Duration of antiviral immunity after smallpox vaccination (original) (raw)
Main
Current views on smallpox immunity suggest that it is low or nonexistent in today's population because of the lack of mass vaccination since the mid 1970s and the total eradication of this disease worldwide in 1978 (refs. 1–6). Through this highly successful vaccination program, more than 90% of Americans over the age of 35 (∼140 million people, according to the 2000 United States Census Bureau) have already been vaccinated against smallpox. Is it possible that some of these people still have measurable antiviral immunity? Although the requirements for full protection against orthopoxviruses are not known7, two prospective studies indicate that high levels of neutralizing antibodies may be associated with protective immunity against smallpox8,9. One study showed that contacts of smallpox victims who had neutralizing titers <1:32 against vaccinia virus were more susceptible to smallpox infection (3 of 15 or 20% of contacts infected) than contacts with pre-existing antibody titers ≥1:32 (0 of 127 or <1% of contacts infected)8. A smaller study showed similar results: 6 of 43 (14%) contacts with neutralizing titers <1:20 contracted smallpox, whereas 0 of 13 contacts with titers of 1:20 or higher contracted the disease9. These studies did not prove that neutralizing antibodies were the mechanism of protection, because high amounts of antiviral antibodies may simply have been associated with higher underlying T-cell memory. Nevertheless, these prospective studies8,9, in addition to another study comparing antibody titers to survival during active smallpox infection10, provide evidence that serum antibody levels are a useful biomarker of protective immunity, regardless of whether protection is mediated by B cells, T cells or a combination of both antiviral immune mechanisms. In this study, we evaluated the maintenance of virus-specific immunity after smallpox vaccination by conducting a nonrandomized, cross-sectional analysis of antiviral antibody- and T-cell-mediated immune responses in volunteers examined 1 month to 75 years after vaccination.
Results
Quantitation of virus-specific CD4+ T-cell responses
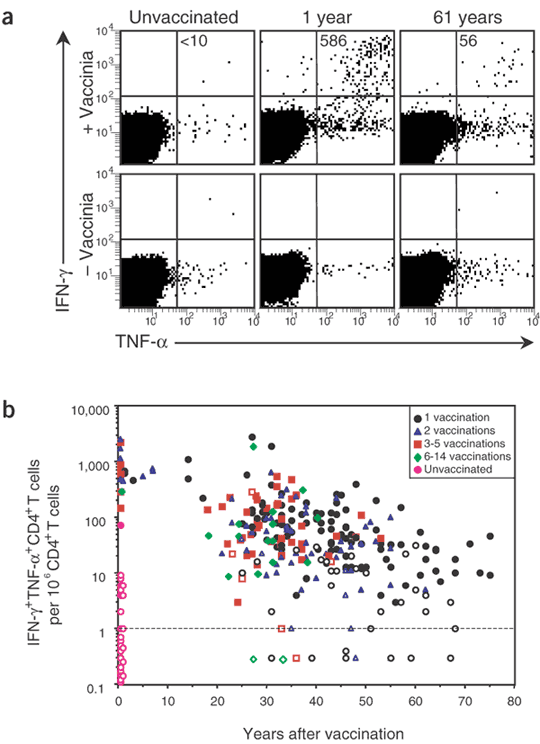
Very little is known about the duration of vaccinia-specific T-cell responses or what proportion of vaccinated individuals will maintain detectable levels of CD4+ or CD8+ T-cell memory. To shed light on this fundamental question, we quantified CD4+ T-cell responses using a highly optimized intracellular cytokine staining (ICCS) protocol that detects vaccinia-specific T cells by virtue of their ability to produce potent antiviral factors, including interferon (IFN)-γ and tumor necrosis factor (TNF)-α, after exposure to vaccinia directly ex vivo (Fig. 1a). After background subtraction, IFN-γ+TNF-α+CD4+ T cells were below detection in the representative unvaccinated control (<10 per 106 CD4+ T cells) but were readily observed at 1 year after vaccination (586 per 106 CD4+ T cells) and at 61 years after vaccination (56 per 106 CD4+ T cells). In both of these latter cases, the number of IFN-γ+TNF-α+ events in the vaccinia-stimulated samples was more than tenfold higher than those observed in the unstimulated controls cultured in parallel. In seven consecutive experiments, samples from the same volunteer at 1 year after vaccination averaged 622 ± 125 IFN-γ+TNF-α+CD4+ T cells per million CD4+ T cells, indicating that this is a highly reproducible assay. Approximately 90% of IFN-γ+ vaccinia-specific CD4+ T cells expressed TNF-α, indicating that they maintained a 'memory' phenotype of dual cytokine expression11. We also observed subpopulations of IFN-γ+TNF-α− and IFN-γ−TNF-α+ T cells in some, but not all, individuals (Fig. 1a and data not shown). To quantify the duration of CD4+ T-cell memory, we relied on the most conservative estimates obtained by enumeration of functional T cells that were capable of dual IFN-γ and TNF-α production. After vaccination or revaccination, virus-specific CD4+ T cells were present in 18 of 18 vaccinees at 27–34 d after immunization (average of 900 per 106 CD4+ T cells); they then declined slowly with a half-life of 8–12 years (Fig. 1b and Table 1). Although multiple vaccinations are believed to provide maximal long-term protection12,13, repeated exposure to vaccinia did not greatly alter the magnitude (Fig. 1b) or the half-life of T-cell memory (Table 1). Although the frequency of virus-specific CD4+ T cells waned slowly over time, T-cell responses in most subjects remained within one to two orders of magnitude of those achieved at up to 7 years after vaccination and could be detected as late as 75 years after immunization.
Figure 1: Virus-specific CD4+ T-cell memory after smallpox vaccination.
(a) Representative flow cytometry dot plot, gated on CD4+CD8− T cells, showing the number of IFN-γ+TNF-α+ events per million CD4+ T cells (+ Vaccinia) after background subtraction (− Vaccinia) in PBMC samples from an unvaccinated volunteer or from volunteers analyzed at 1 or 61 years after vaccination. (b) Quantitation of virus-specific CD4+ T cells as a function of time after vaccination. Filled symbols represent twofold or higher over background; open symbols represent samples that were not twofold over background. Samples that scored below detection were graphed with values of <1 per 106.
Table 1 Estimated survival of virus-specific T-cell memory after smallpox vaccination
Quantitation of virus-specific CD8+ T-cell responses
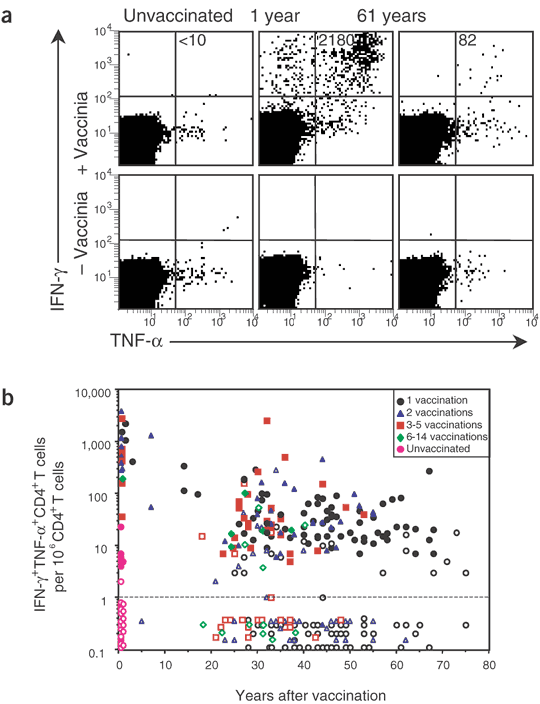
Antiviral CD8+ T-cell responses were quantified by ICCS after direct ex vivo stimulation with vaccinia-infected cells (Fig. 2a). Although a recent study has identified two HLA-A*0201-restricted T-cell epitopes14, these epitopes measure only a subpopulation of the total T-cell response14. Thus, we chose to stimulate T cells with live virus in this cross-sectional study so we could identify the global antiviral CD8+ T-cell response irrespective of the HLA type of the donor15 and to allow side-by-side comparisons with CD4+ T-cell responses (to which no epitopes have yet been mapped). Most IFN-γ+CD8+ T cells expressed TNF-α, and again we used dual cytokine production as the functional criterion for quantifying virus-specific T-cell memory. Samples from one volunteer (1 year after vaccination) averaged 2,215 ± 325 IFN-γ+TNF-α+CD8+ T cells per million CD8+ T cells in seven consecutive experiments. At 27–34 d after vaccination or revaccination, robust CD8+ T-cell responses (average of 870 per 106 CD8+ T cells) were identified in 18 of 18 vaccinees (Fig. 2b). Similar to CD4+ T cells (Fig. 1b), CD8+ T-cell memory declined slowly with a half-life of 8–15 years (Table 1). Comparison of CD8+ T-cell numbers after booster vaccination did not show any substantial improvements in long-term T-cell memory above that observed after a single vaccination (Fig. 2b and Table 1).
Figure 2: Virus-specific CD8+ T-cell memory after smallpox vaccination.
(a) Representative flow cytometry dot plot gated on CD8+CD4− T cells showing the number of IFN-γ+TNF-α+ events per million CD8+ T cells (+ Vaccinia) after background subtraction (− Vaccinia) in PBMC samples from an unvaccinated volunteer or from volunteers analyzed at 1 or 61 years after vaccination. (b) Quantitation of virus-specific CD8+ T cells as a function of time after vaccination. Filled symbols represent more than twofold or greater over background and open symbols represent samples that were not twofold over background. Samples that scored below detection were graphed with values of <1 per 106.
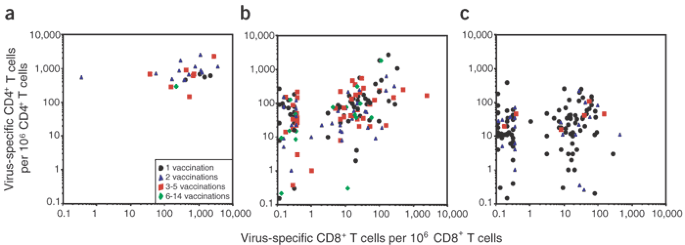
Direct comparisons between virus-specific CD4+ and CD8+ T-cell numbers within individual vaccinees revealed dynamic and independently regulated changes in T-cell memory over time (Fig. 3). At early time points ranging from 27 d to 7 years after vaccination, nearly all volunteers possessed strong CD4+ and CD8+ T-cell responses (Fig. 3a). At later time points of 14–40 years after vaccination (Fig. 3b) or 41–75 years after vaccination (Fig. 3c), many individuals still maintained both CD4+ and CD8+ T-cell memory (albeit at lower levels than the earlier time points observed in Fig. 3a), but other individuals preferentially lost CD8+ T-cell memory while leaving the antiviral CD4+ T-cell compartment intact. In rare cases, CD8+ T-cell responses remained elevated while CD4+ T-cell responses dropped below detection. Further studies will be necessary to determine why virus-specific CD8+ T cells, or in some cases CD4+ T cells, are disproportionately lost over prolonged periods of time, but the overall shift in T-cell memory seems to reflect the survival rates of antiviral CD4+ and CD8+ T cells (Table 1).
Figure 3: Relationship between vaccinia-specific CD4+ and CD8+ T-cell memory over time.
(a–c) Comparisons were made between the number of antiviral CD4+ and CD8+ T cells from the same individual at 1 month to 7 years after vaccination (a), 14–40 years after vaccination (b), or 41–75 years after vaccination (c). Virus-specific T cells are shown as the number per million CD4+ or CD8+ T cells, respectively, and samples that scored below detection were graphed with values of <1 per 106.
Duration of antiviral antibody production
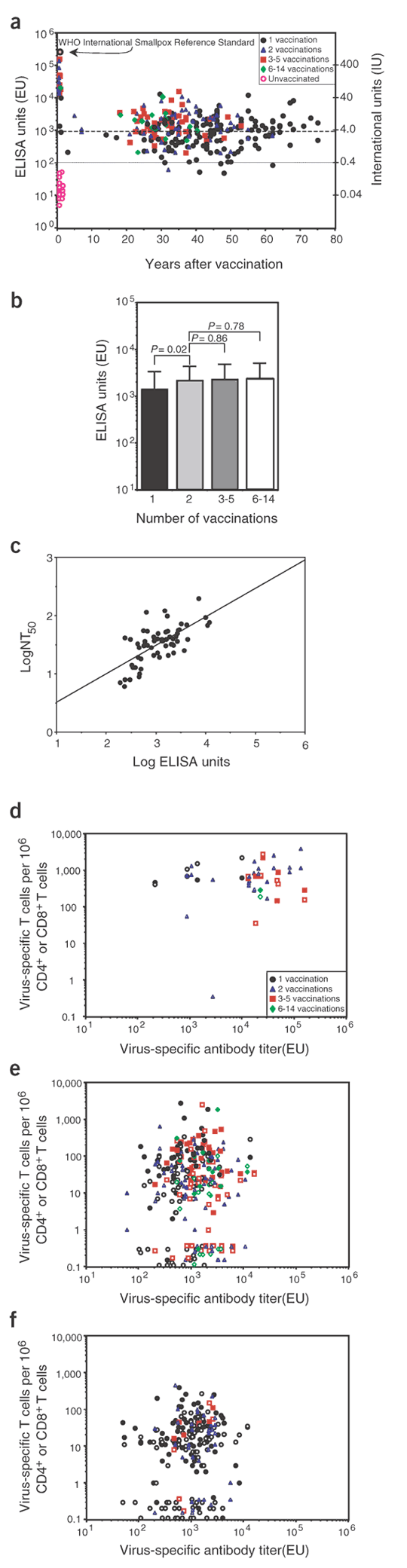
Vaccinia-specific neutralizing antibody titers have been the cardinal feature used to estimate the immunity afforded by smallpox vaccination7,10,16,17,18,19. To examine this issue in more detail, we developed a sensitive, reproducible and validated vaccinia-specific ELISA for high-throughput analysis of humoral immunity after smallpox vaccination (Fig. 4a). Using 100 ELISA units (EU) as the lowest titer considered to be positive, we observed 100% specificity (0 of 26 unvaccinated controls scored ≥100 EU) and 98% sensitivity (288 of 293 samples from volunteers vaccinated against smallpox scored ≥100 EU). One representative positive control (scoring 644 EU) was repeated more than 40 times and varied by less than 12% within a single assay and less than 18% between assays, with no false-negative results. Likewise, a representative negative control sample from an unvaccinated volunteer was repeated in more than 40 assays and in each case scored less than 50 EU, with no false-positive results.
Figure 4: Long-lived antiviral antibody responses induced by smallpox vaccination.
(a) Quantitation of vaccinia-specific antibody responses by ELISA. International units (IU) were calibrated based on the World Health Organization International Standard for smallpox antiserum (1,000 IU/ml). All serum samples from unvaccinated volunteers (n = 26) scored less than 100 EU, which was then used as the lowest positive titer (dotted line); 944 EU indicates the predicted antibody levels equivalent to a neutralizing titer of 1:32 (dashed line). (b) Vaccinia-specific antibody titers (1–75 years after vaccination) were compared with the total number of vaccinations received. Bars show the average and s.d. of antibody production by each group. (c) The correlation between virus-specific antibody titers determined by ELISA and neutralizing assays was determined by linear regression analysis after plotting the log values obtained from serum samples of volunteers vaccinated one or two times against smallpox. The slope of the line was defined as log NT50 = 0.056 + 0.487(log ELISA units) (_R_2 = 0.450; P < 0.0001). (d–f) The relationship between virus-specific CD4+ (filled symbols) or CD8+ (open symbols) T cells (per million CD4+ or CD8+ T cells, respectively) with virus-specific antibody titers was determined 1 month to 7 years after vaccination (d; CD4+, P = 0.67; CD8+, P = 0.39), 14–40 years after vaccination (e; CD4+, P = 0.72; CD8+, P = 0.89) or 41–75 years after vaccination (f; CD4+, P = 0.77; CD8+, P = 0.06*). *, P value derived from the data set after removing the high proportion of negative samples in which there were no measurable CD8+ T-cell response. The P values correspond to the association between CD4+ T-cell numbers and antiviral antibody production or CD8+ T-cell numbers and antiviral antibody production.
In contrast to vaccinia-specific T-cell memory, which declined steadily over time (Figs. 1 and 2), vaccinia-specific serum antibody levels were remarkably stable between 1 and 75 years after vaccination. We were thus unable to determine the half-life of antibody decay. Comparison of antiviral antibody titers elicited by one or more vaccinations showed a very small (less than twofold), but statistically significant, increase in the mean antibody titer that was produced after two vaccinations compared with only one vaccination (P = 0.02; Fig. 4b). Additional vaccinations, however, which ranged from 3–5 to as many as 6–14 immunizations, did not result in any further increase in long-term antibody production. This indicates that booster vaccination may increase a previously suboptimal antibody response but is unlikely to induce prolonged synthesis of higher antibody numbers above a certain threshold.
ELISA assays do not directly measure neutralizing antibodies and must therefore be validated alongside neutralizing assays if they are to be useful for quantifying biologically relevant antibody titers. A previous study determined an experimental value for protective immunity against smallpox, defined as serum antibody levels with half-maximal neutralizing titer (NT50), greater than or equal to 1:32 (ref. 8). By conducting our neutralizing assays in essentially the same manner, we can directly relate our data to historical findings that cannot be repeated now that natural smallpox is extinct. We found a direct linear relationship (P < 0.0001) between neutralizing titers and virus-specific antibodies quantified by ELISA (Fig. 4c). Based on this analysis, an NT50 of 1:32 equals 944 EU (Fig. 4a) and indicates that ∼50% of volunteers at more than 20 years after a single vaccination have neutralizing antibody titers greater than or equal to 1:32. Neutralizing antibodies were below detection (NT50 < 1:4) in 16 of 16 samples from unvaccinated volunteers (data not shown).
An important point to consider is whether or not high antibody responses are correlated with increased T-cell memory, because this would shed light on whether high neutralizing antibody titers were directly involved with protective immunity against smallpox or whether they are simply a surrogate marker indicative of increased antiviral T-cell responsiveness. We compared antiviral T-cell responses with their accompanying antibody titers in individuals who had been vaccinated up to 7 years previously (cohorts similar to that described in ref. 8; Fig. 4d) as well as in individuals vaccinated 14–40 years ago or 41–75 years ago (a cohort that might be similar to contemporary populations; Fig. 4e,f). We found no correlation between virus-specific T-cell numbers and antibody titers at early or late time points, thus indicating that humoral and cellular immunity are independently regulated. If our early cohort (≤7 years after vaccination) is comparable to the smallpox contacts examined in ref. 8, then our results would also indicate that high neutralizing antibody titers are still an effective biomarker of protective immunity but are not necessarily indicative of enhanced T-cell memory. This also implies that high neutralizing antibody titers have a more direct role in protective immunity against smallpox than previously realized.
Discussion
In this study, we examined the levels and duration of antiviral immunity after smallpox vaccination. The combined total of vaccinia- specific IFN-γ-producing CD4+ and CD8+ T cells identified by ICCS at 1 month after vaccination was similar to that calculated in previous studies by ELISPOT analysis14,20,21, indicating that the ICCS technique is an effective and sensitive means for quantifying virus-specific T cells directly ex vivo. We found that virus-specific T-cell memory was maintained for decades but declined slowly with an estimated half-life of 8–15 years. Increasing the number of smallpox vaccinations did not result in higher vaccinia-specific T-cell memory, which may explain why protection against smallpox was still maintained in many vaccinated individuals living in nonendemic countries22,23 even though they were not repeatedly exposed to smallpox or repeatedly vaccinated. In contrast to slowly declining T-cell memory, most volunteers maintained virus-specific antibody responses in the same range as that observed between 1 and 7 years after vaccination. These amounts of antiviral antibody production were readily detected for up to 75 years after vaccination, indicating essentially a lifetime of antiviral antibody production after a single acute viral infection.
Immunity against smallpox has been believed to persist for only 3–5 years after vaccination1,2,3,4,5,6. But contrary to this perspective, several large epidemiological studies have shown that smallpox vaccination provided 90–95% of vaccinees with protection against lethal smallpox infection for many years24 and possibly for life25,26. One interpretation is that the long-lived protection noted in these early studies was due to repeated exposure to smallpox, resulting in subclinical infections that continued to boost immunity over time. Later epidemiological studies in Europe (1950–1971), however, came to similar conclusions even though smallpox was not endemic and occurred only sporadically by importation22,23. The epidemiology of 49 cases of imported smallpox is particularly revealing23. Unvaccinated individuals were highly susceptible to smallpox: 22 of 40 (55%) individuals (hospital staff and general public) exposed to the virus died. In contrast, there was only a 2% fatality rate in contacts vaccinated 0–10 years previously; the fatality rate was only slightly increased in individuals vaccinated 11–20 years, or more than 20 years previously (6% and 7% mortality, respectively). The mortality rates among vaccinated individuals probably underestimate the true extent of protective immunity because these are based only on people whose immunity had waned to the point that they became infected and showed clinical signs of disease. These statistics do not take into account that ∼50% of previously vaccinated smallpox contacts have such high immunity that smallpox infection is clinically inapparent and can only be diagnosed by serology27, a phenomenon documented in some cases even 13 to more than 40 years after vaccination22. Together, these studies provide convincing evidence that vaccination confers long-term protection against lethal smallpox infection and dovetails well with our data demonstrating long-term maintenance of antiviral antibody production and T-cell-mediated immunity.
In humans, it is likely that both humoral and cell-mediated immunity are important in protecting against orthopoxviruses. Patients with genetic defects in either B-cell or T-cell immunity are at increased risk of complications after smallpox vaccination28, and T-cell- deficient patients are far more susceptible than agammaglobulinemic patients to vaccinia as well as many other viral infections. This is sometimes mistaken as proof that humoral immunity is not as important for protection against viral infections. It is not surprising, however, that these differences exist, because the loss of B-cell immunity leaves the T-cell compartment relatively intact, whereas the loss of T-cell immunity almost invariably results in severely impaired antiviral antibody responses as well. This is perhaps most dramatically illustrated in cases of vaccinia necrosum, a severe and life-threatening complication of smallpox vaccination. Even though this disease is almost universally associated with defects in cell-mediated immunity, 100% (18 of 18) of these patients failed to mount a neutralizing antibody response of more than 1:4 after vaccinia infection28. Vaccinia necrosum was essentially 100% fatal if left untreated28. With the advent of vaccinia immune globulin (VIG) therapy, however, nearly 80% of affected children survived, even though they were often genetically incapable of mounting an antiviral T-cell response28. Adoptive transfer of vaccinia-immune lymphocytes from recently vaccinated donors eventually cured one particularly severe case of advanced vaccinia necrosum that was not controllable using VIG. This suggests that either cellular immunity or humoral immunity can be important in protecting against normally lethal poxvirus infections28. Various animal models have also shown that adoptive transfer of either neutralizing antibodies29,30,31 or virus-specific T cells32,33 can provide full protection against vaccinia infection.
With the extinction of naturally occurring smallpox, it may be impossible to definitively establish the immunological correlates of protection against this disease in humans. By relating our current research findings to historical evidence of protection, we may, however, be able to identify long-term immune responses that correlate with protective immunity. Several studies indicate that vaccination affords at least 90–95% of vaccinees with protection against lethal smallpox infection for more than 20 years after vaccination23; this occurs even in the absence of revaccination or smallpox outbreaks in the preceding 30 years22. Our findings on antiviral CD8+ T-cell memory indicate that it might be maintained in only ∼50% of volunteers by 20 years after vaccination, regardless of how many booster vaccinations were received (Fig. 2b and Table 1). This implies that CD8+ T-cell memory is not absolutely required for protection and that CD4+ T-cell memory or neutralizing antibody responses (or both) may constitute the main components of long-term immunity against smallpox after CD8+ T-cell memory has faded. Human CD4+ T cells can exert perforin-dependent lysis of susceptible target cells34,35, and studies in both humans36,37,38 and chimpanzees39 have identified vaccinia-induced cytolytic cells that are major histocompatibility complex class II–restricted CD4+ T lymphocytes. Orthopoxviruses are highly cell-associated, and smallpox is believed to disseminate by phagocytic cells such as macrophages as well as by release of free infectious virus particles7,40. Because macrophages are major histocompatibility complex class II–positive, cytolytic CD4+ T cells might be able to at least partially compensate for the loss of CD8+ T-cell memory by decreasing viral dissemination. Vaccinia-specific antibody and natural killer cells have been implicated in strong, direct ex vivo lytic activity after smallpox vaccination41,42, suggesting that antiviral antibodies may also contribute to the cellular immune response by aiding in antibody-dependent cellular cytotoxicity.
It is difficult to dissect the relative roles of neutralizing antibody and T-cell memory because both are maintained for prolonged periods of time and both are probably involved with long-term protection against smallpox. Although little is known about T-cell immunity during smallpox infection, there is both indirect and direct evidence to support the protective antiviral role of neutralizing antibody. The most convincing data stems from two independent studies, which concluded that smallpox contacts with neutralizing titers of greater than 1:20 or 1:32 were later protected against smallpox8,9. Our analysis shows that there is no direct correlation between T-cell memory and antibody titers (Fig. 4), indicating that high neutralizing titers are not necessarily a biomarker of heightened T-cell immunity. This does not, however, preclude the possibility that high neutralizing titers may be protective only if a minimal number of antiviral T cells are also present. A directly protective effect of VIG therapy during human smallpox infection had been noted in early studies43,44, and a large study involving 705 smallpox contacts demonstrated that administration of VIG, in addition to immediate smallpox vaccination, decreased disease transmission by nearly 70% as compared with vaccination alone45. If one assumes that all of the VIG (10 ml injected intramuscularly) was able to reach the blood stream of a 75-kg adult (∼5,250 ml blood volume) and was not absorbed by other tissues, it would have been diluted from a concentrated NT50 of 1:512 (standard VIG45) to 1:1. Because the amount of VIG administered in these studies would have resulted in neutralizing antibodies that were considerably lower than 1:32, this provides remarkable proof that even low levels of antibody can yield a substantial degree of protection against smallpox. Again, the protective effect of neutralizing antibodies is unlikely to act in isolation from the contributions of antiviral T cells, and it remains to be seen what combination of these complementary mechanisms of adaptive immunity are required for full and unambiguous protection against smallpox.
Many acute viral infections such as measles, mumps, rubella, polio and yellow fever result in long-term immunity46. Measles, mumps and rubella, in particular, were often described as “childhood diseases”, mainly because those who were infected once during childhood were often afforded protection against reinfection for life. Based on our analysis described here, and the indisputable epidemiological evidence showing the long-term protective effects of vaccination22,23,24,25,26, vaccinia joins this list of viruses that induce prolonged and, in many cases, lifelong immunity. In this regard, our study demonstrates that >90% of volunteers maintain measurable humoral or T-cell-mediated immunity for up to 75 years after smallpox vaccination—a result that will affect future models of potential smallpox outbreaks in contemporary populations.
Methods
Demographics of study population.
Unvaccinated controls (n = 26) included 54% female and 46% male participants. Our cohort of 306 vaccinees (66% female, 34% male) included 241 volunteers vaccinated against smallpox in the United States (representing 42 states and Washington, D.C.) and 65 volunteers vaccinated in foreign countries (representing 34 countries). Although most people were vaccinated only once or twice, volunteers with multiple vaccinations were specifically recruited to determine whether this would have a significant effect on the magnitude or duration of antiviral immunity. Number of vaccinations (with number of volunteers in parentheses) are as follows: 1 (n = 160), 2 (n = 73), 3 (n = 26), 4 (n = 18), 5 (n = 11), 6 (n = 4), 7 (n = 5), 8 (n = 3), 9 (n = 2), 10 (n = 2), 11 (n = 0), 12 (n = 0), 13 (n = 1), 14 (n = 1). After one or more vaccinations, no correlation between vaccination scar size and antiviral T-cell immunity or antiviral antibody responses was observed (data not shown). Each volunteer provided written informed consent before participating in the study and all human studies were approved by the Institutional Review Board of Oregon Health & Science University.
Intracellular cytokine staining analysis.
Peripheral blood mononuclear cells (PBMCs) were cultured at 37 °C and 6% CO2 in RPMI containing 20 mM HEPES, L-glutamine, antibiotics and 5% heat-inactivated FBS (HyClone), with or without a pretitered optimal amount of vaccinia (sucrose gradient–purified intracellular mature virus (IMV), vaccinia strain Western Reserve) at an MOI of 0.1. After 12 h of culture, we added Brefeldin A (ICN) at a final concentration of 2 μg/ml for 6 h and stained the cells overnight at 4 °C with antibodies specific for CD8β (clone 2ST8.5H7; Beckman Coulter) and CD4 (clone L200; PharMingen). Cells were washed, fixed, permeabilized and stained intracellularly as previously described11 using antibodies to IFN-γ (clone 4S.B3) and TNF-α (clone Mab11; both from PharMingen). We analyzed samples on a FACSCaliber (Becton Dickinson) using CellQuest software (Becton Dickinson), with 1–2 million events acquired per sample. Live cell gating was performed based on forward and side scatter characteristics. Quantitation of cytokine-positive cells was determined after gating on CD4+CD8− T cells or CD8+CD4− T cells. PBMCs from a positive control (1 year after vaccination) and a negative control (unvaccinated) were included in every assay. We did not use CD8α-specific antibodies for ICCS as previously described47 because human natural killer cells can express low levels of CD8α (but not CD8β). Also, a recent study using ICCS has shown that IFN-γ+CD8αlo cells are likely to be natural killer cells responding to vaccinia in vitro because gating out CD56+ cells selectively removed the CD8αloIFN-γ+ population without reducing the CD8αhiIFN-γ+ cell population15.
ELISA and neutralization assays.
We carried out vaccinia-specific ELISA as previously described48 using a vaccinia-infected cell lysate to coat 96-well flat-bottomed plates. Serial threefold dilutions of sera were incubated on preblocked ELISA plates for 1 h. The plates were washed and incubated with horseradish peroxidase–conjugated mouse antibodies to human IgG (clone G18-145; PharMingen). After another washing step, detection reagents were added and samples were analyzed on a VersaMax ELISA plate reader (Molecular Devices). The World Health Organization International Standard for smallpox antiserum49 was used to calibrate antiviral IgG as measured by ELISA, and an internal positive control was included on every plate to normalize ELISA values between plates and between assays done on different days. We determined antibody titers by logarithmic transformation of the linear portion of the curve, with 0.1 optical density units used as the endpoint to convert the final values.
We carried out neutralization assays following an optimized protocol similar to that previously described8,50. Several threefold dilutions (beginning at 1:4 or 1:12) of heat-inactivated serum were incubated with vaccinia (∼100 plaque-forming units) for 2 h at 37 °C before incubating the virus with Vero cells for 1 h, overlaying with 0.5% agarose and incubating for 3.5 d to allow plaque formation. We fixed cells with 75% methanol and 25% acetic acid. After removing the agarose, we visualized plaques by staining with 0.1% crystal violet in PBS containing 0.2% formaldehyde. The NT50 was defined as the reciprocal of the serum dilution required for 50% reduction in vaccinia plaques. We used logarithmic transformation of the data to calculate the titer and to convert the final values, excluding those for which ≥85% neutralization occurred. Antibodies against extracellular enveloped virus are highly protective in vivo30, but so are antibodies against IMV29,31; we chose IMV for our neutralization studies because there is a precedent for protective immunity against smallpox if the individuals have pre-existing neutralizing antibody titers (against IMV) that are ≥1:20 (ref. 9) or ≥1:32 (ref. 8). Extracellular enveloped virus would be unsuitable for these assays because we would not be able to compare our results with these historical values.
Statistical analysis.
Statistics were done using Microsoft Excel and JMP software (SAS). We log-transformed CD4+, CD8+, ELISA and NT50 values to linearize the relationship between these variables and the time after vaccination, and substituted 0.5/106 as a value for the T-cell samples that scored below the limits of detection. The _t_1/2 of T-cell memory was determined by linear regression analysis, and differences in antibody titers after 1, 2, 3–5, or 6–14 vaccinations were determined by a two-talied Student _t_-test. P ≤ 0.05 indicates statistical significance.
References
- Henderson, D.A. The looming threat of bioterrorism. Science 283, 1279–1282 (1999).
Article CAS Google Scholar - Meltzer, M.I., Damon, I., LeDuc, J.W. & Millar, J.D. Modeling potential responses to smallpox as a bioterrorist weapon. Emerg. Infect. Dis. 7, 959–969 (2001).
Article CAS Google Scholar - Gani, R. & Leach, S. Transmission potential of smallpox in contemporary populations. Nature 414, 748–751 (2001).
Article CAS Google Scholar - Kaplan, E.H., Craft, D.L. & Wein, L.M. Emergency response to a smallpox attack: the case for mass vaccination. Proc. Natl. Acad. Sci. USA 99, 10935–10940 (2002).
Article CAS Google Scholar - O'Toole, T., Mair, M. & Inglesby, T.V. Shining light on “Dark Winter”. Clin. Infect. Dis. 34, 972–983 (2002).
Article Google Scholar - Smith, G.L. & McFadden, G. Science and society. Smallpox: anything to declare? Nat. Rev. Immunol. 2, 521–527 (2002).
Article CAS Google Scholar - Fenner, F., Henderson, D.A., Arita, I., Jezek, Z. & Ladnyi, I.D. in The Pathogenesis, Immunology, and Pathology of Smallpox and Vaccinia (World Health Organization, Geneva, 1988).
Google Scholar - Mack, T.M., Noble, J. Jr & Thomas, D.B. A prospective study of serum antibody and protection against smallpox. Am. J. Trop. Med. Hyg. 21, 214–218 (1972).
Article CAS Google Scholar - Sarkar, J.K., Mitra, A.C. & Mukherjee, M.K. The minimum protective level of antibodies in smallpox. Bull. World Health Organ. 52, 307–311 (1975).
CAS PubMed PubMed Central Google Scholar - Downie, A.W. & McCarthy, K. The antibody response in man following infection with viruses of the pox group. III. Antibody response in smallpox. J. Hyg. 56, 479–487 (1958).
Article CAS Google Scholar - Slifka, M.K. & Whitton, J.L. Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J. Immunol. 164, 208–216 (2000).
Article CAS Google Scholar - Nyerges, G., Hollos, I., Losonczy, G., Erdos, L. & Petras, G. Development of vaccination immunity in hospital personnel revaccinated at three-year intervals. Acta Microbiol. Acad. Sci. Hung. 19, 63–68 (1972).
CAS PubMed Google Scholar - el-Ad, B. et al. The persistence of neutralizing antibodies after revaccination against smallpox. J. Infect. Dis. 161, 446–448 (1990).
Article CAS Google Scholar - Terajima, M. et al. Quantitation of CD8+ T cell responses to newly identified HLA-A*0201-restricted T cell epitopes conserved among vaccinia and variola (smallpox) viruses. J. Exp. Med. 197, 927–932 (2003).
Article CAS Google Scholar - Speller, S.A. & Warren, A.P. Ex vivo detection and enumeration of human antigen-specific CD8+ T lymphocytes using antigen delivery by a recombinant vaccinia expression vector and intracellular cytokine staining. J. Immunol. Methods 262, 167–180 (2002).
Article CAS Google Scholar - McCarthy, K. & Downie, A.W. The antibody response in man following infection with viruses of the pox group. II. Antibody response following vaccination. J. Hyg. 56, 466–478 (1958).
Article CAS Google Scholar - Stienlauf, S. et al. Kinetics of formation of neutralizing antibodies against vaccinia virus following re-vaccination. Vaccine 17, 201–204 (1999).
Article CAS Google Scholar - Centers for Disease Control. Vaccinia (smallpox) vaccine: recommendations of the Advisory Committee on Immunization practices (ACIP). Morb. Mortal. Wkly. Rep. 50, 1–25 (2001).
- Frey, S.E., Newman, F.K., Yan, L. & Belshe, R.B. Response to smallpox vaccine in persons immunized in the distant past. J. Am. Med. Assoc. 289, 3295–3299 (2003).
Article Google Scholar - Frey, S.E. et al. Dose-related effects of smallpox vaccine. N. Engl. J. Med. 346, 1275–1280 (2002).
Article CAS Google Scholar - Ennis, F.A., Cruz, J., Demkowicz, W.E. Jr, Rothman, A.L. & McClain, D.J. Primary induction of human CD8+ cytotoxic T lymphocytes and interferon-γ-producing T cells after smallpox vaccination. J. Infect. Dis. 185, 1657–1659 (2002).
Article Google Scholar - CDC. Smallpox - Stockholm, Sweden, 1963. Morb. Mortal. Wkly. Rep. 12, 172, 174–176, 183, 188, 191, 220, 236 (1963).
- Mack, T.M. Smallpox in Europe, 1950–1971. J. Infect. Dis. 125, 161–169 (1972).
Article CAS Google Scholar - Gayton, W. in The value of vaccination as shown by an analysis of 10,403 cases of smallpox (Gillett & Henty, London, 1885).
Google Scholar - Hanna, W. in Studies in smallpox and vaccination (William Wood and Company, New York, 1913).
Book Google Scholar - Hanna, W. & Baxby, D. Studies in smallpox and vaccination. 1913. Rev. Med. Virol. 12, 201–209 (2002).
Article CAS Google Scholar - Heiner, G.G. et al. A study of inapparent infection in smallpox. Am. J. Epidemiol. 94, 252–268 (1971).
Article CAS Google Scholar - Kempe, C.H. Studies on smallpox and complications of smallpox vaccination. Pediatrics 25, 176–189 (1960).
Google Scholar - Czerny, C.P. & Mahnel, H. Structural and functional analysis of orthopoxvirus epitopes with neutralizing monoclonal antibodies. J. Gen. Virol. 71 (Pt. 10), 2341–2352 (1990).
Article CAS Google Scholar - Galmiche, M.C., Goenaga, J., Wittek, R. & Rindisbacher, L. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology 254, 71–80 (1999).
Article CAS Google Scholar - Ramirez, J.C., Tapia, E. & Esteban, M. Administration to mice of a monoclonal antibody that neutralizes the intracellular mature virus form of vaccinia virus limits virus replication efficiently under prophylactic and therapeutic conditions. J. Gen. Virol. 83, 1059–1067 (2002).
Article CAS Google Scholar - Zinkernagel, R.M. & Althage, A. Antiviral protection by virus-immune cytotoxic T cells: infected target cells are lysed before infectious virus progeny is assembled. J. Exp. Med. 145, 644–651 (1977).
Article CAS Google Scholar - Derby, M., Alexander-Miller, M., Tse, R. & Berzofsky, J. High-avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low-avidity CTL. J. Immunol. 166, 1690–1697 (2001).
Article CAS Google Scholar - Appay, V. et al. Characterization of CD4(+) CTLs ex vivo. J. Immunol. 168, 5954–5958 (2002).
Article CAS Google Scholar - Yanai, F. et al. Essential roles of perforin in antigen-specific cytotoxicity mediated by human CD4+ T lymphocytes: analysis using the combination of hereditary perforin-deficient effector cells and Fas-deficient target cells. J. Immunol. 170, 2205–2213 (2003).
Article CAS Google Scholar - Littaua, R.A., Takeda, A., Cruz, J. & Ennis, F.A. Vaccinia virus-specific human CD4+ cytotoxic T-lymphocyte clones. J. Virol. 66, 2274–2280 (1992).
CAS PubMed PubMed Central Google Scholar - Erickson, A.L. & Walker, C.M. Class I major histocompatibility complex-restricted cytotoxic T cell responses to vaccinia virus in humans. J. Gen. Virol. 74, 751–754 (1993).
Article Google Scholar - Demkowicz, W.E.J., Littaua, R.A., Wang, J. & Ennis, F.A. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J. Virol. 70, 2627–2631 (1996).
CAS PubMed PubMed Central Google Scholar - Zarling, J.M. et al. Proliferative and cytotoxic T cells to AIDS virus glycoproteins in chimpanzees immunized with a recombinant vaccinia virus expressing AIDS virus envelope glycoproteins. J. Immunol. 139, 988–990 (1987).
CAS PubMed Google Scholar - Breman, J.G. & Henderson, D.A. Diagnosis and management of smallpox. N. Engl. J. Med. 346, 1300–1308 (2002).
Article Google Scholar - Perrin, L.H., Reynolds, D., Zinkernagel, R. & Oldstone, M.B. Generation of virus-specific cytolytic activity in human peripheral lymphocytes after vaccination with vaccinia virus and measles virus. Med. Microbiol. Immunol. (Berl.) 166, 71–79 (1978).
Article CAS Google Scholar - Perrin, L.H., Zinkernagel, R.M. & Oldstone, M.B. Immune response in humans after vaccination with vaccinia virus: generation of a virus-specific cytotoxic activity by human peripheral lymphocytes. J. Exp. Med. 146, 949–969 (1977).
Article CAS Google Scholar - Kempe, C.H., Berge, T.O. & England, B. Hyperimmune vaccinial γ globulin. Pediatrics 18, 177 (1956).
CAS PubMed Google Scholar - Peirce, E.R., Melville, F.S., Downie, A.W. & Duckworth, M.J. Antivaccinial γ-globulin in smallpox prophylaxis. Lancet 2, 635–638 (1958).
Article CAS Google Scholar - Kempe, C.H. et al. The use of vaccinia hyperimmune γ-globulin in the prophylaxis of smallpox. Bull World Health Organ. 25, 41–48 (1961).
CAS PubMed PubMed Central Google Scholar - Slifka, M.K. & Ahmed, R. Long-term humoral immunity against viruses: revisiting the issue of plasma cell longevity. Trends Microbiol. 4, 394–400 (1996).
Article CAS Google Scholar - Frelinger, J.A. & Garba, M.L. How durable are the immune responses after smallpox vaccination? N. Engl. J. Med. 347, 689–690 (2002).
Article Google Scholar - Slifka, M.K. & Ahmed, R. Limiting dilution analysis of virus-specific memory B cells by an ELISPOT assay. J. Immunol. Meth. 199, 37–46 (1996).
Article CAS Google Scholar - Anderson, S.G. & Skegg, J. The international standard for anti-smallpox serum. Bull. World Health Organ. 42, 515–523 (1970).
CAS PubMed PubMed Central Google Scholar - Cutchins, E., Warren, J. & Jones, W.P. The antibody response to smallpox vaccination as measured by a tissue culture plaque technique. J. Immunol. 85, 275–283 (1960).
CAS PubMed Google Scholar
Acknowledgements
We thank the many volunteers for the generous gift of their time and their unselfish participation in this research study; S. Tofte, T. Gromlich and K. Buxton for technical assistance; J. Kravitz, A. Melnick and G. Oxman for their support; and J.L. Whitton for insightful discussions. This work was supported by Oregon Health and Science University postdoctoral fellowship T32HL07781 (to S.G.H.), National Institutes of Health grants AI21640 (to J.A.N.) and AI051346 (to M.K.S.), Public Health Service grant 5 M01 RR00334 (to M.K.S.) and Oregon National Primate Research Center grant RR00163 (to M.K.S.).
Author information
Authors and Affiliations
- Oregon Health & Science University Vaccine and Gene Therapy Institute, 505 NW 185th Avenue, Beaverton, 97006, Oregon, USA
Erika Hammarlund, Matthew W Lewis, Scott G Hansen, Lisa I Strelow, Jay A Nelson & Mark K Slifka - Clinical Research Center, 3181 SW Sam Jackson Park Road, Portland, 97201, Oregon, USA
Gary J Sexton - Department of Dermatology, Oregon Health & Science University School of Medicine, Clinical Research Center, 3181 SW Sam Jackson Park Road, Portland, 97201, Oregon, USA
Jon M Hanifin
Authors
- Erika Hammarlund
You can also search for this author inPubMed Google Scholar - Matthew W Lewis
You can also search for this author inPubMed Google Scholar - Scott G Hansen
You can also search for this author inPubMed Google Scholar - Lisa I Strelow
You can also search for this author inPubMed Google Scholar - Jay A Nelson
You can also search for this author inPubMed Google Scholar - Gary J Sexton
You can also search for this author inPubMed Google Scholar - Jon M Hanifin
You can also search for this author inPubMed Google Scholar - Mark K Slifka
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toMark K Slifka.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Hammarlund, E., Lewis, M., Hansen, S. et al. Duration of antiviral immunity after smallpox vaccination.Nat Med 9, 1131–1137 (2003). https://doi.org/10.1038/nm917
- Received: 13 May 2003
- Accepted: 20 July 2003
- Published: 17 August 2003
- Issue Date: 01 September 2003
- DOI: https://doi.org/10.1038/nm917