Hypomethylation at multiple maternally methylated imprinted regions including PLAGL1 and GNAS loci in Beckwith–Wiedemann syndrome (original) (raw)
Introduction
The expression of imprinted genes in the mammalian genome is dependent on parental origin. These genes have key roles in the control of foetal growth and many of them have been implicated in human growth disorders and cancer.1, 2, 3 Epigenetic modifications including DNA methylation differentially mark imprinted genes in egg and sperm and lead to the unequal expression of parental alleles in somatic cells.1 Maintenance of differential DNA methylation between maternal and paternal alleles of specific sequences is crucial to proper imprinting control, and methylation defects at imprinted loci have been described in human imprinting disorders.4
Beckwith–Wiedemann syndrome (BWS, OMIM 13650) is a tumour-associated overgrowth disorder that is mainly caused by the dysregulation of a cluster of imprinted genes located at chromosome 11p15.5.5 Although some familial cases with dominant maternal inheritance have been described,6, 7 BWS cases are mostly sporadic and are characterised by methylation abnormalities at one of the two imprinting control regions (ICRs) (H19 and KCNQ1OT1 ICR, also known as IC1 and IC2) or paternal uniparental disomy (UPD) at 11p15.5. Several genes with growth-regulatory properties are part of the 11p15.5 cluster, including IGF2 and H19, which are controlled by the H19 ICR, and CDKN1C and KCNQ1OT1, which are controlled by the KCNQ1OT1 ICR. We recently demonstrated that gain of methylation at the H19 ICR (IC1 defect, ICD1) is independent of the DNA sequence context in several BWS patients and proposed that these molecular defects generally arise as de novo epimutations in early embryogenesis.8
A subset of BWS patients with hypomethylation at the KCNQ1OT1 ICR (IC2 defect, ICD2) have loss of methylation at other imprinted loci.9 Hypomethylation of multiple maternally methylated ICRs including KCNQ1OT1 has also been demonstrated in cases of transient neonatal diabetes10, 11 (TND, OMIM 601410). TND is a disease characterised by intrauterine growth retardation and transient hyperglycaemia that results from dysregulation of the imprinted growth inhibitor and antiapoptotic PLAGL1 (ZAC) gene located at chromosome 6q24.12 A minority of TND patients have BWS features, such as macroglossia and abdominal wall defects, suggesting an interaction between the 6q24 and 11p15.5 loci. However, no molecular defect at PLAGL1 has been reported in BWS patients so far and the relationship between BWS and TND remains undefined.9, 13
We have further investigated the role of the imprinted genes other than the 11p15.5 cluster in BWS, by analysing DNA methylation at 11 ICRs with multiple techniques and screening a large number of cases through a multicentre study. The results obtained demonstrate that a subset of BWS patients display hypomethylation at multiple maternally methylated ICRs, including those involved in TND and pseudohypoparathyroidism type 1b (PHP1b), and support the hypothesis that multiple maternal hypomethylation may present with different clinical phenotypes.
Patients and methods
Patients
The patients comprised 149 individuals with clinical diagnosis of BWS according to the criteria reported by DeBaun et al.14 These included 105 patients from Italy and 44 from the Netherlands (Table 1). Their clinical features are summarised in Table 2. Genetic analyses of the Italian cohort were performed with the informed consent of the parents of the patients. The experimental plan was approved by the ethical committees of the Second University of Naples, Italy. The collection of clinical data in the Netherlands was approved by the ethical committee of the Academic Medical Centre in Amsterdam (MEC 99/030), and the experiments were performed in accordance with the local (ethical) protocols for research on patient material.
Table 1 Classification of epigenetic defects found in 149 BWS patients
Table 2 Clinical features of the BWS patients with multiple maternal hypomethylation
DNA methylation analysis of the imprinted loci
The DNA methylation of ICRs at various imprinted loci was analysed in DNA derived from peripheral blood leukocytes using four different techniques. The H19 ICR and the KCNQ1OT1 ICR were analysed by Southern blotting hybridisation, as described earlier.17 The KCNQ1OT1 ICR and all the other ICRs were analysed by combined bisulphite restriction analysis (COBRA) and methylation-specific PCR (MS-PCR). For the COBRA, 2 _μ_g of genomic DNA was treated with sodium bisulphite, PCR amplified, the PCR product digested with a restriction enzyme containing a CpG dinucleotide in its target sequence and the fragments separated on a polyacrylamide gel. In some individuals, the methylation of the KCNQ1OT1, MEST(PEG1) and PLAGL1 ICRs was also analysed by bisulphite sequencing. In this case, the PCR products were cloned in Topo pCR2.1 vector (Invitrogen) and the clones sequenced. Primer sequences, genomic locations and amplicon sizes of relevant assays are detailed in Supplementary Table 1; further experimental details are available on request. MS-PCR was performed exactly as described.18
Methylation analysis of classical satellite DNA
DNA methylation of classical satellites 2 and 3 was analysed by Southern blotting, as described.19 Two micrograms of DNA were digested overnight with _Hpa_II, _Msp_I and _Mcr_BC, in separate tubes. Blots were hybridised with oligonucleotide probes specific for satellite 2 of chromosomes 1 and 16 and satellite 3 of chromosome 9.
Mutation analysis of the DNMT3L gene
The DNMT3L gene was analysed by sequencing all exons and their flanking intronic regions. The primers and PCR conditions used are as described;20 DNA sequencing was obtained from PRIMM (Italy).
Statistical analysis
Fisher's exact testing and _χ_2 analysis were used as appropriate. Statistical significance was taken at the 5% level.
Results
DNA methylation defects at multiple imprinted loci in BWS
We investigated 149 individuals affected with BWS for the presence of DNA methylation defects at imprinted loci other than the 11p15.5 cluster. In all cases, leukocyte-derived DNA was analysed. The BWS samples were classified for the presence of hypermethylation at the H19 ICR (ICD1), hypomethylation at the KCNQ1OT1 ICR (ICD2), paternal UPD at 11p15.5 (UPD11) or no epigenetic defect at 11p15.5, as described earlier5, 21, 22 (see Table 1 and Supplementary Figure 1). The same samples were then analysed for the presence of methylation defects at other imprinted loci. One ICR normally methylated on the paternal allele, GTL2-IG (14q32), and eight maternally methylated ICRs, PLAGL1 (6q24), IGF2R (6q25.3), GRB10 (7p21), MEST (7q32.2), SNRPN (15q11), PEG3 (19q13), GNAS (20q13.32) and NESPAS (20q13.32), were investigated. At these loci, methylation levels were determined by using both COBRA and MS-PCR (Table 3).
Table 3 Loss of methylation values of various imprinted loci in BWS patients with multiple maternal hypomethylation
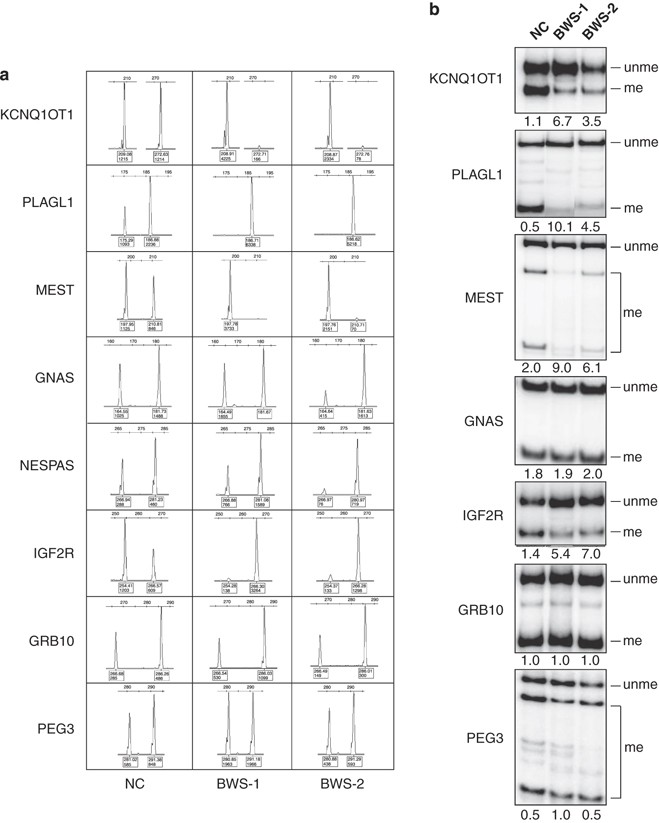
Abnormal methylation at loci other than 11p15.5 was detected in 17 patients with ICD2 (21%, see Table 1). The results were reported as unme/me ratios and named ‘loss of methylation values’ (Table 3). In patients with multiple methylation anomalies, only maternally methylated ICRs were affected. No methylation defect of imprinted loci other than 11p15 was detected in patients with ICD1, or in those with UPD11, or in those with no detected anomaly of chromosome 11p15.5. No methylation changes were observed in 120 normal controls with MS-PCR and 100 normal controls with COBRA. The number and type of loci involved and the extent of hypomethylation varied from patient to patient (Table 3). Apart from KCNQ1OT1, methylation defects were found at MEST in six to seven cases, PLAGL1 in seven cases, IGF2R in six cases, GNAS in eight cases, NESPAS in 10 cases and GRB10 in four cases. The observed hypomethylation defects were strikingly mosaic in form; individual patients showed epimutations ranging from modest to total hypomethylation at different loci. Generally, MS-PCR appeared to be more sensitive to allelic imbalances. Nevertheless, qualitatively, the results obtained with the two different methods were comparable (Table 3 and Figure 1a and b).
Figure 1
Examples of the assays used for the methylation analysis. The methylation of nine maternally methylated ICRs was analysed by MS-PCR (a) and COBRA (b) in the DNAs of a normal individual and two BWS patients with multiple methylation defects (BWS1 and BWS2). The peaks of the MS-PCR corresponding to unmethylated and methylated DNA are indicated by blue and red filled circles, respectively, and the bands of the COBRA corresponding to the unmethylated and methylated DNA are indicated at the right side of the panels. The area of the MS-PCR peaks and the non-normalised unme/me ratios of intensities of the COBRA bands are also indicated below each panel. Details are given in Patients and methods and Supplementary Tables 1 and 2. Note that the results obtained with the two techniques are highly concordant.
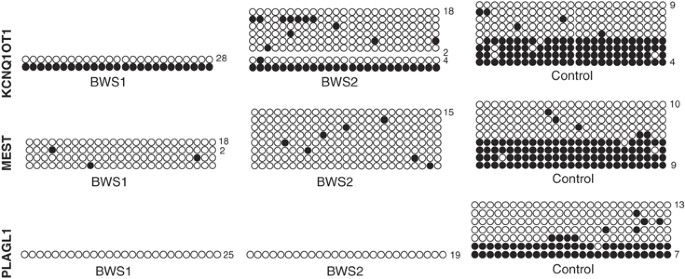
As MS-PCR and COBRA assay the methylation of only a few CpGs at each site, we investigated the extent of the methylation losses, by bisulphite-sequencing analysis of two cases from the Italian cohort showing significant hypomethylation of the MEST and PLAGL1 ICRs (Figure 2). The patient DNA showed essentially complete hypomethylation within the amplicons sequenced. Demonstration of heterozygosity by the analysis of closely linked SNPs and microsatellites excluded the deletion of the methylated allele in the cases with complete absence of methylation at the ICR (data not shown).
Figure 2
Multiple hypomethylated ICRs, as analysed by bisulphite sequencing. The methylation of the three maternally methylated ICRs was analysed by bisulphite sequencing in the DNAs of a normal individual and two BWS patients with multiple methylation defects (BWS1 and BWS2). Twenty-five CpGs of the KCNQ1OT1 ICR, 26 CpGs of the PLAGL1 ICR and 59 CpGs (only the first 25 CpGs are shown) of the MEST ICR were analysed. Each line corresponds to a single template DNA molecule cloned; each circle corresponds to a CpG dinucleotide. Filled circles designate methylated cytosines; open circles, unmethylated cytosines. Numbers at the right border of the CpG circles indicate multiple sequenced clones with identical methylation pattern. Note that the hypomethylation extends over the entire amplicons sequenced.
The specificity of the methylation defects was investigated by the analysis of repetitive sequences. We analysed the methylation of satellites 2 and 3, by Southern blotting, in four patients with multiple hypomethylation. The results showed that methylation was present at essentially control levels in all individuals investigated (Supplementary Figure 2).
Overall, these results demonstrate that a subset of BWS patients display a complex but specific hypomethylation defect involving multiple maternally methylated imprinted loci. Interestingly, the hypomethylated loci included the PLAGL1 locus implicated in TND, and the GNAS and NESPAS loci implicated in PHP1b.
Clinical characteristics of patients with multiple maternal hypomethylation
The clinical features of the patients with multiple hypomethylation were compared with those of the patients with loss of methylation restricted to KCNQ1OT1 (Table 2). The birth weight of the patients with multiple hypomethylation was on average lower than that of the subgroup with isolated KCNQ1OT1 hypomethylation (58th±35 vs 79th±21 centile). This difference is just over the limits of statistical significance (_P_=0.06). However, the low frequency of neonatal macrosomia was particularly evident among the cases with severe hypomethylation (38th±34, see Table 3, in bold). Intriguingly, some of these individuals showed postnatal overgrowth. The frequencies of nevus flammeus and hemihypertrophy among the patients with multiple defects were lower than those observed among the patients with only KCNQ1OT1 hypomethylation (_P_=0.03 and _P_=0.04, respectively). In addition, some patients with multiple hypomethylation displayed characteristics not usually associated with BWS, such as speech retardation (BWS3), peri/postnatal apnoea, feeding difficulties, hearing problems (BWS11) and premature birth (28 weeks of gestation, BWS12 and BWS16). Overall, these data suggest that maternal hypomethylation in addition to that at 11p15.5 may modify the clinical presentation of BWS.
Mutation analysis of DNMT3L
In the mouse, Dnmt3L is required for establishing maternal imprints.23 We, therefore, looked for mutations at the DNMT3L locus in the mothers of two patients (BWS1 and BWS2) with severe hypomethylation at multiple imprinting loci. We sequenced all exons and flanking intronic regions. Only four single-nucleotide variations with respect to the reference sequence (NT_011515) were detected, but all of them corresponded to polymorphisms also found in the normal population (db8129767, db2014457, db762424 and db2014264).
Discussion
Rossignol et al9 reported that a subgroup of BWS patients displayed maternal methylation defects at imprinted loci in addition to the KCNQ1OT1 ICR, raising the hypothesis that genes other than those located at chromosome 11p15.5 were involved in the pathogenesis of BWS. In a larger cohort of patients, we have demonstrated that the epigenetic defects at loci other than 11p15.5 were limited to loss of methylation at maternally methylated ICRs, and that these abnormalities were restricted to the individuals with loss of KCNQ1OT1 methylation. We also observed that a specific set of ICRs were found more frequently affected than others and that the patients with multiple methylation defects often displayed atypical BWS phenotypes.
The percentage of ICD2 patients with multiple hypomethylation was reported by Rossignol et al9 to be 25% in a study of the KCNQ1OT1, IGF2R, MEST and SNRPN loci in 40 BWS cases. We have found a 20% frequency of multiple methylation among nine maternally methylated and two paternally methylated loci analysed, including a hypomethylation frequency of only 11% among the loci investigated by Rossignol et al.9 This lower frequency may reflect variations in the clinical inclusion criteria for the two cohorts investigated; this is particularly significant bearing in mind that multiple hypomethylation cases may have a BWS presentation modified by the effects of loci other than 11p15.5. Differently from Rossignol et al,9 we used multiple techniques to analyse DNA methylation. Notably, there was a broad concordance between the results of methylation analysis by Southern blotting, COBRA and MSP. In some cases, there was a variation in the degree of hypomethylation detected by the different techniques (for example, the hypomethylation of KCNQ1OT1 in BWS1–4). In two cases (MEST, BWS16 and GNAS, BWS2), the discrepancy caused an apparently divergent determination of hypomethylation, with MSP detecting marginal hypomethylation not detected by COBRA. In general, it appears that MSP is more sensitive than COBRA to allelic imbalance. As MSP is based on simultaneous amplification of methylated and non-methylated DNAs with different primers, it is possible that the different species take different times to reach exponential phase, and this emphasises the differences between methylated and non-methylated DNAs. Aside from this, small discrepancies in calculated methylation ratios may reflect the different positions of the target CpG dinucleotides assayed by the different tests.
The loci most frequently affected in our patient cohort were the NESPAS and GNAS ICRs on chromosome 20, followed by the MEST, PLAGL1 and IGF2R ICRs, whereas GRB10, PEG3 and SNRPN were frequently spared. This may represent clinical ascertainment bias among the patient cohort. Alternatively, the range of loci implicated may indicate underlying mechanistic aetiologies primarily affecting specific groups of imprinted loci. It has been recently demonstrated that TND patients with gene mutations in ZFP57 had imprinting defects chiefly affecting the PLAGL1, PEG3 and GRB10 loci, whereas other loci including KCNQ1OT1, MEST, NESPAS and GNAS were generally spared.18 We suggest, therefore, that there may be an aetiological divergence between that patient group and the cohort described here.
Rossignol et al9 did not identify any significant difference between the clinical features of patients with multiple hypomethylation and those with hypomethylation restricted to 11p15.5. In a more extensive characterisation, we have found that patients with multiple defects were on average smaller at birth than those with ICD2 alone. In addition, some of the individuals with multiple hypomethylation had unusual BWS phenotypes. Similarly to a case of the French study, one patient of our cohort (BWS3) had developmental delay and primary speech retardation associated with MEST hypomethylation. Another patient with multiple methylation defect (BWS11) showed apnoea and feeding and hearing problems. In addition, two patients of our cohort (BWS12 and BWS16) and one patient of the French cohort were born preterm. Therefore, it is possible that dysregulation of additional genes may modify the typical phenotype of BWS. The tumour incidence (low in the BWS cases with ICD2), however, was not affected by the presence of multiple methylation defects.
It is striking that some individuals manifested imprinting defects that would be expected in association with clinical features of another imprinting disorder. For example, four patients (BWS8, BWS14, BWS15 and BWS16) had total or near-total hypomethylation of GNAS and/or NESPAS. The GNAS locus on chromosome 20q13 is a complex imprinted cluster with multiple ICRs and gene products.24 Hypomethylation at these ICRs has also been described in several patients affected with PHP1b.25 Although further investigation is probably needed to exclude all features of PHP1b, no alteration of calcium metabolism has been reported in our BWS patients with multiple hypomethylation so far. However, the two patients with more marked GNAS hypomethylation were not macrosomic at birth (10th and 50–90th centiles, respectively), suggesting that abnormal expression at the GNAS locus may modify the BWS phenotype. Four patients (BWS1, BWS2, BWS9 and BWS10) in this cohort displayed complete or near-complete hypomethylation of PLAGL1 ICR, which is normally associated with TND. Although neonatal diabetes was not described among these cases, they were not macrosomic; this suggests that PLAGL1 hypomethylation may alter the clinical presentation of BWS. Interestingly, TND patients with marked KCNQ1OT1 hypomethylation displayed macroglossia and abdominal wall defects, features typical of BWS.10 These observations suggest that the clinical presentation of these complex cases may be modified to an extent depending on their mosaic epigenotype. Possibly, the lack of presentation reflects the somatic mosaicism of the patients, with critical target tissues being spared. Alternatively, the interaction of genetic pathways affected by these different imprinted genes gives rise to a primary clinical presentation, with other clinical disorders being ameliorated. Also, it is not possible to exclude that the observed loss of methylation at ICRs other than KCNQ1OT1 is limited to blood leukocytes. Clearly larger cohort studies, with extensive clinical and epigenetic characterisations, are warranted.
Consistent with the French study, hypomethylation was incomplete in most cases, indicating epigenetic mosaicism, probably arising postfertilisation.9 It cannot be excluded, however, that an imprinting defect originating in the gametes results in unstable methylation of the ICRs afterwards. The loss of methylation may arise stochastically or as a consequence of defective trans-acting factor or environmental cause. The use of assisted reproduction technology (ART) can be excluded as a cause, as the frequency of ART-associated cases is not increased among the patients with multiple hypomethylation in both the French9 and our studies (Table 2). Our results also exclude defects in the DNMT3L gene as a common cause of multiple hypomethylation in the BWS. The multi-zinc finger ZFP-57 gene has been implicated in the aetiology of multiple hypomethylation in TND.18 This protein, which is enriched in undifferentiated ES cells, may be required for the maintenance of DNA methylation at specific ICRs in early embryogenesis. It is possible that a defect in a similar regulatory protein is involved in multiple hypomethylation in BWS.
Functional interactions between imprinted genes controlling embryo growth have been proposed on the basis of similar expression patterns.13, 26 In addition, the product of the PLAGL1 gene has been shown to bind in vitro the KCNQ1OT1 ICR and activate the expression of the non-coding KCNQ1OT1 RNA.13 Also, extensive networks of intra- and interchromosomal interactions were demonstrated among imprinted domains.27 As a consequence of these interactions, it is possible that epigenetic alterations at one locus may result in abnormalities at other loci.
In conclusion, hypomethylation at multiple maternally methylated imprinted loci, including KCNQ1OT1, MEST, GNAS/NESPAS, PLAGL1 and IGF2R, is associated with atypical BWS presentations. These cases may be an example of a group of more generalised imprinting disorders resulting from defects in the imprinting maintenance factors. Further investigation will be necessary to identify the involved gene(s) and better define the phenotypes associated with these complex disorders.
References
- Reik W, Walter J : Genomic imprinting: parental influence on the genome. Nat Rev Genet 2001; 2: 21–32.
CAS Google Scholar - Delaval K, Wagschal A, Feil R : Epigenetic deregulation of imprinting in congenital diseases of aberrant growth. Bioessays 2006; 28: 453–459.
Article CAS Google Scholar - Feinberg AP : Phenotypic plasticity and the epigenetics of human disease. Nature 2007; 447: 433–440.
Article CAS Google Scholar - Tycko B : Imprinted genes in placental growth and obstetric disorders. Cytogenet Genome Res 2006; 113: 271–278.
Article CAS Google Scholar - Weksberg R, Shuman C, Smith AC : Beckwith–Wiedemann syndrome. Am J Med Genet C Semin Med Genet 2005; 137: 12–23.
Article Google Scholar - Sparago A, Russo S, Cerrato F et al: Mechanisms causing imprinting defects in familial Beckwith–Wiedemann syndrome with Wilms’ tumour. Hum Mol Genet 2007; 16: 254–264.
Article CAS Google Scholar - Prawitt D, Enklaar T, Gärtner-Rupprecht B et al: Microdeletion of target sites for insulator protein CTCF in a chromosome 11p15 imprinting center in Beckwith–Wiedemann syndrome and Wilms’ tumor. Proc Natl Acad Sci USA 2005; 102: 4085–4090.
Article CAS Google Scholar - Cerrato F, Sparago A, Verde G et al: Different mechanisms cause imprinting defects at the IGF2/H19 locus in Beckwith–Wiedemann syndrome and Wilms’ tumour. Hum Mol Genet 2008; 17: 1427–1435.
Article CAS Google Scholar - Rossignol S, Steunou V, Chalas C et al: The epigenetic imprinting defect of patients with Beckwith–Wiedemann syndrome born after assisted reproductive technology is not restricted to the 11p15 region. J Med Genet 2006; 43: 902–907.
Article CAS Google Scholar - Mackay DJ, Hahnemann JM, Boonen SE et al: Epimutation of the TND locus and the Beckwith–Wiedemann syndrome centromeric locus in individuals with transient neonatal diabetes mellitus. Hum Genet 2006a; 119: 179–184.
Article CAS Google Scholar - Mackay DJG, Boonen SE, Clayton-Smith J et al: A maternal hypomethylation syndrome presenting as transient neonatal diabetes mellitus. Hum Genet 2006b; 120: 262–269.
Article CAS Google Scholar - Temple IK, Shield JP : Transient neonatal diabetes, a disorder of imprinting. J Med Genet 2002; 39: 872–875.
Article CAS Google Scholar - Arima T, Kamikihara T, Hayashida T et al: ZAC, LIT1 (KCNQ1OT1) and p57KIP2 (CDKN1C) are in an imprinted gene network that may play a role in Beckwith–Wiedemann syndrome. Nucleic Acids Res 2005; 33: 2650–2660.
Article CAS Google Scholar - DeBaun MR, Tucker MA : Risk of cancer during the first four years of life in children from The Beckwith–Wiedemann Syndrome Registry. J Pediatr 1998; 132: 398–400.
Article CAS Google Scholar - Kramer MS, Platt RW, Wen SW et al: A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 2001; 108: E35.
Article CAS Google Scholar - Buckler JM, Green M : Birth weight and head circumference standards for English twins. Arch Dis Child 1994; 71: 516–521.
Article CAS Google Scholar - Sparago A, Cerrato F, Vernucci M et al: Microdeletions in the human H19 DMR result in loss of IGF2 imprinting and Beckwith–Wiedemann syndrome. Nat Genet 2004; 36: 958–960.
Article CAS Google Scholar - Mackay DJG, Callaway JLA, Marks SM et al: Hypomethylation of multiple imprinted loci in patients with transient neonatal diabetes is associated with mutations in ZFP57. Nat Genet 2008; 40: 949–951.
Article CAS Google Scholar - Jiang YL, Rigolet M, Bourc’his D et al: DNMT3B mutations and DNA methylation defect define two types of ICF syndrome. Hum Mutat 2005; 25: 56–63.
Article CAS Google Scholar - Hayward BE, De Vos M, Judson H et al: Lack of involvement of known DNA methyltransferases in familial hydatidiform mole implies the involvement of other factors in establishment of imprinting in the human female germline. BMC Genet 2003; 4: 2.
Article CAS Google Scholar - Bliek J, Maas SM, Ruijter JM et al: Increased tumour risk for BWS patients correlates with aberrant H19 and not KCNQ1OT1 methylation: occurrence of KCNQ1OT1 hypomethylation in familial cases of BWS. Hum Mol Genet 2001; 10: 467–476.
Article CAS Google Scholar - Cooper WN, Luharia A, Evans GA et al: Molecular subtypes and phenotypic expression of Beckwith–Wiedemann syndrome. Eur J Hum Genet 2005; 13: 1025–1032.
Article CAS Google Scholar - Bourc’his D, Xu GL, Lin CS, Bollman B, Bestor TH : Dnmt3L and the establishment of maternal genomic imprints. Science 2001; 294: 2536–2539.
Article Google Scholar - Peters J, Williamson CM : Control of imprinting at the Gnas cluster. Adv Exp Med Biol 2008; 626: 16–26.
Article CAS Google Scholar - Bastepe M : The GNAS locus and pseudohypoparathyroidism. Adv Exp Med Biol 2008; 626: 27–40.
Article CAS Google Scholar - Varrault A, Gueydan C, Delalbre A et al: Zac1 regulates an imprinted gene network critically involved in the control of embryonic growth. Dev Cell 2006; 11: 711–722.
Article CAS Google Scholar - Zhao Z, Tavoosidana G, Sjölinder M et al: Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions. Nat Genet 2006; 38: 1341–1347.
Article CAS Google Scholar
Acknowledgements
We thank all the patients and their families for their participation in this study. This study was supported by grants from MIUR PRIN 2005 (to AR), Telethon-Italia Grant no. GGP07086 (to AR), Associazione Italiana Ricerca sul Cancro (to AR), Istituto Superiore di Sanità (to LL), Diabetes UK BDA:RD04/0002932 (to DJGM) and Diabetes UK BDA:RD06/0003185 (to JLAC). Flavia Cerrato was the recipient of a fellowship founded by Fondazione Pezcoller and Società Italiana di Cancerologia.
Author information
Author notes
- Jet Bliek and Gaetano Verde: These authors contributed equally to this work.
Authors and Affiliations
- Department of Clinical Genetics, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands
Jet Bliek, Saskia M Maas & Marcel M A M Mannens - Institute of Genetics and Biophysics ‘A. Buzzati-Traverso’, CNR, Naples, Italy
Gaetano Verde, Angela Sparago & Andrea Riccio - Department of Environmental Science, Second University of Naples, Caserta, Italy
Gaetano Verde, Agostina De Crescenzo, Angela Sparago, Flavia Cerrato & Andrea Riccio - Division of Human Genetics, University of Southampton, Southampton, UK
Jonathan Callaway & Deborah J G Mackay - Wessex Regional Genetics Laboratory, Salisbury District Hospital, Salisbury, UK
Jonathan Callaway & Deborah J G Mackay - Department of Pediatrics, Academic Medical Center, Amsterdam, The Netherlands
Saskia M Maas & Lidia Larizza - Laboratorio di Citogenetica e Genetica Molecolare, Istituto Auxologico Italiano, Milano, Italy
Silvia Russo & Serena Ferraiuolo - AORNA Cardarelli UOC Genetica Medica, Napoli, Italy
Maria Michela Rinaldi - UO Metabolic Disease-Medical Genetics, PO Giovanni XXIII-AOU Policlinico Consorziale, Bari, Italy
Rita Fischetto - Department of Obstetrics and Pediatrics, Clinical Genetic Unit, Fondazione Ospedale Maggiore Policlinico Mangiagalli e Regina Elena, Milan, Italy
Faustina Lalatta - Neuropsichiatria Infantile, Spedali Civili, Brescia, Italy
Lucio Giordano - Department of Pediatrics, University of Modena, Italy
Paola Ferrari - Dipartimento di Biologia Strutturale e Funzionale, Università di Napoli ‘Federico II’, Naples, Italy
Maria Vittoria Cubellis - Division of Medical Genetics, San Paolo School of Medicine, University of Milan, Milano, Italy
Lidia Larizza - Academic Unit of Genetic Medicine, Wessex Clinical Genetics Service, Southampton University Hospitals Trust, Southampton, UK
I Karen Temple
Authors
- Jet Bliek
You can also search for this author inPubMed Google Scholar - Gaetano Verde
You can also search for this author inPubMed Google Scholar - Jonathan Callaway
You can also search for this author inPubMed Google Scholar - Saskia M Maas
You can also search for this author inPubMed Google Scholar - Agostina De Crescenzo
You can also search for this author inPubMed Google Scholar - Angela Sparago
You can also search for this author inPubMed Google Scholar - Flavia Cerrato
You can also search for this author inPubMed Google Scholar - Silvia Russo
You can also search for this author inPubMed Google Scholar - Serena Ferraiuolo
You can also search for this author inPubMed Google Scholar - Maria Michela Rinaldi
You can also search for this author inPubMed Google Scholar - Rita Fischetto
You can also search for this author inPubMed Google Scholar - Faustina Lalatta
You can also search for this author inPubMed Google Scholar - Lucio Giordano
You can also search for this author inPubMed Google Scholar - Paola Ferrari
You can also search for this author inPubMed Google Scholar - Maria Vittoria Cubellis
You can also search for this author inPubMed Google Scholar - Lidia Larizza
You can also search for this author inPubMed Google Scholar - I Karen Temple
You can also search for this author inPubMed Google Scholar - Marcel M A M Mannens
You can also search for this author inPubMed Google Scholar - Deborah J G Mackay
You can also search for this author inPubMed Google Scholar - Andrea Riccio
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toDeborah J G Mackay.
Additional information
Conflict of interest
The authors had no conflict of interest in connection with this paper.
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary information
Rights and permissions
About this article
Cite this article
Bliek, J., Verde, G., Callaway, J. et al. Hypomethylation at multiple maternally methylated imprinted regions including PLAGL1 and GNAS loci in Beckwith–Wiedemann syndrome.Eur J Hum Genet 17, 611–619 (2009). https://doi.org/10.1038/ejhg.2008.233
- Received: 10 September 2008
- Revised: 30 October 2008
- Accepted: 31 October 2008
- Published: 17 December 2008
- Issue Date: May 2009
- DOI: https://doi.org/10.1038/ejhg.2008.233