Identification and characterization of phosphodiesterases that specifically degrade 3′3′-cyclic GMP-AMP (original) (raw)
Introduction
Cyclic di-nucleotides (c-di-NMPs) act as intracellular second messengers in bacteria, modulating a variety of cellular activities1,2. As the first identified c-di-NMP in bacteria3, cyclic di-GMP (c-di-GMP) has been implicated in the regulation of biofilm formation, bacterial dispersion and motility, virulence, cell cycle progression and differentiation4,5,6. It is now recognized as a second messenger universal in bacteria since enzymes for its synthesis and degradation are found in almost all major bacterial phyla. Gram-positive bacteria mainly utilize c-di-GMP, and also produce cyclic di-AMP (c-di-AMP) that is involved in the regulation of cell growth7, cell size8 and cell wall homeostasis9,10. Recently, 3′3′-cGAMP, generated from GTP and ATP by Vibrio cholerae (V. cholerae) dinucleotide cyclase DncV, was shown to be responsible for efficient intestinal colonization and chemotaxis regulation in the bacterium V. cholerae11. Soon after this, 2′3′-cGAMP was found as a product of cGAMP synthase cGAS in mammals12, which serves as a second messenger to activate the innate immunity by pathogenic DNA13,14,15. Notably, both c-di-GMP and c-di-AMP are able to activate mammalian innate immune responses by binding to STING (also named MITA or ERIS), the ER-bound protein responsible for the detection of c-di-NMPs and subsequent activation of TBK1 kinase16,17.
Cellular c-di-GMP is generated by diguanylate cyclases containing the GGDEF domain, and degraded by specific phosphodiesterases (PDEs) with the EAL or HD-GYP domain2. The EAL domain-containing PDE is a monomeric enzyme that linearizes c-di-GMP into 5′-pGpG, which is further degraded by nonspecific cellular PDEs18,19,20. Alternatively, the HD-GYP domain-containing PDE breaks the phosphodiester bond in c-di-GMP to produce 5′-pGpG, and further degrades 5′-pGpG into GMP. HD-GYP domains are a subclass of the HD domain superfamily, characterized by a His-Asp (HD) divalent metal-binding motif, a conserved His, His, Glu (HHE) motif and a Gly-Tyr-Pro (GYP) motif21,22,23. Although a lot of genetic evidence supports the c-di-GMP PDE function of HD-GYP domains, only a few HD-GYP proteins with c-di-GMP PDE activity have been reported23,24. It was reported that overexpression of an HD-GYP protein VCA0681 decreased the c-di-GMP level in V. cholera, but deletion of VCA0681 did not affect biofilm formation presumably controlled by c-di-GMP24, suggesting that the physiological role of this protein is yet to be identified.
Similarly, the levels of c-di-AMP are raised by diadenylate cyclases through condensation of two ATP moieties, and reduced by DHH-DHHA (DHH-associated) domain-containing PDEs that cleave c-di-AMP into pApA or AMP25,26. DHH domain proteins, which are named after the conserved Asp-His-His (DHH) motif in the active site, have been shown to function as phosphatases or phosphoesterases that hydrolyze many substrates such as inorganic pyrophosphate or single-stranded DNA27.
Although the degradation of c-di-GMP and c-di-AMP has been extensively studied, no PDEs have been reported to be responsible for cGAMP hydrolyzation. Given the fact that cGAMP plays critical roles in bacterial chemotaxis and infectivity, and in activating mammalian innate immunity in response to foreign DNA during microbial or viral infections, cellular levels of cGAMP must be tightly regulated. We hypothesized that 3′3′-cGAMP-specific PDE(s) should exist in V. cholera, which harbor the EAL, HD-GYP or DHH domain. V. cholerae is predicted to contain 22 EAL-GAF proteins and 9 HD-GYP proteins28. Three Phosphodiest domain proteins, one DHH-DHHA domain protein and one HDOD domain protein were further revealed when we searched the genome of V. cholerae strain El Tor Inaba N16961 (Supplemetnary information, Figure S2A). In this study, we tested all these 36 genes for their PDE activities to degrade cGAMP in a eukaryotic screening system and identified three novel 3′3′-cGAMP-specific PDEs. VCA0681 (herein designated as V-cGAP1) breaks 3′3′-cGAMP into 5′-ApG via an intermediate 5′-pApG, whereas VCA0210 and VCA0931 (designated as V-cGAP2 and 3, respectively) linearize 3′3′-cGAMP into 5′-pApG. Interestingly, V-cGAP1 functions as both a PDE to break 3′3′-cGAMP into 5′-pApG, and a 5′-nucleotidase to remove the phosphate at carbon 5′ of pApG. The HD-GYP domain is required for cGAMP degradation as mutations in either the HD motif or the GYP motif completely abolished the PDE activity of all three V-cGAPs. The expression of V-cGAPs was differentially induced by 3′3′-cGAMP production as demonstrated at both RNA and protein levels in V. cholera. Finally, in vivo experiments revealed that V-cGAPs enhanced bacterial chemotaxis and suppressed the bacterial infectivity by inhibiting intestinal colonization.
Results
A eukaryotic screening system to identify PDEs in V. cholerae
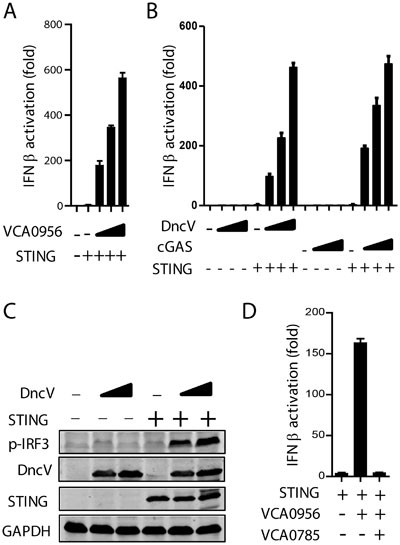
In mammalian cells, cytosolic DNA is sensed by its cytoplasmic receptor cGAS, which produces 2′3′-cGAMP to activate STING, leading to the phosphorylation and activation of transcription factor IRF3 and the subsequent upregulation of type I interferon production13. It has been demonstrated that STING is responsive not only to c-di-AMP/GMP, but also to all four forms of cGAMP, namely 3′3′-, 3′2′-, 2′3′- and 2′2′-cGAMPs13,14. In order to establish a screening system to detect the activation of STING by cyclic dinucleotides, we overexpressed VCA0956, a known dinucleotide cyclase in V. cholerae which produces c-di-GMP29, in 293T cells (Supplementary information, Figure S1A and S1B). VCA0956 overexpression led to a strong IFN-β promoter activation in the presence of STING expression (Figure 1A). Similarly, overexpression of the 3′3′-cGAMP synthetase DncV also strongly activated IFN-β promoter and induced IRF3 phosphorylation in a STING-dependent manner (Figure 1B and 1C). These data suggest that overexpression of V. cholerae dinucleotide cyclases mimics the effects of ectopic expression of their mammalian counterparts on activation of innate immune responses. Importantly, when VCA0785, a PDE known to hydrolyze c-di-GMP30, was co-expressed with VCA0956, IFN-β promoter activation was completely suppressed (Figure 1D). These results suggest that the luciferase reporter assay was sensitive enough to identify V. cholerae PDE(s) that could degrade cGAMP produced by DncV overexpression.
Figure 1
Establishment of a eukaryotic screening system to identify PDEs in V. cholera. (A) 293T cells were transiently transfected with increasing amounts (100, 200, 300 ng; wedges) of VCA0956 and 50 ng of STING, along with IFN-β-Luc reporter. The luciferase assay was performed after 24 h. (B, C) 293T cells were transfected with increasing amounts of DncV or cGAS with or without STING, along with the IFN-β-Luc reporter. The luciferase assay (B) or immunoblot analysis of p-IRF3 (C) was performed after 24 h. GAPDH was used as the loading control. (D) VCA0956 (50 ng) was transfected into 293T cells with or without PDE VCA0785 (300 ng), along with STING (50 ng) and IFN-β-Luc reporter. Twenty-four hours later, luciferase assay was performed. Unless otherwise noted, all results in this work were representative of at least three independent experiments.
Identification of cGAMP PDEs in V. cholerae (V-cGAPs) that inhibit DncV-induced IRF3 activation
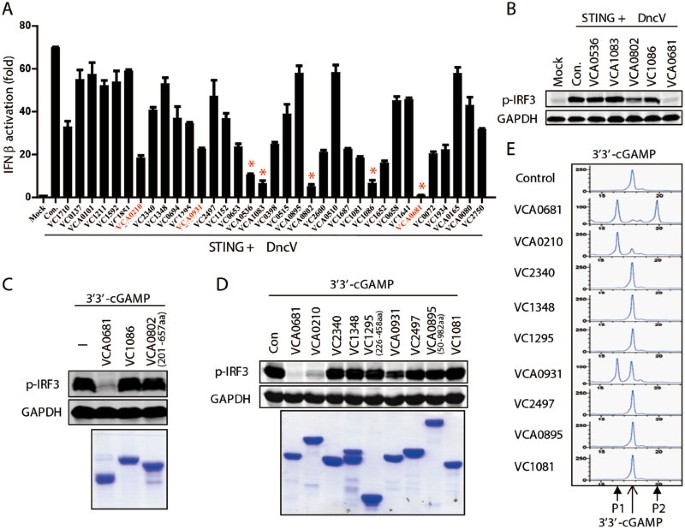
V. cholerae strain El Tor Inaba N16961 encodes 36 proteins with possible PDE activity (Supplementary information, Figure S2A). Each of these genes was cloned into a mammalian expression vector and co-transfected with DncV. Several proteins were found to significantly inhibit IFN-β luciferase activity induced by DncV, with five genes harboring the strongest inhibitory effects as marked by asterisks (Figure 2A and Supplementary information, Figure S2A and S2C). To exclude false-positive genes, these five genes were further tested for their ability to affect IRF3 phosphorylation induced by DncV. VCA0681 overexpression almost completely abrogated DncV-induced IRF3 activation, and VC1086 and VCA0802 showed minor effects. However, overexpression of VCA0536 or VCA1083 barely affected DncV-induced IRF3 phosphorylation (Figure 2B and Supplementary information, Figure S2D). Therefore, we focused on VCA0681, VC1086 and VCA0802 in the subsequent studies. In order to directly analyze the potential PDE activity of these three proteins, we utilized Perfringolysin O (PFO)-permeabilized THP-1 cells, which are able to respond to extracellular cGAMP with detectable IRF3 phosphorylation12, to set up an in vitro cGAMP activity assay. Bacterially expressed VCA0802, VC1086 and VCA0681 were purified and incubated with chemically synthesized 3′3′-cGAMP for 2 h. These treated 3′3′-cGAMP molecules were then added to PFO-permeabilized THP-1 cells to analyze its ability to induce IRF3 phosphorylation. Surprisingly, only VCA0681 destroyed 3′3′-cGAMP activity (Figure 2C). VCA0681 is an HD-GYP-containing protein, and has been reported to degrade c-di-GMP in vitro24. Notably, two other HD-GYP proteins, VCA0210 and VCA0931, the function of which has never been reported, were also shown to inhibit DncV-induced IFN-β promoter activation, although not as potent as VCA0681 (Figure 2A). To figure out whether other HD-GYP proteins identified in V. cholerae are also capable of inhibiting 3′3′-cGAMP activity, they were purified and subjected to the in vitro cGAMP activity assay (Supplementary information, Figure S2B). It was found that besides VCA0681 which completely abrogated cGAMP-induced IRF3 activation, VCA0210 and VCA0931 also inhibited 3′3′-cGAMP activity (Figure 2D). Next, high-performance liquid chromatography (HPLC) was used to determine whether 3′3′-cGAMP was degraded after incubation with each of these HD-GYP proteins, and we found that after 2-hour incubation, most cGAMP was eluted as the control sample, but cGAMP incubated with VCA0681, VCA0210 or VCA0931 was eluted quite differently. cGAMP was converted to one common product by all three PDEs, to which we named product 1 (P1). Among these three proteins, VCA0931 has the lowest activity as only half of cGAMP was converted to P1 at this point, consistent with its minimal PDE activity in the in vitro cGAMP activity assay (Figure 2D). Nevertheless, VCA0931 is able to degrade 3′3′-cGAMP completely given enough time (Figure 3B and Supplementary information, Figure S4A). In addition, VCA0681 produced another peak, which was eluted slower than 3′3′-cGAMP. This product was named P2. Since these HD-GYP proteins are supposed to function as PDEs, and all produced P1 at earlier points, we hypothesized that P1 is the linearized cGAMP, whereas P2 is the further hydrolyzed product. In fact, it has been shown by different groups that 5′-pApA or 5′-pGpG was eluted faster than c-di-AMP or c-di-GMP in HPLC18,19,25. These data suggest that these three HD-GYP proteins may antagonize 3′3′-cGAMP activity through their PDE activity to degrade cGAMP. We thus named these proteins as V ibrio cholerae cGAMP PDEs, V-cGAPs. VCA0681, VCA0210 and VCA0931 were designated as V-cGAP1, 2 and 3, respectively. To our knowledge, this is the first report on enzymes catalyzing 3′3′-cGAMP degradation.
Figure 2
Identification of cGAMP PDEs in V. cholerae (V-cGAPs) that inhibit DncV-induced IRF3 activation. (A) 293T cells were transiently transfected with 50 ng of DncV and 150 ng of the indicated Flag-tagged PDE candidates, along with STING and IFN-β-Luc reporter construct. Luciferase assay was performed after 24 h. Candidates with the most significant difference were marked with an asterisk (*) and selected for further analysis. Genes in red are HD-GYP proteins fully characterized in this study. (B) Flag-tagged PDE candidates as indicated were transfected into 293T cells, along with STING and DncV. p-IRF3 levels were then analyzed. (C) Chemically synthesized 3′3′-cGAMP was incubated with the indicated purified proteins (bottom) for 2 h. cGAMP activity assay (top) was performed in permeabilized THP-1 cells. (D, E) All indicated HD-GYP domain-containing proteins in V. cholerae were expressed and purified from E.coli (D, bottom), and incubation with 3′3′-cGAMP for 2 h. cGAMP activity assay (D, top) or HPLC assay (E) was then performed. P1: the first product; P2: the second product (also see Figure 4A).
Figure 3
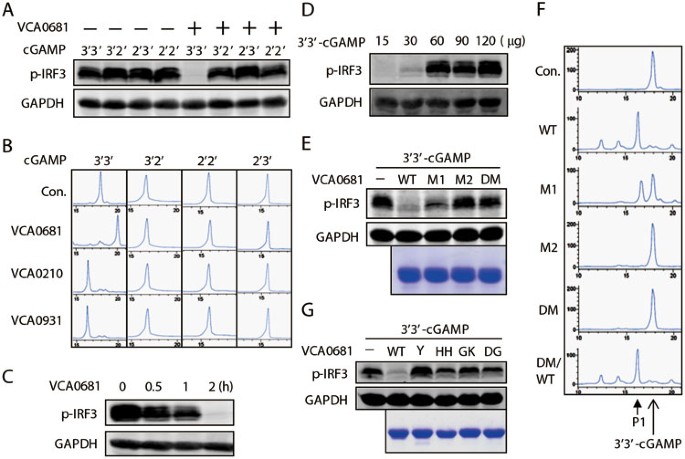
V-cGAPs specifically degrade 3′3′-cGAMP. (A) Four chemically synthesized cGAMP isoforms were incubated with purified VCA0681 for 2 h, and then the cGAMP activity assay was performed. (B) HPLC assay was performed after incubation of cGAMPs with the indicated purified V-cGAPs for 10 h. (C, D) 2.5 μg of 3′3′-cGAMP (C) or indicated amounts of 3′3′-cGAMP (D) was incubated with 3 μg of purified VCA0681 for the indicated times (C) or 10 h (D). The cGAMP activity assay was then performed. (E, F) VCA0681 HD/AA mutants as indicated were expressed and purified from E. coli(E, bottom), and incubated with 3′3′-cGAMP for 2 h (E) or 40 min (F). The cGAMP activity assay (E, top) or HPLC analysis (F) was then performed. M1: H74A/D75A; M2: H288A/D289A; DM: H74A/D75A/H288A/D289A; DM/WT: A74H/A75D/A288H/A289D. (G) Similar to E, purified VCA0681 mutants as indicated (bottom) were incubated with 3′3′-cGAMP for 2 h, and then the cGAMP activity assay (top) was performed. Y: Y350A; HH: H341A/H342A/E343A; GK: G291A/K292A; DG: D346A/G347A.
V-cGAPs specifically degrade 3′3′-cGAMP.
Next, we tested the specificity of V-cGAPs to other forms of cGAMP. Interestingly, V-cGAPs only worked on 3′3′-cGAMP but not other forms of cGAMP as revealed by the in vitro cGAMP activity assay (Figure 3A and Supplementary information, Figure S3A) and the HPLC analysis (Figure 3B). This result coincides with the fact that only 3′3′-cGAMP has been discovered in prokaryotic organisms11, and suggests the high specificity of V-cGAPs. Dosage- and time-dependent feature further confirmed that V-cGAPs act as PDEs to degrade 3′3′-cGAMP (Figure 3C, 3D and Supplementary information, Figure S3B). Consistent with previous work, VCA0681 also hydrolyzed c-di-GMP but not c-di-AMP24, so did VCA0210 and VCA0931 (Supplementary information, Figure S3C and data not shown). These results suggest a similar spatial conformation between 3′3′-cGAMP and c-di-GMP. Notably, prolonged treatment of c-di-GMP with VCA0681, but not with VCA0210 or VCA0931, further hydrolyzed GMP into the nucleoside G (Supplementary information, Figure S3C). To determine whether the HD-GYP domain contributes to the PDE activity of V-cGAPs, we replaced the conserved HD residues with AA residues in these proteins (Supplementary information, Figure S3D). In vitro cGAMP activity assay and HPLC analysis of these mutants indicated that the HD-GYP domain is absolutely essential for the PDE activity of all three V-cGAPs; however, mutation of the N-terminal HD domain of VCA0681 (M1) showed a minor effect (Figure 3E,3F, and Supplementary information, Figure S3D-S3F). To confirm that the loss of inhibition was indeed caused by the mutation, HD mutants were reverted to their WT version by site-directed mutagenesis, and the PDE activity of V-cGAPs was recovered (Figure 3F and Supplementary information, Figure S3D). Similarly, mutation of conserved amino acids (Y350A, G291A/K292A, D346A/G347A, etc) in the GYP motif of the HD-GYP domain22 on V-cGAP1 (VCA0681) also completely destroyed the PDE activity (Figure 3G). These data collectively demonstrate that V-cGAPs are 3′3′-cGAMP-specific PDEs.
V-cGAPs break 3′3′-cGAMP into 5′-ApG via the intermediate 5′-pApG
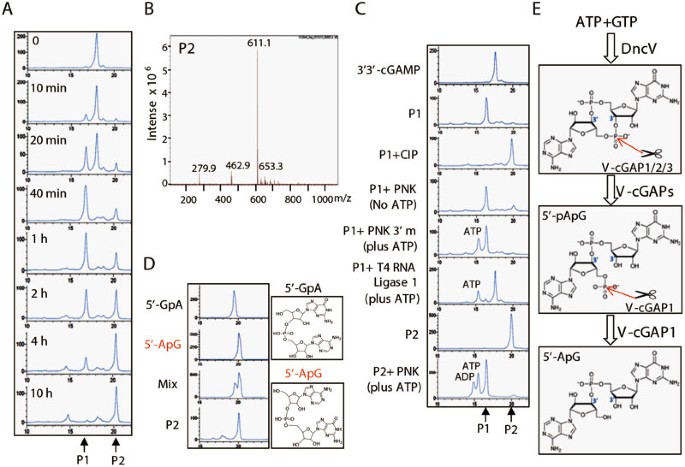
To determine how V-cGAPs degrade 3′3′-cGAMP, we carried out detailed HPLC analysis of the 3′3′-cGAMP products generated by purified V-cGAPs. It was found that VCA0210 or VCA0931 broke 3′3′-cGAMP into P1 with similar kinetics, though VCA0210 appeared to work more efficiently (Supplementary information, Figure S4A). P1 was quite stable even after prolonged incubation (10 h) with VCA0210 or VCA0931 (Figure 3B). Notably, treatment of P1 with T4 RNA ligase 1 converted P1 back to 3′3′-cGAMP (data not shown), confirming that VCA0210 and VCA0931 only linearize cGAMP as a PDE. Surprisingly, VCA0681 worked on 3′3′-cGAMP with a much higher activity. P1 appeared at 10 min and its level peaked at 40 min. Moreover, VCA0681 further converted P1 into P2 in 4 h (Figure 4A), suggesting that VCA0681 has activity other than PDE. Different from other nucleases, such as ribonuclease T2 and snake venom PDE I, that directly hydrolyze 3′3′-cGAMP into two nucleosides15, VCA0681 degraded 3′3′-cGAMP into an unidentified molecule that is neither a nucleoside (G or A) nor a nucleotide mono-phosphate (AMP or GMP) (Supplementary information, Figure S4B). Mass spectra (MS) analysis of P2 revealed a molecular weight of 611.1 Da, lower than that of 3′3′-cGAMP, which is 673.1 Da (Figure 4B). Notably, the lost weight is equal to the molecular weight of one phosphorus atom plus two oxygen atoms, suggesting that VCA0681 might also act as a nucleotidase. Based on this observation, we hypothesized that 3′3′-cGAMP was first opened up to generate P1, a linear GpAp, ApGp, pGpA or pApG. P1 was then hydrolyzed into P2, a GpA or ApG. To test this, we performed the following enzymatic treatments (Figure 4C). T4 RNA ligase 1 treatment converted P1 back to 3′3′-cGAMP, suggesting that P1 is indeed the linearized cGAMP. Calf intestinal phosphatase (CIP) treatment which removes 5′-phosphate group turned P1 into P2, suggesting that P1 is either a pGpA or a pApG, which was confirmed by the result that polynucleotide kinase (PNK) converted P2 into P1 through its ATP-dependent 5′-hydroxyl kinase activity. Consistently, in the absence of ATP, PNK did not catalyze P1 into P2 via its 3′-phosphatase activity, excluding the possibility that P1 is GpAp or ApGp. Importantly, the HPLC retention time of chemically synthesized 5′-GpA and 5′-ApG confirmed that P2 is indeed a 5′-ApG (Figure 4D and Supplementary information, Figure S4C and S4D). These results collectively suggest that VCA0681 linearizes 3′3′-cGAMP into 5′-pApG, and further hydrolyzes 5′-pApG to produce 5′-ApG, during which VCA0681 functions as both a PDE and a 5′-nucleotidase (Figure 4E). These results agree with the previous work showing that HD-GYP proteins may have multiple enzymatic activities31. Treatment of P1 produced by other two PDEs, VCA0210 and VCA0931, with CIP also converted P1 to P2, which had a same HPLC retention time as P2 produce by VCA0681 (Supplementary information, Figure S4A), suggesting that these two PDEs also linearize 3′3′-cGAMP into 5′-pApG.
Figure 4
V-cGAP1 degrades 3′3′-cGAMP into 5′-ApG via 5′-pApG. (A) 3′3′-cGAMP was incubated with purified VCA0681 for the indicated times, followed by HPLC analysis. (B) P2 (as shown in A) was purified and enriched by C18 reverse-phase HPLC system, followed by MS (ESI) analysis: (M−H) = 611.1. (C) Purified P1 and P2 were treated with the indicated enzymes for 2 h, followed by HPLC analysis. CIP, calf intestinal phosphatase; PNK (3′m), T4 polynucleotide kinase (3′ phosphatase minus). (D) Chemically synthesized 5′-GpA and 5′-ApG as illustrated (right) were fractionated by HPLC (left). Retention time: 5′-GpA, 19.449 min; 5′-ApG, 20.058 min; P2, 20.058 min. (E) Model for 3′3′-cGAMP degradation by V-cGAPs. V-cGAP1/2/3 first linearize 3′3′-cGAMP (produced by DncV from ATP and GTP) into 5′-pApG (P1), followed by further hydrolysis to produce 5'-ApG (P2) only by V-cGAP1.
V-cGAPs play a vital role in V. cholerae chemotaxis and colonization in vivo
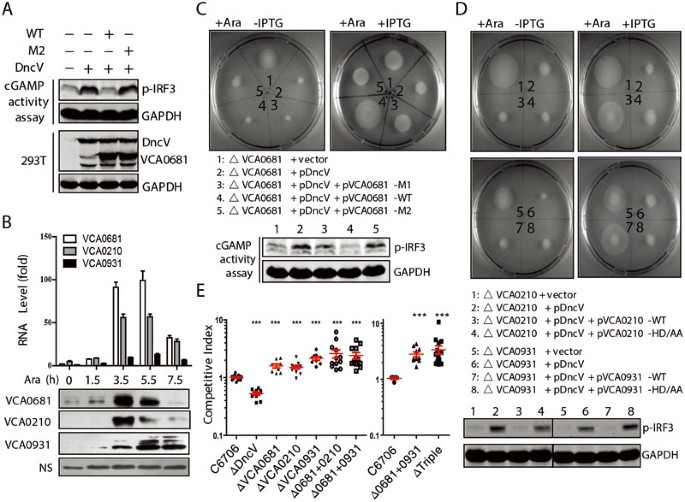
To assess the impact of V-cGAPs on DncV function in cells, we tested whether V-cGAPs could impair 3′3′-cGAMP activity under more physiological conditions. DncV or cGAS was first overexpressed in 293T cells, 3′3′-cGAMP (produced by DncV expression) or 2′3′-cGAMP (produced by cGAS expression) was isolated from the transfected cells after 24 h, and the cGAMP activity assay was performed in PFO-permeabilized THP-1 cells. It was found that, like cGAS, DncV-transfected cells produced cGAMPs which induced robust IRF3 activation in THP-1 cells (Supplementary information, Figure S5A). However, upon the forced expression of wild-type (WT) VCA0681, but not its mutant M2, DncV overexpression-caused IRF3 activation was completely abolished (Figure 5A). This result confirms that VCA0681 functions to antagonize the activity of 3′3′-cGAMP produced in mammalian cells. Previous work showed that DncV is an inducible gene, requiring outside signals such as the interaction with host cells to trigger its expression. When overexpressed, DncV was shown to contribute to an enhanced intestinal colonization and a hyperinfectious state of V. cholerae in vivo due to elevated 3′3′-cGAMP production11. As V-cGAPs are 3′3′-cGAMP-specific PDEs, we reasoned that their expression levels might also be regulated by DncV expression. To test this, we first analyzed V-cGAP expression at the mRNA level by quantitative realtime RT-PCR (qRT-PCR), and found that V-cGAP expression was greatly and readily enhanced after arabinose-induced DncV expression in Δ_dncV_ mutant V. cholerae (Figure 5B). Interestingly, the extent and dynamics of expression were quite different among these three PDEs. VCA0681 showed the strongest but also the most transient induction while VCA0931 showed a slow but steady induction. We also generated mouse anti-sera against these PDEs. Immunoblot analysis revealed that V-cGAP proteins were also greatly induced by arabinose treatment (Figure 5B), suggesting that V-cGAP expression is indeed induced by 3′3′-cGAMP production.
Figure 5
V-cGAPs regulate bacterial chemotaxis and intestinal colonization in vivo. (A) 293T cells were transfected with VCA0681 (WT or M2) and DncV. After 24 h, extracts from these cells were assayed for the presence of cGAMP activity, which was measured by detecting IRF3 phosphorylation after delivery to permeabilized THP-1 cells. (B) Quantitative RT-PCR assay (top) or immunoblot analysis (bottom) was performed to analyze the RNA or protein levels of V-cGAPs at the indicated times after arabinose (Ara) treatment in V. cholerae Δ_dncV_ mutants expressing DncV from an arabinose-inducible plasmid. NS (nonspecific band) served as the loading control. (C, D) Examination of chemotactic behavior (top) of V. cholerae_Δ_v-cGAPs mutants expressing control vector, arabinose-inducible DncV, and DncV together with IPTG-inducible V-cGAPs (WT or mutant) after induction by arabinose (left) or both arabinose and IPTG (right). Endogenous cGAMP activity assay (bottom) was performed as in A. VCA0210-HD/AA: H382A/D383A; VCA0931-HD/AA: H317A/D318A. (E) In vivo competition experiments measuring the ability of the indicated mutant strains to colonize the infant mouse intestine compared to the parental strain (C6706). Triple indicates the deletion of three V-cGAPs. Statistical significance was determined by comparing colonization ratio of mutants vs the parental strain WT C6706 (Student's _t_-test, ***P < 0.001).
Based on these observations and our in vitro results, we proposed that V-cGAPs counteract DncV function in vivo. To test this hypothesis, we generated DncV deletion mutant (Δ_dncV_), V-cGAP deletion mutants (ΔVCA0681, ΔVCA0210 and ΔVCA0931), and combined V-cGAP deletions (Δ0681+0210, Δ0681+0931 and ΔTriple: 0681+0931+0210) by allelic exchange in strain C6706, and performed the chemotactic assay. Consistent with previously reported data, no obvious growth difference was observed between the WT strain and Δ_dncV_ mutant in a semisolid agar chemotaxis plate (data not shown). Overexpression of DncV in the Δ_dncV_ strain (dncV_-pDncV) resulted in inhibition of chemotaxis. Interestingly, enforced DncV expression in VCA0681 deletion mutant (ΔVCA0681-pDncV) even further enhanced chemotactic inhibition (Supplementary information, Figure S5B). Consistently, cGAMP activity assay demonstrated that cell extracts isolated from ΔVCA0681-pDncV caused the strongest IRF3 activation, with Δ_dncV_-pDncV in the middle (Supplementary information, Figure S5B), suggesting that VCA0681 regulates the level of 3′3′-cGAMP in bacteria. As expected, the induced expression of WT, but not the HD-mutant, V-cGAPs antagonized chemotactic suppression caused by DncV expression (Figure 5C and 5D). This is consistent with the cGAMP activity assay showing that cell extracts from bacteria with induced V-cGAP (WT) expression completely lost the 3′3′-cGAMP activity (Figure 5C and 5D). Importantly, deletion of any V-cGAPs in V. cholerae did not affect biofilm formation (Supplementary information, Figure S5C), although enforced expression showed some inhibitory effects (data not shown), which is most likely due to protein overexpression. As bacterial chemotaxis correlates with its infectivity, we next performed the infant mouse colonization competition assay to determine whether V-cGAPs were involved in V. cholerae pathogenesis. Consistent with previous data reported by Mekalanos' group11, Δ_dncV bacteria showed a notable defect in intestinal colonization. In sharp contrast, V. cholerae deficient for any V-cGAPs showed a significant increase in the ability to colonize the small intestine compared to the WT strain (Figure 5E). Importantly, combined V-cGAP deletion (Δ0681+0210 and Δ0681+0931) showed enhanced bacterial infectivity, and the triple deletion (ΔTriple) had the highest infectivity, suggesting that these PDEs all contribute to the degradation of cGAMPs, probably under different physiological conditions. This was also inferred from their expression pattern after DncV-caused 3′3′-cGAMP production (Figure 5B). These data collectively suggest that V-cGAPs exert crucial function in regulating bacterial infectivity through catalyzing 3′3′-cGAMP degradation.
Discussion
In this work, we developed a screening system that combined the IFN-β luciferase reporter assay with the in vitro cGAMP activity assay, and for the first time identified and characterized three 3′3′-cGAMP-specific PDEs V-cGAP1/2/3. Previously, Bassler's group24 reported that V-cGAP1 (VCA0681) functions as a c-di-GMP PDE as overexpression of this protein reduced c-di-GMP level and biofilm formation. However, deletion of V-cGAP1 from the V. cholerae El genome was not observed to alter biofilm formation. Consistently, we did not observe any difference in biofilm formation between WT and the V-cGAP1 deletion (ΔVCA0681) strains. Instead, enforced DncV expression in ΔVCA0681 caused an enhanced inhibition of chemotaxis, and the ΔVCA0681 strain showed a significant increase in the ability to colonize small intestine. These results suggest that V-cGAP1 has more important physiological functions in 3′3′-cGAMP regulation than in c-di-GMP regulation. Interestingly, it has been demonstrated that VC0179, encoding DncV, locates in the Vibrio 7th pandemic island-1 (VSP-1 island) that is a genetic characteristic distinguishing the 7th pandemic El Tor biotype V. cholerae from former classical pandemic strains32. However, V-cGAP1/2/3 exist in conservative chromosome regions in all strains of V. cholera, suggesting that 3′3′-cGAMP might also be produced by other dinucleotide cyclase(s) and that its utilization as a second messenger is an ancient strategy. In line with this, VSP or VSP-like islands were also found in non-O1/non-O139 V. cholerae strains and across both the 7th pandemic El Tor biotype V. cholerae and V. mimicus33,34.
In addition, we identified two other previously uncharacterized HD-GYP PDEs, VCA0210 and VCA0931, both functioning only as PDEs to linearize 3′3′-cGAMP. Given the fact that they are both HD-GYP domain-containing proteins, degrading c-di-GMP but not c-di-AMP, we propose that the P1 product generated by VCA0210 or VCA0931 would also be 5′-pApG. In fact, from enzymatic manipulations, P1 produced after VCA0210/VCA0931 treatment were converted to 5′-ApG, but not 5′-GpA, indicating that all V-cGAPs linearize 3′3′-cGAMP into 5′-pApG. It was recently reported that DncV generates 3′3′-cGAMPs in two steps, with adenosine 3-OH attack of the guanosine a-phosphate, generating the linear dinucleotide intermediate pp(c)pA[3′–5′]pG, followed by the guanosine 3′-OH attack of the adenosine a-phosphate35. Our data showed that V-cGAPs hydrolyze 3′3′-cGAMP by attacking the chemical bond formed at the second step of cyclization, suggesting that this bond might be more vulnerable or conformationally suitable to PDEs. Nevertheless, these PDEs likely play non-redundant roles in 3′3′-cGAMP degradation as the expression of three PDEs is differentially regulated by 3′3′-cGAMP production and the combined gene deletion caused enhanced bacterial infectivity. Although VCA0931 showed the lowest PDE activity in in vitro cGAMP activity assay, sustained VCA0931 expression correlated perfectly with the most prominent bacterial infectivity in the VCA0931 deletion mutant, suggesting the fine tuning feedback regulation of this important protein.
The degradation of 3′3′-cGAMP by V-cGAP1 is unique in a way that 3′3′-cGAMP is first linearized and then dephosphorylated. Consistently, c-di-GMP was also first linearized and further hydrolyzed into nucleoside G by V-cGAP1, but not by V-cGAP2/3. In contrast, previous work demonstrated that HD-GYP PDEs hydrolyze c-di-GMP to produce 5′-pGpG, which is further broken into GMP23. Whether 5′-ApG is further hydrolyzed into the nucleoside (G or A) or nucleotide mono-phosphate by other enzymes is currently unknown. It is highly possible that there are PDEs specifically involved in breaking 5′-pApG or 5′-ApG. The HD-GYP domain of V-cGAPs is absolutely required for their PDE activity as mutations in the HD-GYP domain completely destroyed PDE activity of V-cGAPs. Further crystallographic study would provide a better insight into how V-cGAPs interact with 3′3′-cGAMP.
Nothing is known about the degradation of 2′3′-cGAMP, which plays a vital role in innate immune responses to pathogenic DNA in mammalian cells. The high specificity of V-cGAPs on 3′3′-cGAMP suggests that there should be 2′3′-cGAMP-specific PDE(s). Although the conserved HD domain was found in a variety of mammalian PDEs that degrade cyclic nucleotide, like cAMP, there is no HD-GYP domain protein found in mammals36. Although previous work from Walsh's group37 showed that mutations in the GYP motif of _Pm_GH in Persephonella marina did not affect its catalytic activity as a PDE to hydrolyze c-di-GMP, we found that the GYP motif is essential for 3′3′-cGAMP degradation, suggesting that the HD domain alone is not sufficient for cGAMP hydrolysis. Moreover, recent work by Doudna's group35 showed that DncV and human cGAS generate cGAMPs in sequential reactions that proceed in opposing directions. Given the fact that all V-cGAPs attack 3′3′-cGAMP on the phosphodiester bond formed at the second step of cyclization, it is plausible to speculate that mammalian cGAMP PDE(s) would also attack the phosphodiester bond vulnerable and/or more accessible to PDEs. These results will provide valuable information for the identification and characterization of mammalian 2′3′-cGAMP-specific PDEs in future studies.
Materials and Methods
Plasmids
cDNAs encoding VC0179, VCA0956, VCA0785 and all PDE candidates tested in this work were amplified from the genomic DNA of V. cholerae strain N16961, and cloned into p3×Flag-CMV-14 (Sigma) or p3×Flag-CMV-7.1 (Sigma) to generate Flag-tagged proteins. Deleted, truncated and point mutants were generated by the KOD-Plus-Mutagenesis Kit (TOYOBO Bio-Technology). The construct coding the WT protein served as the template. Homo Flag-cGAS expression vector was kindly provided by Dr Zhijian J Chen (University of Texas Southwestern Medical Center, Dallas, TX, USA). For ectopic expression, DncV was cloned into plasmids pBAD24 and pBAD33. VCA0681, VCA0210 and VCA0931 were cloned into plasmid pMalc2x. The resulting plasmids were electroporated into receptor V. cholerae strains. Primers are listed in Supplementary information, Table S1B. Other expression plasmids were described previously38. Each construct was confirmed by sequencing.
In vitro cGAMP activity assay
Purified V-cGAPs were incubated with different cGAMPs in the reaction mixture (20 mM HEPES, pH 7.0, 1 mg/ml bovine serum albumin, 1 mM DTT and 5 mM MgCl2) at 30 °C for the indicated time. After heating at 95 °C for 5 min, the mixture was centrifuged at 12 000 rpm for 5 min. A total of 2 μl supernatant was mixed with 106 PFO-permeabilized THP1 cells to detect the phosphorylation of IRF3 as reported13. To isolate 3′3′-cGAMP from 293T cells expressing DncV, the supernatant (S100) of dounced cells was prepared according to the reported protocol13, and concentrated by drying at 65 °C before the in vitro cGAMP activity assay.
To isolate endogenous 3′3′-cGAMP from V. cholerae colonies on chemotaxis plate, colonies of equal wet weight were re-suspended in the culture medium. After ultrasonication, the mixture was heated at 95 °C for 5 min and centrifuged at 14 000 rpm for 5 min before the in vitro cGAMP activity assay.
HPLC and MS
HPLC was carried out on a Shimadzu LC-20A HPLC system. Samples were purified or fractionated by analytical Inertsil ODS-3 C18 column (250 × 4.6 mm, 5 μm) at 1 ml/min flow rate, with a gradient of B (CH3CN) in A (50 mM TEAA pH 7.2; 0%-20% of B over 15 min, 20%-30% of B over 10 min). MS (ESI) was obtained by using Bruker APEX IV instrument.
Construction of mutants and DNA manipulations in V. cholerae.
DncV (VC0179) and V-cGAP deletion mutants were constructed by allelic exchange using strain C6706 as WT precursor (Supplementary information, Table S1A). All primers were designed based on the DNA sequence of the V. cholerae N16961 genome downloaded from the TIGR database (available at: http://cmr.tigr.org). The flanking sequences of the target genes were amplified from genomic DNA of strain N16961. The chromosomal fragments containing a deletion of the target genes were obtained with bridge PCR using the mixture of the up and down flanking sequences products as templates and cloned into suicide vector pWM91. The resulting suicide plasmid was constructed in E. coli SM10λpir and mobilized into receptor strain C6706. Exconjugants were selected on LB agar containing PolB (100 units/ml) and Amp (150 μg/ml) and streaked on LB agar containing 15% (w/v) sucrose. Sucrose-resistant colonies were tested for Amp sensitivity and deletion mutants were confirmed by DNA sequencing.
Quantitative PCR
Total RNA from V. cholerae was extracted using the Trizol Plus RNA purification system (TaKaRa) followed by the DNase treatment (Ambion) to remove residual chromosomal DNA contamination. The purity and integrity of RNA samples were verified by UV spectrophotometry and agarose gel electrophoresis. cDNA was synthesized by reverse transcription with Superscript II reverse transcriptase (Invitrogen). cDNA equivalent to 20 ng total RNA was analyzed by qRT-PCR with SYBR Green (TaKaRa). Relative expression values (R) were calculated using the equation R = 2−(ΔCt target −ΔCt reference)where Ct is the fractional threshold cycle. The recA mRNA was used as reference. A control mixture lacking reverse transcriptase was performed for each reaction to exclude chromosomal DNA contamination.
Examination of chemotactic behavior of V. cholerae
Chemotactic behavior of V. cholerae was examined in AKI39 or LB medium containing 0.25% agar. Taking V-cGAP1 for example, briefly, overnight culture of V. cholerae Δ_dncV_, Δ_V-cGAP1_, Δ_dncV_ and Δ_V-cGAP1_ expressing DncV from arabinose-inducible plasmid, Δ_V-cGAP1_ expressing DncV from arabinose-inducible plasmid and V-cGAP1 (WT or mutant) from IPTG-inducible plasmid with and without induction were stabbed in motility or chemotaxis plates and incubated at 30 °C for 24-48 h.
Biofilm formation
Biofilm formation was measured by the crystal violet staining method and the results were normalized for growth and expressed as the A570/OD600 ratio. Briefly, overnight culture from fresh colonies was diluted 1:50 in fresh medium and transferred to glass tubes. These tubes were incubated for 24 h at 30 °C for biofilm development.
Infant mouse colonization assays
Intestinal colonization competition assay in 4-5-day-old CD-1 suckling mice (Beijing HFK Bioscience) was performed basically as described previously11. Briefly, strains were grown on LB-agar plates with Sm overnight at 30 °C. WT and mutant strains were mixed together in PBS. 100 μl of this competition mixture (105 bacteria) was inoculated into a 4-5-day-old CD-1 mouse pup. One strain carried an active lacZ allele. 100 μl of serial dilutions of the competition mixture were dropped in LB + Sm100 + X-gal and enumerated to determine the input ratio of WT and mutant strains. After incubation at 30 °C for 18 h, the mouse pups were killed and small intestines were removed and homogenized in 4 ml of PBS. 100 μl of serial dilutions were dropped in LB + Sm100 + Xgal and enumerated to determine the output ratio of WT and mutant strains. The in vivo competitive indices were defined as the output ratio of mutant/WT strain divided by the input ratio of mutant/WT strain. Statistical significance was determined by comparing the resulting ratio to the ratio of WT versus WT _lacZ_−.
Statistical analysis
We performed statistical analysis by using an unpaired Student's _t_-test for all studies unless otherwise indicated. We considered P < 0.05 to be statistically significant.
Cells, recombinant proteins, nucleotides, primers, chemical synthesis of cGAMPs, 5′-ApG and 5′-GpA, other reagents and detailed experimental procedures can be found in Supplementary information, Data S1.
References
- Hengge R . Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol 2009; 7:263–273.
Article CAS Google Scholar - Romling U, Galperin MY, Gomelsky M . Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 2013; 77:1–52.
Article Google Scholar - Ross P, Weinhouse H, Aloni Y, et al. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 1987; 325:279–281.
Article CAS Google Scholar - Simm R, Morr M, Kader A, Nimtz M, Romling U . GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol Microbiol 2004; 53:1123–1134.
Article CAS Google Scholar - Tischler AD, Camilli A . Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol Microbiol 2004; 53:857–869.
Article CAS Google Scholar - Tischler AD, Camilli A . Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect Immun 2005; 73:5873–5882.
Article CAS Google Scholar - Mehne FM, Gunka K, Eilers H, Herzberg C, Kaever V, Stulke J . Cyclic di-AMP homeostasis in Bacillus subtilis: both lack and high level accumulation of the nucleotide are detrimental for cell growth. J Biol Chem 2013; 288:2004–2017.
Article CAS Google Scholar - Corrigan RM, Abbott JC, Burhenne H, Kaever V, Grundling A . c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog 2011; 7:e1002217.
Article CAS Google Scholar - Witte CE, Whiteley AT, Burke TP, Sauer JD, Portnoy DA, Woodward JJ . Cyclic di-AMP is critical for Listeria monocytogenes growth, cell wall homeostasis, and establishment of infection. MBio 2013; 4:e00282–e00213.
Article CAS Google Scholar - Luo Y, Helmann JD . Analysis of the role of Bacillus subtilis sigma(M) in beta-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis. Mol Microbiol 2012; 83:623–639.
Article CAS Google Scholar - Davies BW, Bogard RW, Young TS, Mekalanos JJ . Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell 2012; 149:358–370.
Article CAS Google Scholar - Sun L, Wu J, Du F, Chen X, Chen ZJ . Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013; 339:786–791.
Article CAS Google Scholar - Wu J, Sun L, Chen X, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 2013; 339:826–830.
Article CAS Google Scholar - Gao P, Ascano M, Wu Y, et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 2013; 153:1094–1107.
Article CAS Google Scholar - Ablasser A, Goldeck M, Cavlar T, et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 2013; 498:380–384.
Article CAS Google Scholar - Burdette DL, Monroe KM, Sotelo-Troha K, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature 2011; 478:515–518.
Article CAS Google Scholar - Woodward JJ, Iavarone AT, Portnoy DA . c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 2010; 328:1703–1705.
Article CAS Google Scholar - Christen M, Christen B, Folcher M, Schauerte A, Jenal U . Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J Biol Chem 2005; 280:30829–30837.
Article CAS Google Scholar - Schmidt AJ, Ryjenkov DA, Gomelsky M . The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J Bacteriol 2005; 187:4774–4781.
Article CAS Google Scholar - Tamayo R, Tischler AD, Camilli A . The EAL domain protein VieA is a cyclic diguanylate phosphodiesterase. J Biol Chem 2005; 280:33324–33330.
Article CAS Google Scholar - Galperin MY, Koonin EV . Divergence and convergence in enzyme evolution. J Biol Chem 2012; 287:21–28.
Article CAS Google Scholar - Ryan RP, Dow JM . Intermolecular interactions between HD-GYP and GGDEF domain proteins mediate virulence-related signal transduction in Xanthomonas campestris. Virulence 2010; 1:404–408.
Article Google Scholar - Ryan RP, Fouhy Y, Lucey JF, et al. Cell-cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover. Proc Natl Acad Sci USA 2006; 103:6712–6717.
Article CAS Google Scholar - Hammer BK, Bassler BL . Distinct sensory pathways in Vibrio cholerae El Tor and classical biotypes modulate cyclic dimeric GMP levels to control biofilm formation. J Bacteriol 2009; 191:169–177.
Article CAS Google Scholar - Bai Y, Yang J, Eisele LE, et al. Two DHH subfamily 1 proteins in Streptococcus pneumoniae possess cyclic di-AMP phosphodiesterase activity and affect bacterial growth and virulence. J Bacteriol 2013; 195:5123–5132.
Article CAS Google Scholar - Rao F, See RY, Zhang D, Toh DC, Ji Q, Liang ZX . YybT is a signaling protein that contains a cyclic dinucleotide phosphodiesterase domain and a GGDEF domain with ATPase activity. J Biol Chem 2010; 285:473–482.
Article CAS Google Scholar - Aravind L, Koonin EV . A novel family of predicted phosphoesterases includes Drosophila prune protein and bacterial RecJ exonuclease. Trends Biochem Sci 1998; 23:17–19.
Article CAS Google Scholar - Galperin MY, Nikolskaya AN, Koonin EV . Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol Lett 2001; 203:11–21.
Article CAS Google Scholar - Hunter JL, Severin GB, Koestler BJ, Waters CM . The Vibrio cholerae diguanylate cyclase VCA0965 has an AGDEF active site and synthesizes cyclic di-GMP. BMC Microbiol 2014; 14:22.
Article Google Scholar - Lim B, Beyhan S, Yildiz FH . Regulation of Vibrio polysaccharide synthesis and virulence factor production by CdgC, a GGDEF-EAL domain protein, in Vibrio cholerae. J Bacteriol 2007; 189:717–729.
Article CAS Google Scholar - Yakunin AF, Proudfoot M, Kuznetsova E, et al. The HD domain of the Escherichia coli tRNA nucleotidyltransferase has 2′,3′-cyclic phosphodiesterase, 2′-nucleotidase, and phosphatase activities. J Biol Chem 2004; 279:36819–36827.
Article CAS Google Scholar - Dziejman M, Balon E, Boyd D, Fraser CM, Heidelberg JF, Mekalanos JJ . Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc Natl Acad Sci USA 2002; 99:1556–1561.
Article CAS Google Scholar - Grim CJ, Choi J, Chun J, et al. Occurrence of the Vibrio cholerae seventh pandemic VSP-I island and a new variant. OMICS 2010; 14:1–7.
Article CAS Google Scholar - Cho YJ, Yi H, Lee JH, Kim DW, Chun J . Genomic evolution of Vibrio cholerae. Curr Opin Microbiol 2010; 13:646–651.
Article CAS Google Scholar - Kranzusch PJ, Lee AS, Wilson SC, et al. Structure-guided reprogramming of human cGAS dinucleotide linkage specificity. Cell 2014; 158:1011–1021.
Article CAS Google Scholar - Conti M, Beavo J . Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 2007; 76:481–511.
Article CAS Google Scholar - Bellini D, Caly DL, McCarthy Y, et al. Crystal structure of an HD-GYP domain cyclic-di-GMP phosphodiesterase reveals an enzyme with a novel trinuclear catalytic iron centre. Mol Microbiol 2014; 91:26–38.
Article CAS Google Scholar - Sun W, Li Y, Chen L, et al. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci USA 2009; 106:8653–8658.
Article CAS Google Scholar - Iwanaga M, Yamamoto K . New medium for the production of cholera toxin by Vibrio cholerae O1 biotype El Tor. J Clin Microbiol 1985; 22:405–408.
CAS PubMed PubMed Central Google Scholar
Acknowledgements
We thank Dr Yulong Li for critical discussion and Dr Zhijian J Chen for human cGAS expression vector and helpful technical assistance. This work was supported by the National Natural Science Foundation of China (31025010, 31230023, 81171640 and 91129000), and the Chinese Ministry of Science and Technology (2011CB911103 and 2014CB542600).
Author information
Author notes
- Juyi Gao, Jianli Tao and Weili Liang: These three authors contributed equally to this work.
Authors and Affiliations
- State Key Laboratory of Protein and Plant Gene Research, School of Life Sciences, Peking University, Beijing, 100871, China
Juyi Gao, Jianli Tao, Xiaoxia Du, Xiaodong Su & Zhengfan Jiang - Key Laboratory of Cell Proliferation and Differentiation of the Ministry of Education, School of Life Sciences, Peking University, Beijing, 100871, China
Juyi Gao, Jianli Tao & Zhengfan Jiang - Biodynamic Optical Imaging Center (BIOPIC), School of Life Sciences, Peking University, Beijing, 100871, China
Shan Cui, Haifeng Duan & Xiaodong Su - Peking-Tsinghua Center for Life Sciences, Beijing, China
Juyi Gao, Jianli Tao & Zhengfan Jiang - State Key Laboratory for Infectious Disease Prevention and Control, National Institute for Communicable Disease Control and Prevention, Chinese Centre for Disease Control and Prevention, Beijing, 102206, China
Weili Liang, Meng Zhao & Biao Kan - Collaborative Innovation Centre for Diagnosis and Treatment of Infectious Diseases, Hangzhou, 310003, China
Weili Liang & Biao Kan
Authors
- Juyi Gao
- Jianli Tao
- Weili Liang
- Meng Zhao
- Xiaoxia Du
- Shan Cui
- Haifeng Duan
- Biao Kan
- Xiaodong Su
- Zhengfan Jiang
Corresponding authors
Correspondence toXiaodong Su or Zhengfan Jiang.
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0
About this article
Cite this article
Gao, J., Tao, J., Liang, W. et al. Identification and characterization of phosphodiesterases that specifically degrade 3′3′-cyclic GMP-AMP.Cell Res 25, 539–550 (2015). https://doi.org/10.1038/cr.2015.40
- Received: 22 October 2014
- Revised: 12 January 2015
- Accepted: 11 February 2015
- Published: 03 April 2015
- Issue Date: May 2015
- DOI: https://doi.org/10.1038/cr.2015.40