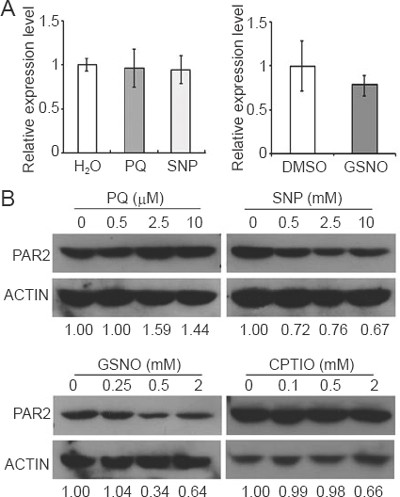
Because GSNOR1/HOT5/PAR2 directly modulates the NO level, which, in turn, correlates with resistance to paraquat that induces ROS generation, it is likely that expression of GSNOR1/HOT5/PAR2 is subjected to the regulation by oxidative stresses and the NO level. To explore this possibility, we analyzed GSNOR1/HOT5/PAR2 expression by quantitative RT-PCR (qRT-PCR) under different conditions. The steady-state level of GSNOR1/HOT5/PAR2 mRNA did not appear to be altered by a variety of treatments, including paraquat, SNP and GSNO (Figure 6A). We next examined the GSNOR1/HOT5/PAR2 protein level by western blot. We observed that paraquat treatment caused an increased accumulation of GSNOR1/HOT5/PAR2 protein (Figure 6B). Conversely, the GSNOR1/HOT5/PAR2 protein level was reduced when treated by NO donors, SNP and GSNO (Figure 6B). The GSNOR1/HOT5/PAR2 protein level did not appear to be significantly altered when treated with CPTIO alone or in combination with GSNO (Figure 6B and data not shown). These results indicate that the accumulation of GSNOR1/HOT5/PAR2 protein is enhanced by paraquat, but is negatively regulated by NO donors, suggesting possible reciprocal regulation on the accumulation of GSNOR1/HOT5/PAR2 protein by ROS and NO.
Figure 6
The accumulation of GSNOR1/HOT5/PAR2 protein is regulated by paraquat and NO donors. (A) Expression of GSNOR1/HOT5/PAR2 analyzed by qRT-PCR. Wild type (Col-0) seedlings (2-week-old) germinated and grown on MS medium were treated with paraquat (1 μM), SNP (200 μM) or GSNO (400 μM) for 12 h, and then used for the preparation of total RNA. Water- or DMSO (dimethyl sulfoxide, a solvent for GSNO)-treated seedlings were used as controls. The relative expression level of GSNOR1/HOT5/PAR2 was determined using Actin7 as an internal control. Data presented are mean values of two independent experiments (biological repeats) with standard derivations (bars in the graph). (B) Effects of paraquat, SNP, GSNO and CTPIO on the accumulation of GSNOR1/HOT5/PAR2 protein analyzed by western blot. Wild type seedlings (2-week-old) were treated with different concentrations of compounds (indicated on the top of the panel) for 12 h. Protein extracts were prepared and then subjected to western blot analysis using an anti-PAR2 antibody. Each lane contains 30 μg total proteins. The blot was striped and reanalyzed by an anti-actin antibody. Relative level of PAR2 protein was normalized with the actin level and indicated at the bottom of the blots.
Discussion
In this study, we show that paraquat-induced cell death is light dependent and shares characteristics of PCD by the induction of superoxide and nuclear DNA fragmentation. Moreover, we have identified and characterized the par2-1 mutant, revealing an important function of GSNOR1/HOT5/PAR2 in the regulation of ROS-mediated cell death. The par2-1 mutant was identified by its resistance to paraquat, which has been known to induce the generation of ROS and free radicals 15, 17, 18. It has been estimated that paraquat treatment on light-grown ryegrass and duckweed produces excessive amount of hydroxyl radicals equivalent to that produced by 10 000 rads of γ irradiation, a dose that is able to induce rapid cell death in plants 17. Thus, the killing effect of paraquat has been mainly attributed to the production of excessive ROS 15, 17, 18. Accordingly, resistance to paraquat in plants can be ascribed to two distinctive mechanisms. One possibility is the detoxifying effect such as by the removal of excessive amount of ROS and reactive hydroxyl radicals. The Arabidopsis rcd1 mutant, for example, displays resistance to paraquat, and this phenotype is associated with the increased expression of plastidic SOD and APX genes 22. Similarly, the paraquat-induced cell death can also be suppressed by overexpression of a chloroplast-targeted SOD gene 20 or a thylakoidal APX gene 51. When treated with paraquat, the par2-1 mutant displays reduced cell death, but accumulates superoxide at a level similar to that in wild type, suggesting that GSNOR1/HOT5/PAR2 may not be directly involved in the regulation of superoxide production or turnover.
A second possibility is that GSNOR1/HOT5/PAR2 acts downstream of superoxide to regulate a cell-death signaling pathway, and the par2-1 mutation blocks this signaling pathway that is activated by paraquat-induced superoxide or free radicals. Alternatively, GSNOR1/HOT5/PAR2 may regulate a superoxide-independent cell-death signaling pathway. Because a major physiological effect of paraquat is the induction of superoxide and free radicals in plant cells, it is reasonable to assume that PAR2 functions downstream of superoxide to regulate cell death. This view is supported by the observation that par2-1 displays resistance to FB1, which also induces the formation of ROS 8, 9, 11.
The superoxide-insensitivity phenotype of gsnor1/hot5/par2 mutants suggests that the wild-type allele is a component involved in a superoxide-mediated cell-death signaling pathway, and that the anti-cell death phenotype results from blocked superoxide signaling. What is the possible mechanism of the reduced superoxide-sensitivity of par2-1? PAR2 encodes a GSNOR involved in the regulation of a wide range of biotic and abiotic stress responses as well as plant growth and development. Consistent with the biochemical nature of GSNOR, multiple gsnor1/hot5/par2 mutant alleles show an increased NO level, whereas overexpression of GSNOR1/HOT5/PAR2 causes a reduced NO level 42, 43, 46. These results imply that the altered NO level may suppress cell death induced by paraquat in par2-1. Consistent with this notion, properly balanced homeostasis of ROS and NO has been found to play a critical role in the regulation of HR cell death 27, 28, 29. Although the underpinning mechanism of the ROS-NO interaction remains elusive, NO donors have been demonstrated to regulate ROS levels by inhibiting two antioxidant enzymes, catalase and APX, in tobacco 31. Moreover, reciprocal scavenging of NO and O2– was shown to cause the formation of peroxynitrite anion ONOO–, a compound that is less toxic in plants 28. Likewise, a similar mechanism may be operating in par2-1, and the NO donor SNP confers paraquat resistance, presumably by exerting an antagonistic effect against the oxidative stress through increasing the NO level. This view is supported by the observation that SNP also confers paraquat resistance to potato leaves 52, and acts as an antioxidant to delay PCD induced by gibberellin in barley aleurone layers 33. Again, consistent with the reciprocal regulation of NO and ROS, the accumulation of GSNOR1/HOT5/PAR2 protein is induced by paraquat, but is reduced when treated by SNP and GSNO. This result may partly explain the paraquat-resistance phenotype of par2-1 and SNP-treated wild-type plants, both of which have reduced GSNOR1/HOT5/PAR2 protein levels, which may act as a key regulator of the NO-ROS interaction to regulate cell death in plants.
Finally, GSNOR1/HOT5/PAR2 may also regulate plant cell death through _S_-nitrosylation. In animal cells, the regulatory role of _S_-nitrosylation in apoptosis has been well appreciated 53, 54, 55. In Arabidopsis, a number of _S_-nitrosylated proteins have been identified under different conditions using proteomic approaches 56, 57, 58. In particular, _S_-nitrosylation of peroxiredoxin II E has been demonstrated to inhibit its activity involved in hydroperoxide reduction and peroxynitrite detoxification 37, and _S_-nitrosylation of NPR1 is critical for the regulation of redox-based conformational changes that directly determine the NPR1 activity 35. In addition, the Arabidopsis METACASPASE9 activity is regulated by _S_-nitrosylation, providing a possible link between the redox-based posttranslational modification mechanism and biochemical execution of cell death in plant cells 38. Among these proteins, whereas the level of biologically active monomeric NPR1 is reduced in gsnor1-3, which is presumably caused by an altered _S_-nitrosylation pattern 35, it is unclear whether _S_-nitrosylation of other proteins and additional unidentified targets plays a regulatory role in PCD. It will be challenging and of great interest to clarify these questions, which should shed new light on the molecular mechanism of plant PCD.
Materials and Methods
Plant materials, growth conditions and genetic screen for par mutants
The Col-0, WS and L_er_ accessions of wild-type Arabidopsis were used in this study. Seeds of gsnor1-3 42 were kindly provided by Dr Gary Loake. Plants were grown under a 16 h light/8 h dark cycle or continuous white light (120∼130 μmol m-2 s-1) at 22 °C in soil or on an MS medium 48 containing 3% sucrose and 0.8% agar.
To screen par mutants, EMS-mutagenized M2 seeds, ∼15 000 seeds in the Col-0 background and 45 000 seeds in the WS background, were germinated and grown on MS agar plates containing 2 μM paraquat (Sigma, Hong Kong, China) for 7-10 days. Upon germination, whereas majority of seedlings were bleached or yellowish, putative par mutants became green or light green. These putative par mutant seedlings were transferred onto a fresh MS medium without paraquat and then to soil until maturation. M3 seeds of the putative par mutants were rescreened under the same conditions, and 12 mutants (2 in Col-0 and 10 in WS background) were recovered. Genetic and allelism analyses of the par mutants were performed by pair-wise crossing of individual mutants, followed by assessing phenotypes and genetic segregation patterns in F1 and F2 generations. In all cases, reciprocal crosses were performed and similar results were obtained.
Genetic mapping of par2-1
F2 seeds derived from crosses between par2-1 (Col-0) and wild-type L_er_ were germinated and grown on MS medium containing 2 μM paraquat for 10-15 days, and paraquat-resistant progenies were selected for genetic mapping. The par2-1 mutation was roughly mapped onto chromosome V, between CIW9 and K9E15 by bulk segregate analysis. An F2 population of 3 363 mutant plants was then analyzed to define the par2-1 mutation between F6B6 and MRH10-5 (see Supplementary information, Table S1 for sequences of all primers), two markers spanning ∼47 kb. PAR2 candidate gene was deduced by DNA sequencing analysis of all annotated genes in this region.
Analysis of cell death, superoxide, hydrogen peroxide and nuclear DNA fragmentation
Detection of cell death, superoxide and hydrogen peroxide was carried out by staining leaves with Evans blue, nitroblue tetrazolium (NBT) and 3,3′-diaminobenzidine, respectively, essentially as previously described 11. All experiments were repeated for 3-5 times (biological repeats), and 10-15 rosette leaves collected from multiple seedlings (4-5-week-old) were analyzed in each experiment.
Preparation of protoplasts and analysis of nuclear DNA fragmentation in protoplasts were performed as previously described 12 with minor modifications. Protoplasts were incubated with or without 0.5 μM paraquat for 6 h at 22 °C under continuous white light, fixed overnight in 2% paraformaldehyde and then subjected to TUNEL analysis, as previously described 12. Nuclear DNA was stained with 4′,6-diamino-2-phenylindole.
In vivo imaging of NO
Analysis of NO contents in Arabidopsis roots were performed by DAF-2DA staining as described 59 with minor modifications. Briefly, 2-3-week-old seedlings were pre-incubated for 2 h at room temperature in a solution of 0.1 mM CaCl2, 10 mM KCl, 10 mM MES-Tris, pH 5, and then stained with 10 μM DAF-2DA for 45 min. After brief rinse with water, the sample was observed and photographed under a fluorescent microscope equipped with a CCD camera (Olympus BX51).
Preparation of anti-PAR2 antibodies and western blot analysis
A GSNOR1/HOT5/PAR2 cDNA fragment (nucleotides 460-951), encoding amino acid residues 154-317 of the protein, was cloned into the _BamH_I and _Sal_I sites of a pET-28a vector (Novagen) to express a 6×His-PAR2 recombinant protein. The purified recombinant protein was used to immunize rabbits to generate the antiserum. The anti-PAR2 antiserum was extensively characterized using protein extracts prepared from wild type, par2-1 and gsnor1-3 plants as well as purified recombinant GSNOR1/HOT5/PAR2 protein, and then was directly used in all experiments in this study.
Protein extracts were prepared by grinding plant materials in liquid nitrogen and immediately mixing with grinding buffer (50 mM Tris, pH7.5, 150 mM NaCl, 10 mM MgCl2, 0.1% Nonidet P-40, 1 mM phenylmethanesulfonyl fluoride). After centrifugation twice at 4 °C for 10 min in a microcentrifuge at full speed, the supernatant was subjected to SDS-PAGE. The protein concentration was determined by the Bradford method using BSA as a standard 60. Typically, 30-60 μg of total proteins were used for SDS-PAGE. After the run, the proteins were electrically transferred onto a PVDF membrane (Whatman), and then detected with the anti-PAR2 antiserum as a primary antibody (∼5 000-fold dilution). After incubation with a secondary antibody (HRP-conjugated goat anti-rabbit IgG; Pierce Biotechnology), the signal was detected using a SuperSignal Western Femto Maximun Sensitivity Substrate kit (Pierce Biotechnology) according to the manufacturers instructions.
Molecular manipulations
All molecular manipulations were performed according to standard methods 61. A GSNOR1/HOT5/PAR2 (At5g43940) genomic DNA fragment of 3.3 kb was obtained by PCR from wild-type (Col-0) plants, verified by restriction digests and DNA sequencing, and then cloned into the _Xho_I and _Spe_I sites of a binary vector pER10 to yield pER10-PAR2. This genomic fragment contained a promoter sequence of 0.8 kb upstream from the putative translation start codon, starting from the 3′-UTR of At5g43935, a gene in a head-to-tail configuration with GSNOR1/HOT5/PAR2 (At5g43940). The pER10-PAR2 was transformed into both par2-1 and gsnor1-3 mutant plants by the floral dip method 62.
Total RNA was prepared using the TRizol reagents (Invitrogen) following the manufacture's instructions. Northern blot, RT-PCR and real-time qRT-PCR were carried out as previously described 12.
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
References
- Pennell RI, Lamb C . Programmed cell death in plants. Plant Cell 1997; 9:1157–1168.
Article CAS Google Scholar - Lam E . Controlled cell death, plant survival and development. Nat Rev Mol Cell Biol 2004; 5:305–315.
Article CAS Google Scholar - Lam E, Kato N, Lawton M . Programmed cell death, mitochondria and the plant hypersensitive response. Nature 2001; 411:848–853.
Article CAS Google Scholar - Dangl JL, Jones JD . Plant pathogens and integrated defence responses to infection. Nature 2001; 411:826–833.
Article CAS Google Scholar - Greenberg JT, Yao N . The role and regulation of programmed cell death in plant-pathogen interactions. Cell Microbiol 2004; 6:201–211.
Article CAS Google Scholar - Greenberg JT, Ausubel FM . Arabidopsis mutants compromised for the control of cellular damage during pathogenesis and aging. Plant J 1993; 4:327–341.
Article CAS Google Scholar - Jabs T, Dietrich RA, Dangl JL . Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 1996; 273:1853–1856.
Article CAS Google Scholar - Asai T, Stone JM, Heard JE, et al. Fumonisin B1-induced cell death in Arabidopsis protoplasts requires jasmonate-, ethylene-, and salicylate-dependent signaling pathways. Plant Cell 2000; 12:1823–1835.
Article CAS Google Scholar - Stone JM, Heard JE, Asai T, Ausubel FM . Simulation of fungal-mediated cell death by fumonisin B1 and selection of fumonisin B1-resistant (fbr) Arabidopsis mutants. Plant Cell 2000; 12:1811–1822.
Article CAS Google Scholar - Stone JM, Liang X, Nekl ER, Stiers JJ . Arabidopsis AtSPL14, a plant-specific SBP-domain transcription factor, participates in plant development and sensitivity to fumonisin B1. Plant J 2005; 41:744–754.
Article CAS Google Scholar - Shi L, Bielawski J, Mu J, et al. Involvement of sphingoid bases in mediating reactive oxygen intermediate production and programmed cell death in Arabidopsis. Cell Res 2007; 17:1030–1040.
Article CAS Google Scholar - Feng H, Chen Q, Feng J, et al. Functional characterization of the Arabidopsis eukaryotic translation initiation factor 5A-2 that plays a crucial role in plant growth and development by regulating cell division, cell growth, and cell death. Plant Physiol 2007; 144:1531–1545.
Article CAS Google Scholar - Teng C, Dong H, Shi L, et al. Serine palmitoyltransferase, a key enzyme for de novo synthesis of sphingolipids, is essential for male gametophyte development in Arabidopsis. Plant Physiol 2008; 146:1322–1332.
Article CAS Google Scholar - Gechev TS, Hille J . Hydrogen peroxide as a signal controlling plant programmed cell death. J Cell Biol 2005; 168:17–20.
Article CAS Google Scholar - Dodge AD . The mode of action of the bipyridylium herbicides, paraquat and diquat. Endeavour 1971; 30:130–135.
Article CAS Google Scholar - Suntres ZE . Role of antioxidants in paraquat toxicity. Toxicology 2002; 180:65–77.
Article CAS Google Scholar - Babbs CF, Pham JA, Coolbaugh RC . Lethal hydroxyl radical production in paraquat-treated plants. Plant Physiol 1989; 90:1267–1270.
Article CAS Google Scholar - Fujii T, Yokoyama E, Inoue K, Sakurai H . The sites of electron donation of photosystem I to methyl viologen. Biochim Biophys Acta 1990; 1015:41–48.
Article CAS Google Scholar - Arisi A-CM, Cornic G, Jouanin L, Foyer CH . Overexpression of iron superoxide dismutase in transformed poplar modifies the regulation of photosynthesis at low CO2 partial pressures or following exposure to the prooxidant herbicide methyl viologen. Plant Physiol 1998; 117:565–574.
Article CAS Google Scholar - Gupta AS, Heinen JL, Holaday AS, Burke JJ, Allen RD . Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase. Proc Natl Acad Sci USA 1993; 90:1629–1633.
Article CAS Google Scholar - Bowler C, Slooten L, Vandenbranden S, et al. Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO J 1991; 10:1723–1732.
Article CAS Google Scholar - Fujibe T, Saji H, Arakawa K, et al. A methyl viologen-resistant mutant of Arabidopsis, which is allelic to ozone-sensitive rcd1, is tolerant to supplemental ultraviolet-B irradiation. Plant Physiol 2004; 134:275–285.
Article CAS Google Scholar - Crawford NM, Guo F-Q . New insights into nitric oxide metabolism and regulatory functions. Trends Plant Sci 2005; 10:195–200.
Article CAS Google Scholar - Romero-Puertas MC, Perazzolli M, Zago ED, Delledonne M . Nitric oxide signalling functions in plant-pathogen interactions. Cell Microbiol 2004; 6:795–803.
Article CAS Google Scholar - Neill S, Barros R, Bright J, et al. Nitric oxide, stomatal closure, and abiotic stress. J Exp Bot 2008; 59:165–176.
Article CAS Google Scholar - Delledonne M . NO news is good news for plants. Curr Opin Plant Biol 2005; 8:390–396.
Article CAS Google Scholar - Delledonne M, Xia Y, Dixon RA, Lamb C . Nitric oxide functions as a signal in plant disease resistance. Nature 1998; 394:585–588.
Article CAS Google Scholar - Delledonne M, Zeier J, Marocco A, Lamb C . Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 2001; 98:13454–13459.
Article CAS Google Scholar - Zaninotto F, Camera SL, Polverari A, Delledonne M . Cross talk between reactive nitrogen and oxygen species during the hypersensitive disease resistance response. Plant Physiol 2006; 141:379–383.
Article CAS Google Scholar - Wendehenne D, Durner J, Klessig DF . Nitric oxide: a new player in plant signalling and defence responses. Curr Opin Plant Biol 2004; 7:449–455.
Article CAS Google Scholar - Clark D, Durner J, Navarre DA, Klessig DF . Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol Plant Microbe Interact 2000; 13:1380–1384.
Article CAS Google Scholar - Guo F-Q, Crawford NM . Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell 2005; 17:3436–3450.
Article CAS Google Scholar - Beligni MV, Fath A, Bethke PC, Lamattina L, Jones RL . Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers. Plant Physiol 2002; 129:1642–1650.
Article CAS Google Scholar - Wang Y, Yun B-W, Kwon E, et al. _S_-nitrosylation: an emerging redox-based post-translational modification in plants. J Exp Bot 2006; 57:1777–1784.
Article CAS Google Scholar - Tada Y, Spoel SH, Pajerowska-Mukhtar K, et al. Plant immunity requires conformational charges of NPR1 via _S_-nitrosylation and thioredoxins. Science 2008; 321:952–956.
Article CAS Google Scholar - Wang Y-Q, Feechan A, Yun B-W, et al. _S_-nitrosylation of AtSABP3 antagonizes the expression of plant immunity. J Biol Chem 2009; 284:2131–2137.
Article CAS Google Scholar - Romero-Puertas MC, Laxa M, Matte A, et al. _S_-nitrosylation of peroxiredoxin II E promotes peroxynitrite-mediated tyrosine nitration. Plant Cell 2007; 19:4120–4130.
Article CAS Google Scholar - Belenghi B, Romero-Puertas MC, Vercammen D, et al. Metacaspase activity of Arabidopsis thaliana is regulated by _S_-nitrosylation of a critical cysteine residue. J Biol Chem 2007; 282:1352–1358.
Article CAS Google Scholar - Stamler JS . Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell 1994; 78:931–936.
Article CAS Google Scholar - Liu L, Hausladen A, Zeng M, et al. A metabolic enzyme for _S_-nitrosothiol conserved from bacteria to humans. Nature 2001; 410:490–494.
Article CAS Google Scholar - Martínez MC, Achkor H, Persson B, et al. Arabidopsis formaldehyde dehydrogenase. Molecular properties of plant class III alcohol dehydrogenase provide further insights into the origins, structure and function of plant class p and liver class I alcohol dehydrogenases. Eur J Biochem 1996; 241:849–857.
Article Google Scholar - Feechan A, Kwon E, Yun B-W, et al. A central role for _S_-nitrosothiols in plant disease resistance. Proc Natl Acad Sci USA 2005; 102:8054–8059.
Article CAS Google Scholar - Rusterucci C, Espunya MC, Diaz M, Chabannes M, Martinez MC . _S_-nitrosoglutathione reductase affords protection against pathogens in Arabidopsis, both locally and systemically. Plant Physiol 2007; 143:1282–1292.
Article CAS Google Scholar - Achkor H, Diaz M, Fernandez MR, et al. Enhanced formaldehyde detoxification by overexpression of glutathione-dependent formaldehyde dehydrogenase from Arabidopsis. Plant Physiol 2003; 132:2248–2255.
Article CAS Google Scholar - Sakamoto A, Ueda M, Morikawa H . Arabidopsis glutathione-dependent formaldehyde dehydrogenase is an _S_-nitrosoglutathione reductase. FEBS Lett 2002; 515:20–24.
Article CAS Google Scholar - Lee U, Wie C, Fernandez BO, Feelisch M, Vierling E . Modulation of nitrosative stress by _S_-nitrosoglutathione reductase is critical for thermotolerance and plant growth in Arabidopsis. Plant Cell 2008; 20:786–802.
Article CAS Google Scholar - Díaz M, Achkor H, Titarenko E, Martínez MC . The gene encoding glutathione-dependent formaldehyde dehydrogenase/GSNO reductase is responsive to wounding, jasmonic acid and salicylic acid. FEBS Lett 2003; 543:136–139.
Article Google Scholar - Murashige T, Skoog F . A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 1962; 15:473–497.
Article CAS Google Scholar - Jensen DE, Belka GK, Du Bois GC . _S_-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem J 1998; 331:659–668.
Article CAS Google Scholar - Foissner I, Wendehenne D, Langebartels C, Durner J . In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J 2000; 23:817–824.
Article CAS Google Scholar - Murgia I, Tarantino D, Vannini C, et al. Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J 2004; 38:940–953.
Article CAS Google Scholar - Beligni MV, Lamattina L . Nitric oxide protects against cellular damage produced by methylviologen herbicides in potato plants. Nitric Oxide 1999; 3:199–208.
Article CAS Google Scholar - Hara MR, Agrawal N, Kim SF, et al. _S_-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol 2005; 7:665–674.
Article CAS Google Scholar - Mannick JB . Regulation of apoptosis by protein _S_-nitrosylation. Amino Acids 2007; 32:523–526.
Article CAS Google Scholar - Sawa A, Khan AA, Hester LD, Snyder SH . Glyceraldehyde-3-phosphate dehydrogenase: nuclear translocation participates in neuronal and nonneuronal cell death. Proc Natl Acad Sci USA 1997; 94:11669–11674.
Article CAS Google Scholar - Lindermayr C, Saalbach G, Durner J . Proteomic identification of _S_-nitrosylated proteins in Arabidopsis. Plant Physiol 2005; 137:921–930.
Article CAS Google Scholar - Lindermayr C, Saalbach G, Bahnweg G, Durner J . Differential inhibition of Arabidopsis methionine adenosyltransferases by protein _S_-nitrosylation. J Biol Chem 2006; 281:4285–4291.
Article CAS Google Scholar - Romero-Puertas MC, Campostrini N, Mattè A, et al. Proteomic analysis of _S_-nitrosylated proteins in Arabidopsis thaliana undergoing hypersensitive response. Proteomics 2008; 8:1459–1469.
Article CAS Google Scholar - He Y, Tang R-H, Hao Y, et al. Nitric oxide represses the Arabidopsis floral transition. Science 2004; 305:1968–1971.
Article CAS Google Scholar - Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248–254.
Article CAS Google Scholar - Sambrook J, Russell DW . Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, 2001.
- Clough SJ, Bent AF . Floral dip: a simplified method for _Agrobacterium_-mediated transformation of Arabidopsis thaliana. Plant J 1998; 16:735–743.
Article CAS Google Scholar
Acknowledgements
We thank Dr Gary Loake (University of Edinburgh, UK) for providing gsnor1-3 seeds. We are grateful to Drs Chuanyou Li, Shuhua Yang and Yiqin Wang for critically reading the manuscript. This study was supported by grants from the National Natural Science Foundation of China (30330360), the Ministry of Science and Technology of China (2006AA10A112) and the Chinese Academy of Sciences (KSCX2-YW-N-015).
Author information
Author notes
- Ruiqiang Chen, Shulan Sun, Chun Wang, Yansha Li and Yan Liang: These five authors contributed equally to this work.
Authors and Affiliations
- State Key Laboratory of Plant Genomics and National Plant Gene Research Center (Beijing), Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, 100101, China
Ruiqiang Chen, Shulan Sun, Chun Wang, Yansha Li, Yan Liang, Fengying An, Chao Li, Haili Dong, Xiaohui Yang, Jian Zhang & Jianru Zuo - Graduate School, Chinese Academy of Sciences, Beijing, 100049, China
Ruiqiang Chen, Shulan Sun, Chun Wang, Yansha Li, Fengying An, Chao Li & Haili Dong
Authors
- Ruiqiang Chen
You can also search for this author inPubMed Google Scholar - Shulan Sun
You can also search for this author inPubMed Google Scholar - Chun Wang
You can also search for this author inPubMed Google Scholar - Yansha Li
You can also search for this author inPubMed Google Scholar - Yan Liang
You can also search for this author inPubMed Google Scholar - Fengying An
You can also search for this author inPubMed Google Scholar - Chao Li
You can also search for this author inPubMed Google Scholar - Haili Dong
You can also search for this author inPubMed Google Scholar - Xiaohui Yang
You can also search for this author inPubMed Google Scholar - Jian Zhang
You can also search for this author inPubMed Google Scholar - Jianru Zuo
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toJianru Zuo.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, R., Sun, S., Wang, C. et al. The Arabidopsis PARAQUAT RESISTANT2 gene encodes an _S_-nitrosoglutathione reductase that is a key regulator of cell death.Cell Res 19, 1377–1387 (2009). https://doi.org/10.1038/cr.2009.117
- Received: 23 June 2009
- Revised: 29 June 2009
- Accepted: 03 July 2009
- Published: 06 October 2009
- Issue Date: December 2009
- DOI: https://doi.org/10.1038/cr.2009.117