Pleiotropic scaling of gene effects and the ‘cost of complexity’ (original) (raw)
- Letter
- Published: 27 March 2008
- Jane P. Kenney-Hunt2,
- Mihaela Pavlicev2,
- Joel R. Peck3,
- David Waxman3 &
- …
- James M. Cheverud2
Nature volume 452, pages 470–472 (2008)Cite this article
- 2766 Accesses
- 211 Citations
- 16 Altmetric
- Metrics details
Abstract
As perceived by Darwin, evolutionary adaptation by the processes of mutation and selection is difficult to understand for complex features that are the product of numerous traits acting in concert, for example the eye or the apparatus of flight. Typically, mutations simultaneously affect multiple phenotypic characters. This phenomenon is known as pleiotropy. The impact of pleiotropy on evolution has for decades been the subject of formal analysis1,2,3,4,5,6. Some authors have suggested that pleiotropy can impede evolutionary progress (a so-called ‘cost of complexity’5). The plausibility of various phenomena attributed to pleiotropy depends on how many traits are affected by each mutation and on our understanding of the correlation between the number of traits affected by each gene substitution and the size of mutational effects on individual traits. Here we show, by studying pleiotropy in mice with the use of quantitative trait loci (QTLs) affecting skeletal characters, that most QTLs affect a relatively small subset of traits and that a substitution at a QTL has an effect on each trait that increases with the total number of traits affected. This suggests that evolution of higher organisms does not suffer a ‘cost of complexity’ because most mutations affect few traits and the size of the effects does not decrease with pleiotropy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
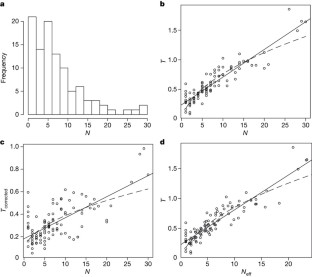
Figure 1: Distribution of QTL effects on 70 skeletal traits in the mouse.
Similar content being viewed by others
References
- Fisher, R. A. The Genetical Theory of Natural Selection (Clarendon, Oxford, 1930)
Book Google Scholar - Rechenberg, I. Evolutionsstrategie: Optimierung technischer Systeme nach Prinzipien der biologischen Evolution (Fromman-Holzboog, Stuttgart, 1973)
Google Scholar - Turelli, M. Effects of pleiotropy on predictions concerning mutation-selection balance for polygenic traits. Genetics 111, 165–195 (1985)
CAS PubMed PubMed Central Google Scholar - Wagner, G. P. The influence of variation and developmental constraints on the rate of multivariate phenotypic evolution. J. Evol. Biol. 1, 45–66 (1988)
Article Google Scholar - Orr, H. A. Adaptation and the cost of complexity. Evolution Int. J. Org. Evolution 54, 13–20 (2000)
Article CAS Google Scholar - Wingreen, N. S., Miller, J. & Cox, E. C. Scaling of mutational effects in models of pleiotropy. Genetics 164, 1221–1228 (2003)
PubMed PubMed Central Google Scholar - Chai, C. K. Analysis of quantitative inheritance of body size in mice II: gene action and segregation. Genetics 41, 165–178 (1956)
CAS PubMed PubMed Central Google Scholar - Eppig, J. T., Bult, C. J., Kadin, J. A., Richardson, J. E. & Blake, J. A. and the members of the Mouse Genome Database Group. The mouse genome data base (MGD): from genes to mice – a community resource for mouse biology. Nucleic Acids Res. 33, D471–D475 (2005)
Article CAS PubMed Google Scholar - Kenney-Hunt, J. P. et al. Pleiotropic patterns of quantitative trait loci for seventy murine skeletal traits. Genetics (in the press)
- Knott, S. A. & Haley, C. S. Multitrait least squares for quantitative trait loci detection. Genetics 156, 899–911 (2000)
CAS PubMed PubMed Central Google Scholar - Xu, S. Theoretical basis of the Beavis effect. Genetics 165, 2259–2268 (2003)
PubMed PubMed Central Google Scholar - Wagner, G. P. Multivariate mutation-selection balance with constrained pleiotropic effects. Genetics 122, 223–234 (1989)
CAS PubMed PubMed Central Google Scholar - Waxman, D. & Peck, J. R. Pleiotropy and preservation of perfection. Science 279, 1210–1213 (1998)
Article CAS ADS PubMed Google Scholar - Waddington, C. H. The Strategy of Genes (Macmillan, New York, 1957)
Google Scholar - Hermisson, J. & Wagner, G. P. The population genetic theory of hidden variation and genetic robustness. Genetics 168, 2271–2284 (2004)
Article PubMed PubMed Central Google Scholar - Wagner, G. P. & Altenberg, L. Complex adaptation and the evolution of evolvability. Evolution Int. J. Org. Evolution 50, 967–976 (1996)
Article Google Scholar - Hansen, T. F. Is modularity necessary for evolvability? Remarks on the relationship between pleiotropy and evolvability. Biosystems 69, 83–94 (2003)
Article PubMed Google Scholar - Wagner, G. P., Pavlicev, M. & Cheverud, J. M. The road to modularity. Nature Rev. Genet. 8, 921–931 (2007)
Article CAS PubMed Google Scholar - Welch, J. J. & Waxman, D. Modularity and the cost of complexity. Evolution Int. J. Org. Evolution 57, 1723–1734 (2003)
Article Google Scholar - Martin, G. & Lenormand, T. A general multivariate extension of Fisher’s geometrical model and the distribution of mutation fitness effects across species. Evolution Int. J. Org. Evolution 60, 893–907 (2006)
Article Google Scholar - Cheverud, J. M. et al. Quantitative trait loci for murine growth. Genetics 142, 1305–1319 (1996)
CAS PubMed PubMed Central Google Scholar - Vaughn, T. T. et al. Mapping quantitative trait loci for murine growth: a closer look at genetic architecture. Genet. Res. 74, 313–322 (1999)
Article CAS PubMed Google Scholar - Cheverud, J. M. et al. Genetic architecture of adiposity in the cross of LG/J and SM/J inbred mice. Mamm. Genome 12, 3–12 (2001)
Article CAS PubMed Google Scholar - Haley, C. S. & Knott, S. A. A simple regression method for mapping quantitative trait loci in line crosses using flanking markers. Heredity 69, 315–324 (1992)
Article CAS PubMed Google Scholar - Cheverud, J. M. A simple correction for multiple comparisons in interval mapping genome scans. Heredity 87, 52–58 (2001)
Article CAS PubMed Google Scholar - Ehrich, T. H. et al. Pleiotropic effects on mandibular morphology I: developmental morphological integration and differential dominance. J. Exp. Zool. B 296, 58–78 (2003)
Article Google Scholar
Acknowledgements
We thank A. Pyle, A. Kondrashov and B. Walsh for suggestions that have improved this manuscript. We thank the members of the Wagner and Cheverud laboratories for critical discussion. J.P. and D.W. thank members of the evolution group at Sussex. J.M.C. is funded by the National Institutes of Health and the National Science Foundation (NSF), G.P.W. acknowledges funding from the NSF, the Humboldt Foundation and the John Templeton Foundation, M.P. is funded by the Austrian Science Foundation (FWF) Fellowship, and the work of D.W. was supported by the Leverhulme Trust.
Author Contributions G.P.W. conceived this study, participated in the statistical analysis and wrote the manuscript. J.P.K.-H. collected the morphological data and performed the QTL analysis. M.P. did the statistical analyses. J.M.C. was responsible for generating the mouse populations and the genotype data used in the original mapping and advised on the pleiotropic scaling analysis. J.P. and D.W. performed a theoretical analysis of the scaling of trait effects with pleiotropy. All authors participated in the preparation of the manuscript.
Author information
Authors and Affiliations
- Department of Ecology and Evolutionary Biology, Yale University, New Haven, Connecticut 06520-8106, USA,
Günter P. Wagner - Department of Anatomy and Neurobiology, Washington University, St Louis, Missouri 63110, USA,
Jane P. Kenney-Hunt, Mihaela Pavlicev & James M. Cheverud - Center for the Study of Evolution, School of Life Sciences, University of Sussex, Brighton BN1 9QG, UK
Joel R. Peck & David Waxman
Authors
- Günter P. Wagner
- Jane P. Kenney-Hunt
- Mihaela Pavlicev
- Joel R. Peck
- David Waxman
- James M. Cheverud
Corresponding authors
Correspondence toGünter P. Wagner or James M. Cheverud.
Supplementary information
Supplementary Information
The file contains Supplementary Figures S1-S4 with Legends, Supplementary Table T1 and Supplementary Notes with additional references. (PDF 356 kb)
Rights and permissions
About this article
Cite this article
Wagner, G., Kenney-Hunt, J., Pavlicev, M. et al. Pleiotropic scaling of gene effects and the ‘cost of complexity’.Nature 452, 470–472 (2008). https://doi.org/10.1038/nature06756
- Received: 23 August 2007
- Accepted: 28 January 2008
- Issue Date: 27 March 2008
- DOI: https://doi.org/10.1038/nature06756