A History of Corticosterone Exposure Regulates Fear Extinction and Cortical NR2B, GluR2/3, and BDNF (original) (raw)
INTRODUCTION
Exposure to adverse life events can disrupt endocrine regulation (de Kloet et al, 2007) and precede the development of psychopathological disorders such as posttraumatic stress disorder (PTSD; Kendler et al, 1999). A history of stress before trauma has been proposed as a risk factor for developing PTSD (Solomon, 1995), particularly with depressive symptoms such as anhedonia (the loss of enjoyment in previously enjoyed activities), as opposed to generalized anxiety/arousal symptoms (Maes et al, 2001). The physiological mechanisms involved in this vulnerability factor are unclear. They may relate to the actions of elevated glucocorticoid levels on cellular mechanisms that support both positive mood and fear extinction. Fear extinction is defined as the process whereby a conditioned stimulus (CS) presented in the absence of aversive consequences reduces CS-elicited fear, as measured by freezing behavior. In rodents, high corticosterone (CORT) doses delay extinction (Kovács et al, 1977), and chronic exposure impairs new learning in several other aversive and nonaversive contexts (Wolf, 2003). Nonetheless, long-term effects of prior CORT exposure on indices of affect and extinction learning in rodents have largely not been reported.
Extinction reflects new learning, not simply memory decay (Rescorla and Heth, 1975). During extinction, excitatory projections from the ventromedial prefrontal cortex (vmPFC)—generally defined as the prelimbic and infralimbic PFC subregions and medial orbitofrontal cortex (OFC)—are thought to synapse onto GABAergic intercalated cells in the amygdala to inhibit fear responding (Quirk et al, 2003). Human imaging studies implicate the vmPFC in recall of extinction (Milad et al, 2007; Kalisch et al, 2006; Phelps et al, 2004), and several anatomically selective neural stimulation, lesion, and inactivation studies in rodents provide compelling evidence that the infralimbic, but not prelimbic, subregion of the vmPFC is the major site of extinction memory consolidation (Milad and Quirk, 2002; Morgan et al, 1993; Quirk et al, 2000; Sierra-Mercado et al, 2006). Thus, although impaired extinction may result from aberrant neural processing in the amygdala (Falls et al, 1992), multiple lines of evidence in rodents point to upstream infralimbic glutamatergic networks driven by AMPA and NMDA receptor signaling as critical mechanisms of plasticity during extinction learning (cf. Sotres-Bayon et al, 2004; Quirk and Mueller, 2008).
The vmPFC is particularly vulnerable to environmental stress. For example, prefrontal activity after symptom provocation or extinction training is decreased in PTSD (Bremner et al, 1999, 2005). Epidemiological evidence suggests that several stress-related psychopathologies may not develop until well after stressful life events (cf. de Kloet et al, 2006). For that reason, we evaluated the long-term effects of chronic glucocorticoid exposure on extinction learning after contextual fear conditioning in rats and examined several targets known to regulate molecular cascades necessary for neuroplasticity and vmPFC-dependent aversive learning (Zhao et al, 2005; Sotres-Bayon et al, 2007; Zushida et al, 2007; Bredy et al, 2007). Prior CORT retarded fear extinction and altered protein expression patterns selectively in cortical tissue samples. These findings, in conjunction with previous work, support a role for these glutamatergic targets in the vmPFC in fear extinction and reveal a novel mechanism by which chronic exposure to elevated corticosteroids selectively disrupts cellular systems subserving extinction learning and behavioral flexibility. These factors may thereby contribute to chronic fear-related psychopathologies such as PTSD.
MATERIALS AND METHODS
CORT (50 μg/ml; 4-pregnen-11_β_ 21-DIOL-3 20-DIONE 21-hemisuccinate; Steraloids Inc., Newport, RI) was dissolved in water and neutralized (pH 7.0–7.4) with HCl (Deroche et al, 1993; Gourley et al, 2008). Pair-housed Sprague–Dawley rats (_n_=106, 10–12 weeks; Charles River Laboratories, Kingston, NY) were given CORT in place of drinking water for 14 days or treated with RU38486 (RU; below). CORT rats were weaned with 3 days with 25 μg/ml and 3 days with 12.5 μg/ml CORT. Solutions were changed within 72 h of dissolution, and bottles were weighed daily. Animals were experimentally naive and maintained on a 12-h light cycle (0700 hours on). Procedures were approved by the Yale University Animal Care and Use Committee.
Fear conditioning experiments used aluminum chambers (30 × 20 × 25 cm) with grid floors controlled by MedPC software (Med Associates Inc., Georgia, VT) housed in a sound-attenuating outer chamber equipped with white noise generator, fan, and houselight. Conditioning sessions included a 3.5-min habituation period, followed by 2-s, 1 mA footshock. One experiment used 1.5 mA footshock to confirm that freezing after CORT was not at a ‘ceiling’ (Figure 1h). After 1.5 min, the shock was repeated, followed by a 1.5-min intertrial interval and shock. After 30 s, lights were extinguished, and rats were removed from the chambers and returned to the colony room. At 1 h after training, rats were returned to the chambers and videotaped for 2 min to evaluate short-term memory (STM) of the CS (the context). At 24 h after training, rats were returned to the chambers, and freezing was videotaped and scored in a long-term memory (LTM) test.
Figure 1
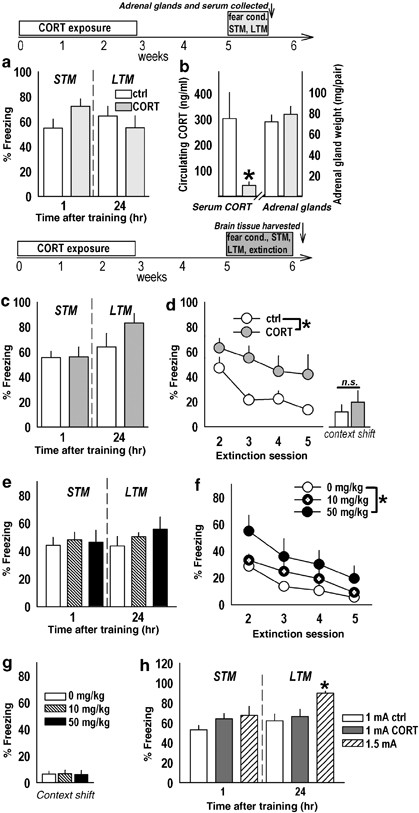
Oral corticosterone (CORT) exposure has long-term consequences for fear extinction. (a) At 2 weeks after CORT exposure, rats were fear conditioned, and freezing to contextual cues was evaluated 1 and 24 h after conditioning, with no differences between groups. (b) Nonetheless, decreased endogenous blood serum CORT was detected in rats with prior exposure to CORT. Adrenal gland weights were unchanged, suggesting the decreased CORT may reflect an acutely blunted response to conditioned stimulus (CS) reexposure. A time course of these experiments is above the plots; ‘Fear cond.’ refers to fear conditioning. (c) Another group of rats was fear conditioned and monitored for freezing to the context 1 and 24 h after training, again revealing no effect of prior CORT. (d) Extinction of freezing was monitored every 24 h for a total of five sessions; here, CORT impaired extinction, but did not influence freezing during a context shift. A time course of these experiments is above the plots. (e) Insufficient glucocorticoid receptor (GR) binding during extinction consolidation could contribute to impeded extinction. We therefore injected RU immediately after the STM test—the first opportunity to learn the context no longer predicted shock—and monitored subsequent extinction learning. As before, freezing during the STM and LTM tests was identical between groups. (f) Posttraining RU38486 (RU; 50 mg/kg) impaired later extinction, though in the absence of a session × RU interaction effect, suggesting acutely impaired GR binding could not fully account for the CORT phenotype. (g) RU did not affect freezing during a context shift. (h) Finally, we confirmed CORT-exposed rats were not at a ‘ceiling’ during the LTM test by comparing these rats (in a and c) to another group conditioned using a 1.0 or 1.5 mA footshock. The 1.0 mA control groups are collapsed. Training with 1.5 mA significantly increased freezing compared to both other groups during the LTM test, suggesting CORT-exposed rats were not at a freezing ceiling. ‘STM’ and ‘LTM’ refer to short-term memory test and long-term memory tests in this and all other figures. Bars and symbols represent group mean±SEM, *_p_⩽0.05.
In experiment 1 (Figure 1a and b), rats were killed immediately after the LTM test, which occurred 2 weeks after CORT exposure, to evaluate the long-term consequences of chronic glucocorticoid exposure, and trunk blood was collected in chilled tubes for blood serum CORT analysis by enzyme-linked immunoassay (Assay Designs, Ann Arbor, MI). After centrifugation at 4°C, samples were diluted 14-fold to fall within the detection limits of the assay and run in duplicate. Data were independently confirmed by analysis in quadruplicate. Adrenal glands were excised from surrounding tissue and weighed as a static reflection of hypothalamic–pituitary–adrenal (HPA) axis activity; one pair was damaged and excluded.
In experiment 2 (Figure 1c and d), rats were fear conditioned 2 weeks after CORT exposure, and extinction after fear conditioning was observed to evaluate the consequences of (1) the blunted CORT response to CS reexposure, or (2) other effects of CORT exposure on vmPFC-dependent learning. Here, animals were fear conditioned as described, and then returned to the conditioning chambers every 24 h for 5 daily sessions, including the LTM test. Freezing during the first 4 min of the 8-min sessions was analyzed, as most freezing occurs during this period. Nonetheless, total freezing scores were later gathered for correlational analyses (Figure 3). One animal was excluded after freezing >2 standard deviations less than the group mean in the LTM test.
Immediately after the last session, rats were placed in a novel context with a different lighting level, background noise, scent, and floor texture than the initial training context. Rats were monitored for freezing for 2 min to quantify the context selectivity of the freezing response (ie context-shift test).
In experiment 3 (Figure 1e–g), to evaluate whether acutely decreased glucocorticoid receptor (GR) binding upon initial extinction learning contributes to increased freezing throughout training, drug-naive rats were fear conditioned and injected with the GR antagonist, RU (10–50 mg/kg; half-life=>30 h; Sigma-Aldrich, St Louis, MO), in 50% polyethylene glycol and saline (2.5 ml/kg, i.p.) immediately after the STM test, the first opportunity to learn that the context no longer predicted shock. A posttraining injection allows for potential disruption of consolidation processes without affecting initial performance. These doses disrupt fear conditioning consolidation (Pugh et al, 1997) and blunt the CORT response to acute stressor (Moldow et al, 2005), respectively. Extinction and context shift protocols proceeded as described.
In experiment 4 (Figure 4), rats were exposed to CORT, allowed 3 weeks recovery, exposed again, allowed an additional 3 weeks recovery, and fear conditioning/extinction training proceeded, as above. To evaluate whether CORT-exposed rats would extinguish with more training, animals were also exposed to an additional three extinction sessions. The goal of this experiment was to confirm the long-lasting effects of CORT by doubling the exposure time and extending the washout period.
Throughout these procedures, freezing was defined as complete immobility except for respiration and scored by a single, blinded rater. STM and LTM freezing were analyzed by _t_-test or one-factor analysis of variance (ANOVA), whereas freezing during extinction training was analyzed by two-factor (drug × session) repeated-measures (RM)-ANOVA (the LTM test was the first session). Data are expressed as percent of session time and were analyzed using SigmaStat 3.1 software (Systat, San Jose, CA). Significance was set at p<0.05; _p_-values >0.05, but <0.1 were considered trends.
Next, we analyzed neural substrates expected to influence fear extinction learning: rats from experiment 2 recovered for 48 h and were killed by rapid decapitation. Brains were placed on dry ice and cut into 2 mm slices using a metal brain matrix (Plastics One, Roanoke, VA). Bilateral punches (1.2 mm diameter, Fine Science Tools, Foster City, CA) were collected from the vmPFC with the caudal-most side ∼+2.2 mm from bregma (Paxinos and Watson, 2005), spanning the length of the infralimbic PFC. It should be noted that some prelimbic tissue contributed to our vmPFC tissue samples to produce a tissue lysate of sufficient protein concentration to conduct our analyses. Lateral OFC and dorsal CA1 hippocampus tissue punches served as comparison sites. Only cortical samples in which tissue was clearly medial or lateral to the ventricles were collected. Because extinction learning is attributed to the vmPFC, the amygdala was not analyzed in the current study.
Samples from one hemisphere per rat were sonicated (200 μl: in 137 mM NaCl, 20 mM Tris-Hcl (pH 8), 1% igepal, 10% glycerol, 1 : 100 Phosphatase Inhibitor 1 and 2 (Sigma)) for Western blotting. Cortical samples from the opposite hemisphere were prepared for quantitative PCR (qPCR; below). The hemisphere used for blotting vs PCR was counterbalanced.
For immunoblotting, tissue was diluted 4 : 1 with 5 × Laemmli buffer (20% glycerol, 2% SDS, bromphenol blue), and an equal amount was loaded onto 8–16% gradient tris-glycine gels (Invitrogen, Carlsbad, CA) for electrophoresis separation. After transfer, nitrocellulose membranes (0.2 μM; Bio-Rad, Hercules, CA) were immunoblotted with anti-NR2B (Rb; 1 : 1000; Novus Biologicals, Littleton CO), anti-GluR2/3 (Rb; 1 : 500; Chemicon, Temecula, CA), anti-GluR1 (Rb; 1 : 500; Chemicon), anti-pGluR1ser-845 (Rb; 1 : 250–1000, PhosphoSolutions, Aurora, CO), and anti-GluR1ser-831 (Rb; 1 : 1000, PhosphoSolutions). For antibody detection by the Odyssey imaging system (LI-COR; Lincoln, NE), membranes were incubated with IRDye700 Dx Anti-Rb IgG and IRDye800 Dx Anti-Ms IgG (1 : 5000; Rockland Immunochemicals, Gilbertsville, PA). Bands were quantified using Odyssey software.
To control for variance within gels, NR2B, GluR2/3, and GluR1 signal values were normalized to GAPDH loading controls (Ms; 1 : 10 000; Advanced Immunochemical Inc., Long Beach, CA). To control for variance between gels, data were converted to percent of control samples on the same gel and analyzed by _t_-test per target (Figure 2).
Figure 2
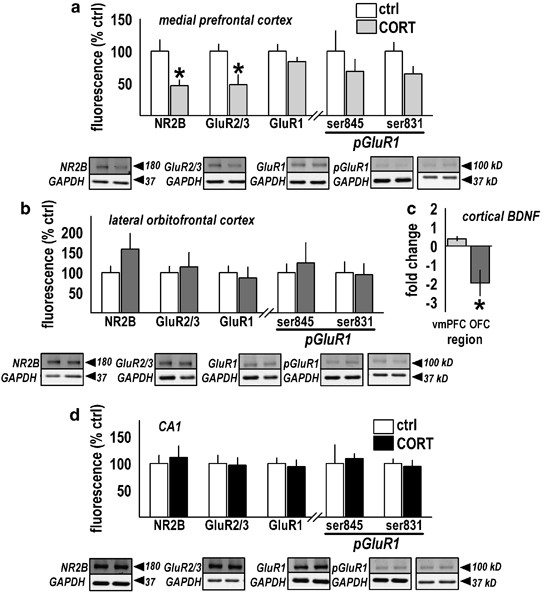
Prior corticosterone (CORT) regulates several cortical targets essential for neuroplastic processes. (a) Rats from Figure 1c and d were killed 2 days after the last extinction session, and ventromedial prefrontal cortex (vmPFC) tissue was analyzed, revealing decreased expression of the NMDA receptor NR2B subunit and AMPA receptor GluR2/3 subunits, and a trend for decreased phosphorylated GluR1 at the ser-831 (protein kinase C) site (_p_=0.08). (b) These findings were not detected in the adjacent orbitofrontal cortex (OFC). (c) Cortical brain-derived neurotrophic factor (BDNF) mRNA was also analyzed, revealing a downward fold change in the OFC, but not vmPFC. Future studies will attempt to evaluate functional consequences. (Data are graphed to represent both magnitude and direction of change with 0 on the y axis representing no change.) (d) The dorsal hippocampal CA1 subregion was also analyzed as a control site, revealing no changes. Bars represent group mean±SEM, *_p_⩽0.02. Representative Western blots are provided below the appropriate bar plot with corresponding GAPDH loading control blots. All proteins fell at the predicted molecular weights. Each lane represents a single subject.
Brain-derived neurotrophic factor (BDNF) mRNA was analyzed by qPCR. RNA was isolated using the mirVana RNA Isolation kit (Ambion, Austin, TX) and reverse transcribed to create cDNA using the First Strand cDNA Synthesis Kit (Invitrogen). Equal amounts of homogenate were analyzed in duplicate using the TaqMan Gene Expression Assay (Applied Biosystems, Foster City, CA) probes for rat BDNF and GAPDH. Cycle threshold (_C_t) values were detected by the 7500Fast Real-Time PCR machine (Applied Biosystems). _C_t values were plotted using the comparative _C_t method (ΔΔ_C_t) to calculate fold change from the control mean, but Δ_C_t values were analyzed by _t_-test to allow for variance within the control group. GAPDH was the loading control.
Behavioral experiment 5 further characterized the chronic effects of CORT using the elevated plus maze, sucrose preference, and locomotor monitoring (Figure 5). In the plus maze, rats were placed in the center of a clean maze elevated 50 cm from the tabletop containing four arms (50 cm long), two of which were enclosed (40 cm walls) for 10 min. Time spent in the open and closed arms was quantified. All four paws were required to be in an arm to be quantified. Ambulation in these rats was also monitored in a clean cage for 1 h with the Digiscan system equipped with 16 photocells (Columbus, OH) to examine whether prior CORT had nonspecific effects on locomotion.
Sucrose preference was assessed using a protocol adapted from Willner et al (1996): pair-housed rats were rehoused individually after CORT to more accurately assess individual sucrose preferences. At 2 weeks after CORT, water bottles were replaced with a 1% (w/v) sucrose solution for 48 h for habituation. Rats were then water-deprived for 4 h, and preference was assessed during a 1-h access period to both sucrose- and water-containing bottles. After 2 days, the deprivation period was extended to 19 h, and rats were again allowed 1-h access to both bottles. Location of sucrose-containing bottle was counterbalanced and reversed to avoid development of side preferences. Preference/entry/activity scores were analyzed by two-factor RM-ANOVA or _t_-test.
RESULTS
Prior CORT Reduces the Endogenous CORT Response to CS Reexposure and Retards Extinction
In these experiments, CORT-exposed rats were fear conditioned 2 weeks after chronic exposure to a dose that is 1/8 of that previously reported to mimic the endogenous CORT response to restraint (Magariños et al, 1998). Testing consisted of an STM test 1 h after conditioning and an LTM test 24 h after conditioning. In initial experiments, freezing after CORT did not significantly differ from control levels (STM: _t_13=−2.0, _p_=0.07; LTM: _t_13=0.75, _p_=0.5; Figure 1a). Trunk blood was collected after the LTM test and was analyzed, revealing decreased endogenous CORT in the previously CORT-exposed group (_t_13=2.7, _p_=0.02; Figure 1b). Adrenal gland weights were indistinguishable (_t_12=−0.68, _p_=0.5; Figure 1b), suggesting endogenous CORT was acutely decreased in experimental rats. At the time of killing, body weights did not significantly differ (control mean=513.3±7.2 g, CORT mean=507±14.7g; _t_13=−0.46, _p_=0.7; data not shown).
To further determine the long-term effects of prior CORT exposure, extinction after contextual fear conditioning was evaluated. Again, rats in this experiment did not significantly differ in the STM (_t_13=0.07, _p_=0.94) or LTM (_t_13=−1.4, _p_=0.18) tests after CORT exposure (Figure 1c). When freezing during extinction (LTM test through session 5) was analyzed, however, significant main effects of both drug (F(1, 13)=4.5, _p_⩽0.05) and session (F(4, 52)=16.6, p<0.001) were found (Figure 1d), with prior CORT-exposed rats displaying increased freezing. Two-factor RM-ANOVA of the first _vs_ last posttraining session revealed an interaction between session and CORT (F(1, 13)=8.2, _p_=0.013) with Tukey's _post hoc_ tests indicating rats differed on the last session (_p_=0.005), but not first (_p_>0.1).
Because endogenous CORT levels were reduced after CS reexposure in previously CORT-exposed rats, acutely decreased GR binding at the time of reexposure may contribute to deficient extinction acquisition. The ability of RU to disrupt extinction consolidation at the first opportunities to learn the context no longer predicted shock—the STM and LTM tests—was therefore tested, with the hypothesis that GR antagonism would block initial consolidation and thereby impair extinction learning in later sessions. Therefore, RU was injected immediately following the STM test to disrupt the earliest possible consolidation of extinction. During this test, the groups designated for veh vs RU (10 or 50 mg/kg, i.p.) injection did not differ (F(2, 24)<1; Figure 1e). LTM freezing scores the following day were also indistinguishable between groups (F(2, 24)<1; Figure 1e). The half-life of RU is >30 h and would therefore be expected to still be on board and disrupt extinction consolidation here, as well. A significant effect of high-dose RU was found for freezing during extinction training (F(2, 24)=5.6, _p_=0.01; vs control _p_=0.008, vs low-dose RU _p_=0.04) (Figure 1f). Freezing scores after RU administration were square-root transformed to preserve required homogeneity of variance.
Despite the main effect of high-dose RU, two-factor RM-ANOVA of the first and last sessions did not reveal an interaction effect (F(2, 24)=1.3, _p_=0.28), suggesting GR inhibition upon consolidation of initial extinction training may contribute to, but could not fully account for, the extinction deficit after CORT exposure. Potential molecular targets of action in the vmPFC were therefore investigated (below).
In both extinction experiments, rats were moved to a novel context immediately after the last extinction session to evaluate whether increased freezing to the training context in CORT-exposed rats could be attributed to factors other than impaired extinction learning, such as enhanced consolidation of the training context. In this instance of ‘context shift,’ CORT-exposed rats would be expected to freeze less when exposed to a novel context than control counterparts. Neither prior CORT exposure nor RU affected freezing in the novel context (_t_13=−0.7, _p_=0.48; F(2, 24)<1, respectively) (Figure 1d and g).
To confirm that freezing after CORT was not at a ceiling, another group of rats was fear conditioned with either 1.0 or 1.5 mA footshock and tested in the STM and LTM tests. As predicted, the 1.0 mA group did not differ from the control groups in experiments 1 and 2, as the testing conditions were identical. This allowed for a one-factor (group) ANOVA of all 1.0 mA control rats, the CORT-exposed rats (trained with a 1.0 mA footshock), and the group of drug-naive rats trained with a 1.5 mA footshock. Although freezing during the STM test did not differ between groups (F(2, 34)=1.8, _p_=0.17), LTM freezing differed (F(2, 34)=3.3, p<0.05) with post hoc tests indicating the 1.5 mA group froze more than the 1.0 mA controls and CORT-exposed rats (_p_s=0.04). This comparison indicates that CORT-exposed rats were not at a ‘ceiling,’ ie a maximum possible freezing level, at the start of extinction training, which could have artificially inflated later freezing during extinction.
Prior CORT Selectively Regulates Cortical Targets
To investigate other potential factors contributing to impaired extinction after chronic CORT exposure, several glutamatergic targets were analyzed in vmPFC tissue lysates —containing infralimbic and some prelimbic cortex—collected from CORT-exposed rats subjected to extinction training (those in Figure 1c and d). The lateral OFC and CA1 subregion of the dorsal hippocampus served as comparison sites. In the vmPFC, decreased NR2B (_t_10=2.7, _p_=0.02) and GluR2/3 (_t_11=2.8, _p_=0.02) were observed, whereas total GluR1 was unchanged (_t_10=1.3, _p_=0.2) (Figure 2a). Given the critical role of GluR1 in activity-driven synaptic plasticity (Shi et al, 2001), we also analyzed phosphorylation levels at the ser-831 and ser-845 residues. GluR1 data from the vmPFC were not normally distributed; therefore, pGluR1 values were normalized to GAPDH loading control values, as opposed to total GluR1. This analysis revealed a trend for a decrease in pGluR1ser-831 (_t_11=1.9, _p_=0.08), but not pGluR1ser-845 (_t_11=0.8, _p_=0.4) (Figure 2a). In the OFC, all of these targets were analyzed, but no significant effects were found (all _p_s⩾0.15; Figure 2b). Further, no significant effects were observed in CA1 (all _p_s⩾0.7; Figure 2d). Although the CA1 field is critical to contextual fear memory retrieval (Hall et al, 2001), alterations in this region would not necessarily be expected to affect extinction learning.
Because increased prefrontal BDNF has been associated with extinction in both appetitive (Berglind et al, 2007) and aversive (Bredy et al, 2007) paradigms, we also analyzed BDNF mRNA with the hypothesis that prior CORT exposure may impair extinction by reducing BDNF expression. We found a marked decrease in BDNF mRNA in the OFC (_t_13=−3.7, _p_=0.003), however, no changes were observed in vmPFC samples (_t_10=−0.1, _p_=0.9) (Figure 2c). BDNF protein expression was not directly analyzed because of the large tissue volume required.
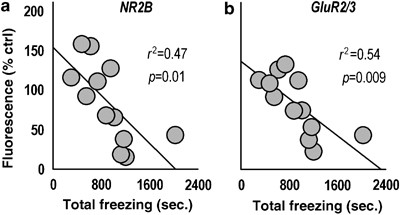
To evaluate whether freezing time could predict vmPFC protein expression, we analyzed covariance between total freezing time (ie the LTM test through extinction session 5) and NR2B, GluR2/3, and pGluR1ser-831 using Pearson's correlation coefficient. Freezing covaried significantly with NR2B (_r_2=0.47, _p_=0.01; Figure 3a) and GluR2/3 (_r_2=0.54, _p_=0.009) expression (Figure 3b).
Figure 3
NR2B and GluR2/3 expression covary with freezing during extinction. (a) Prefrontal NR2B expression levels predicted total time spent freezing during extinction training (the LTM through day 5), suggesting its downregulation by chronic corticosterone (CORT) had functional and long-term consequences for extinction learning. (b) GluR2/3 also predicted total freezing levels with a high degree of significance. Both datasets were analyzed by Pearson's correlation coefficient.
Multiple Exposures to CORT Impair Extinction Learning
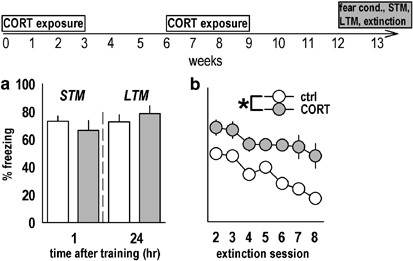
Fear conditioning and extinction learning in rats that were exposed to CORT twice was also evaluated. As before, freezing levels did not differ in initial STM (_t_13=0.77, _p_=0.46) and LTM (_t_13=−0.8, _p_=0.44) tests, despite doubling the CORT exposure regimen (Figure 4a). Freezing across several sessions was increased in CORT-exposed rats, however, to a high degree of significance (main effect: F(1, 13)=13.7, _p_=0.003; Figure 4b). As before, an interaction between session and CORT was found when the first and last sessions were compared (F(1, 13)=7.1, _p_=0.02), with freezing during the first session unchanged (_p_=0.6), whereas CORT-exposed animals froze significantly more on the last session than control rats (_p_=0.002). Although it is tempting to conclude CORT additively confers resistance to extinction training, additional experiments are necessary to directly test this hypothesis.
Figure 4
Multiple exposures to corticosterone (CORT) profoundly impair extinction learning. (a) Rats that had been exposed to CORT twice and then fear conditioned were indistinguishable from control rats in both STM and LTM tests. (b) Freezing during extinction training was, however, significantly increased above control levels (_p_=0.003). A time course of this experiment is above the plots; ‘Fear cond.’ refers to fear conditioning. Bars and symbols represent group mean±SEM, *p<0.05.
Prior CORT Induces Anhedonic, but not Anxiety-Like, Responding
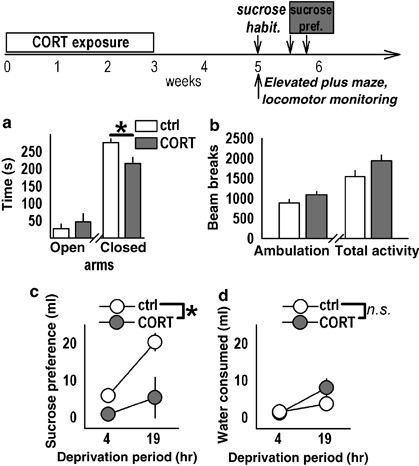
Finally, we evaluated other behavioral consequences of prior CORT exposure relevant to stress-related psychopathology. In the elevated plus maze, two-factor (treatment × maze arm) ANOVA of responding revealed no main effect of group (F(1, 14)=1.4, _p_=0.3; Figure 5a). An interaction between these two factors was found, however; post hoc analyses revealed decreased time spent in the closed arms by CORT-exposed rats (F(1, 28)=5.5, _p_=0.03, _post hoc p_=0.02). There were no differences in time spent in the open arms (_p_=0.4), suggesting CORT-exposed rats spent more time with at least one paw in the center square, as opposed to all paws in open or closed arms. Locomotor activity in a clean cage was also monitored in the same animals, revealing a trend for an increase in both total activity and ambulatory activity counts after CORT (_t_14=1.9, _p_=0.08; _t_14=1.8, _p_=0.1; Figure 5b). These data suggest that nonspecific decreases in activity cannot account for the extinction phenotype.
Figure 5
Prior corticosterone (CORT) induces anhedonic, but not anxiety-like, behavioral responding. (a) To evaluate other relevant consequences of prior CORT exposure, behaviorally naive CORT-exposed rats explored an elevated plus maze, revealing no changes in open arm exploration and a modest decrease in closed arm exploration after CORT. (b) Locomotor monitoring in a clean cage revealed a trend for increased ambulatory and total activity after CORT exposure, suggesting the extinction phenotype cannot be attributed to nonspecifically decreased activity. (c) By contrast, CORT exposure robustly decreased rats' preference for a sucrose solution in a model of anhedonia. (d) No changes in water consumption were observed, indicating a selective deficit in sucrose consumption. A time course of these experiments is above the plots. ‘Habit.’ refers to a sucrose habituation period before testing, and ‘pref.’ refers to preference. Bars and symbols represent group mean±SEM, *p<0.05.
Sucrose preference in a model of anhedonia was also evaluated in CORT-exposed rats after 4- and 19-h water deprivation. Two-factor (treatment × deprivation period) RM-ANOVA revealed main effects of group (F(1, 10)=9.8, _p_=0.01) and deprivation period (F(1, 10)=10.0, _p_=0.01) (Figure 5c), indicating control animals displayed a stronger preference, and that consumption increased as the deprivation period lengthened, as expected. Although water consumption during these tests increased after 19 h of water deprivation (F(1, 10)=10.1, _p_=0.01), there were no differences between the groups (F(1, 10)=2.1, _p_=0.2) (Figure 5d), indicating that CORT exposure selectively decreased sucrose intake, consistent with an anhedonic-like phenotype.
DISCUSSION
We examined extinction of conditioned fear after CORT exposure to evaluate the long-term effects of chronic glucocorticoid exposure on vmPFC-dependent learning and behavioral flexibility. Prior CORT exposure (1) impaired extinction but not recall of fear memory when tested 1 and 24 h after training; (2) did not decrease exploration of the open arms of an elevated plus maze or alter freezing during a context shift, suggesting differences in fear responding during extinction were specific to contextual extinction learning; (3) decreased sucrose preference in a model of anhedonia; and (4) decreased NR2B and GluR2/3 in tissue samples collected from the vmPFC, but not the OFC or CA1, providing evidence for an anatomically selective mechanism by which prior CORT impaired extinction learning. A physiologically relevant dose of RU administered after the first extinction session also increased subsequent freezing (see also Yang et al, 2006). This suggests that an acute reduction in GR activation, due to decreased circulating CORT in prior CORT-exposed fear conditioned rats, contributed to, though could not fully account for, extinction learning deficits. VmPFC NR2B and GluR2/3 levels were also decreased by prior CORT exposure, and protein levels covaried with freezing. These findings support a relationship between selective glutamatergic receptor subunit expression and fear expression. Despite the acute facilitative effects of CORT and other stress factors on various forms of learning (cf. Wolf, 2003), these data demonstrate that a history of chronic CORT exposure persistently impairs extinction learning. Disrupted HPA axis activity and alterations in the expression of distinct glutamate receptor subtypes in the vmPFC may be responsible, at least in part, for these CORT-induced impairments in behavioral flexibility.
Consistent with our finding that NR2B expression correlates with freezing during extinction training, previous studies have selectively implicated this NMDA receptor subunit in cortical long-term potentiation (Zhao et al, 2005), a putative substrate of learning and memory that may contribute to an organism's ability to consolidate fear extinction (Tang et al, 1999; Sotres-Bayon et al, 2007). Consolidation of fear extinction is itself dependent on NMDA receptor-mediated neuronal bursting in the vmPFC (Burgos-Robles et al, 2007). Further, the NMDA receptor antagonist, CPP, impairs long-term extinction learning when microinfused into the vmPFC, while leaving within-session extinction learning intact (Santini et al, 2001; Suzuki et al, 2004). Our data are in agreement with these findings, as between-session freezing was higher in the CORT-exposed group (Figure 1d), but CORT did not change the pattern of freezing across time within the extinction sessions (data not shown). The selective effects of CPP in previous studies were recently attributed to blocking receptors containing the NR2B subunit (Sotres-Bayon et al, 2007). Freezing during extinction training here critically covaried with NR2B expression, supporting a proposed relationship between NR2B- and PFC-dependent learning and memory (Tang et al, 1999; Wong et al, 2002). NR2B-preferring agonists would be hypothesized to reverse these CORT-induced deficits in extinction learning, however, such selective compounds are not yet commercially available.
AMPA receptor expression and phosphorylation events have also been implicated in several forms of learning and memory, yet direct evidence for AMPA receptor activation in fear extinction has been equivocal. The AMPA receptor subunits, GluR1/2, are essential for activity-dependent neuroplasticity, whereas the GluR2/3 heterodimer is constitutively cycled at the cell membrane (Shi et al, 2001). AMPA receptor antagonists and agonists reportedly have no effects on extinction when microinjected into the amygdala (Falls et al, 1992; Lin et al, 2003; Zushida et al, 2007). Nonetheless, a recent study reported that contextual fear extinction could be facilitated with vmPFC microinfusion of the selective agonist, PEPA, which has high affinity for GluR3-containing AMPA receptors (Zushida et al, 2007). Like prior CORT exposure, systemic administration of this compound selectively affected extinction without alterations in initial memory retrieval. Systemic PEPA also activated PFC neural networks more potently than in CA1, where its effects were minimal. Although the hippocampus is involved in recall of extinction (Milad et al, 2007), we similarly found no effects on selected targets in CA1. By contrast, vmPFC GluR2/3 expression was decreased and covaried with freezing, indicating that receptor expression levels fundamentally regulate the ability of the vmPFC to facilitate extinction learning.
We did not find significant changes in vmPFC BDNF mRNA levels. These data are in contrast to previous observations of increased vmPFC BDNF mRNA and distinct patterns of histone acetylation around two BDNF gene promoters after fear extinction (Bredy et al, 2007). These differences could be due to different extinction training protocols: in their study, Bredy et al reported that promoting histone acetylation potentiated fear extinction only when animals received partial, and not full, extinction training. In addition, our vmPFC tissue samples contained some prelimbic tissue—though the infralimbic has been more strongly associated with extinction learning using anatomically selective electrophysiologic techniques by Quirk and colleagues (see Introduction)—and this may also have contributed to our divergent finding. More selective analysis of this subregion in future experiments may reveal anatomically specific alterations in vmPFC BDNF mRNA expression patterns that relate to extinction learning.
Nonetheless, glucocorticoid exposure alone might still be expected to broadly influence prefrontal BDNF levels (Trentani et al, 2002). Glucocorticoid actions on vmPFC BDNF could be transient, however, and therefore nondetectable in rats killed 3 weeks after the last exposure, as was the case here. By contrast, stress-related BDNF regulation appears to be long-lasting in the OFC (Figure 2c). Future studies will employ restricted viral-mediated gene transfer techniques that can be used to evaluate whether OFC-selective BDNF suppression influences fear memory extinction (see Gottfried and Dolan, 2004; Rauch et al, 2005) or performance on other tasks traditionally associated with activity-dependent neuroplasticity in this region.
Here, we found that previously CORT-exposed fear-conditioned rats, which were killed 24 h after training, displayed decreased plasma CORT, yet the same exposure regimen increased subsequent fear responding. How might we resolve these seemingly paradoxical findings? Oral CORT exposure and subsequent fear conditioning in rodents may induce an endocrine, as well as behavioral, state similar to that in PTSD, in which elevated central corticotropin-releasing factor (CRF) and low diurnal CORT have been observed and attributed to exaggerated HPA axis feedback regulation (cf. de Kloet et al, 2006). Further, enhanced cortisol suppression after synthetic glucocorticoid administration remains a highly replicable clinical finding (Yehuda et al, 1993; cf. de Kloet et al, 2006, but see de Kloet et al, 2007) that covaries with the amount of time that has passed since the traumatic event (Yehuda et al, 2002, 2004). Future studies will attempt to characterize the HPA, CRF, and behavioral response to dexamethasone in prior CORT-exposed rats with or without fear conditioning. This distinction may be critical, as we have previously shown that mice administered comparable doses of CORT also display depressive-like behaviors, with no evidence of altered basal (daytime) CORT levels 2–3 weeks after exposure (Gourley et al, in press).
Oral CORT exposure and subsequent fear conditioning in rats may induce an endocrine, as well as behavioral, state similar to that in PTSD, concurrent with selective alterations in vmPFC glutamatergic receptor subunit expression. Notably, we found no long-term anxiety-like consequences for exploration behavior in the elevated plus maze. This finding is in support of a role for vmPFC integrity in learned, but not innate, fear (Corcoran and Quirk, 2007). Our data also complement reports that chronic restraint stress (Radley et al, 2004; Liston et al, 2006), as well as CORT alone (Wellman, 2001; Cerqueira et al, 2007; Liu and Aghajanian, 2008), cause dendritic atrophy and increased segmentation in the vmPFC. Our finding of decreased GluR2/3 in particular could reflect lost synaptic connectivity after morphological reorganization, rendering neurons incapable of engaging in the neuroplasticity essential for new learning. A history of stress before trauma has been suggested to predispose individuals to PTSD with primarily depressive (as opposed to anxiety/arousal) symptoms (Maes et al, 2001). Given anhedonic- but not anxiety-like responding in CORT-exposed rats, our model may be particularly relevant to this clinical subpopulation. This model may therefore provide novel avenues for (1) the basic understanding of fear extinction, (2) further elucidation of the mechanisms regulating behaviorally flexible responding and cognitive deficit in PTSD, and (3) the development of new therapeutic approaches to more effectively treat specific subtypes of stress-related psychiatric illness.
References
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Miller SW et al (2007). A BDNF infusion into the medial prefrontal cortex suppressed cocaine seeking in rats. Eur J Neurosci 26: 757–766.
Article Google Scholar - Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M (2007). Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem 14: 268–276.
Article CAS Google Scholar - Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS (1999). Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry 156: 1787–1795.
CAS PubMed PubMed Central Google Scholar - Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N et al (2005). Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med 35: 791–806.
Article Google Scholar - Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ (2007). Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron 53: 871–880.
Article CAS Google Scholar - Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N (2007). Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex 17: 1998–2006.
Article Google Scholar - Corcoran KA, Quirk GJ (2007). Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci 27: 840–844.
Article CAS Google Scholar - de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HGM (2006). Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res 40: 550–567.
Article CAS Google Scholar - de Kloet CS, Vermetten E, Heijnen CJ, Geuze E, Lenties EG, Westenberg HG (2007). Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology 32: 215–226.
Article CAS Google Scholar - Deroche V, Piazza PV, Deminiere J-M, Le Moal M, Simon H (1993). Rats orally self-administer corticosterone. Brain Res 622: 315–320.
Article CAS Google Scholar - Falls WA, Miserendino MJD, Davis M (1992). Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci 12: 854–863.
Article CAS Google Scholar - Gottfried JA, Dolan RJ (2004). Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci 7: 1145–1153.
Article Google Scholar - Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS et al (2008). Regionally-specific regulation of ERK MAP kinase in a model of antidepressant sensitive chronic depression. Biol Psychiatry 63: 353–359.
Article CAS Google Scholar - Gourley SL, Kiraly DD, Howell JL, Olausson P, Taylor JR (2008). Acute hippocampal BDNF restores motivational and forced swim performance after corticosterone. Biol Psychiatry.
- Hall J, Thomas KL, Everitt BJ (2001). Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during recall of contextual memories. J Neurosci 21: 2186–2193.
Article CAS Google Scholar - Kalisch R, Korenfeld E, Stephan KE, Wieskopf N, Seymour B, Dolan RJ (2006). Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci 26: 9503–9511.
Article CAS Google Scholar - Kendler KS, Karkowski LM, Prescott CA (1999). Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156: 837–841.
Article CAS Google Scholar - Kovács GL, Telegdy G, Lissák K (1977). Dose-dependent action of corticosteroids on brain serotonin context and passive avoidance behavior. Horm Behav 8: 155–165.
Article Google Scholar - Lin C-H, Yeh S-H, Lu H-Y, Gean P-W (2003). The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J Neurosci 23: 8310–8317.
Article CAS Google Scholar - Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR et al (2006). Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 26: 2870–7874.
Article Google Scholar - Liu RJ, Aghajanian GK (2008). Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA 105: 359–364.
Article CAS Google Scholar - Maes M, Mylle J, Delmeire L, Janca A (2001). Pre- and post-disaster negative life events in relation to the incidence and severity of post-traumatic stress disorder. Psychiatry Res 105: 1–12.
Article CAS Google Scholar - Magariños AM, Orchinik M, McEwen BS (1998). Morphological changes in hippocampal CA3 regions induced by non-invasive glucocorticoid administration: a paradox. Brain Res 809: 314–318.
Article Google Scholar - Milad MR, Quirk GJ (2002). Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420: 70–74.
Article CAS Google Scholar - Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL (2007). Recall of fear extinction in humans activates the venotromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 62: 446–454.
Article Google Scholar - Moldow RL, Beck KD, Weaver S, Servatius RJ (2005). Blockage of glucocorticoid, but not mineralocorticoid receptors prevents the persistent increase in circulating basal corticosterone concentrations following stress in the rat. Neurosci Lett 374: 25–28.
Article CAS Google Scholar - Morgan MA, Romanski LM, LeDoux JE (1993). Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett 163: 109–113.
Article CAS Google Scholar - Paxinos G, Watson C (2005). The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego.
Google Scholar - Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43: 897–905.
Article CAS Google Scholar - Pugh CR, Fleshner M, Rudy JW (1997). Type II glucocorticoid receptor antagonists impair contextual but not auditory-cue fear conditioning in juvenile rats. Neurobiol Learn Mem 67: 75–79.
Article CAS Google Scholar - Quirk GJ, Russo GK, Barron JL, Lebron K (2000). The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 20: 6225–6231.
Article CAS Google Scholar - Quirk GJ, Likhtik E, Pelletier JG, Paré D (2003). Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23: 8800–8807.
Article CAS Google Scholar - Quirk GJ, Mueller D (2008). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacol Rev 33: 56–72.
Article Google Scholar - Radley JJ, Sisti HM, Hao I, Rocher AB, McCall T, Hof PR et al (2004). Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons on the medial prefrontal cortex. Neuroscience 125: 1–6.
Article CAS Google Scholar - Rauch SL, Milad MR, Orr SP, Quinn BT, Fischl B, Pitman RK (2005). Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport 28: 1909–1912.
Article Google Scholar - Rescorla RA, Heth CD (1975). Reinstatement of fear to an extinguished conditioned stimulus. J Exp Psychol Anim Behav Process 1: 88–96.
Article CAS Google Scholar - Santini E, Muller RU, Quirk GJ (2001). Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci 21: 9009–9017.
Article CAS Google Scholar - Shi S-H, Hayashi Y, Esteban JA, Malinow R (2001). Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105: 331–343.
Article CAS Google Scholar - Sierra-Mercado M, Corcoran KA, Lebrón-Milad K, Quirk GJ (2006). Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci 24: 1751–1758.
Article Google Scholar - Solomon Z (1995). The effect of prior stressful experience on coping with war trauma and captivity. Psychol Med 25: 1289–1294.
Article CAS Google Scholar - Sotres-Bayon F, Bush DEA, LeDoux JE (2004). Emotional perseveration: an update on prefrontal–amygdala interactions in fear extinction. Learn Mem 11: 525–535.
Article Google Scholar - Sotres-Bayon F, Bush DEA, LeDoux JE (2007). Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology 32: 1929–1940.
Article CAS Google Scholar - Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S (2004). Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci 24: 4787–4795.
Article CAS Google Scholar - Tang Y-P, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M ; et al (1999). Genetic enhancement of learning and memory in mice. Nature 401: 63–69.
Article CAS Google Scholar - Trentani A, Kuipers SD, Ter Horst GJ, Den Boer JA (2002). Selective chronic stress-induced in vivo ERK1/2 hyperphosphorylation in medial prefrontocortical dendrites: implications for stress-related cortical pathology? Eur J Neurosci 15: 1681–1691.
Article CAS Google Scholar - Wellman CL (2001). Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol 49: 245–253.
Article CAS Google Scholar - Willner P, Moreau J-L, Nielsen CK, Papp M, Sluzewska A (1996). Decreased hedonic responsiveness following chronic mild stress is not secondary to loss of body weight. Physiol Behav 60: 129–134.
Article CAS Google Scholar - Wolf OT (2003). HPA axis and memory. Best Prac Res Clin Endocrinol Metabol 17: 287–299.
Article CAS Google Scholar - Wong RW-C, Setou M, Teng J, Takei Y, Hirokawa N (2002). Overexpression of motor protein KIF17 enhances spatial and working memory in transgenic mice. Proc Natl Acad Sci USA 99: 14500–14505.
Article CAS Google Scholar - Yang Y-L, Chao P-K, Lu K-T (2006). Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology 31: 912–924.
Article CAS Google Scholar - Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW (1993). Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry 150: 83–86.
Article CAS Google Scholar - Yehuda R, Halligan SL, Grossman R, Golier JA, Wong C (2002). The cortisol and glucocorticoid receptor response to low dose dexamethasone administration in aging combat veterans and holocaust survivors with and without posttraumatic stress disorder. Biol Psychiatry 52: 393–403.
Article CAS Google Scholar - Yehuda R, Halligan SL, Golier JA, Grossman R, Bierer LM (2004). Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology 29: 389–404.
Article CAS Google Scholar - Zhao M-G, Toyoda H, Lee Y-S, Wu L-J, Ko SW, Zhang X-H et al (2005). Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 47: 859–872.
Article CAS Google Scholar - Zushida K, Sakurai M, Wada K, Sekiguchi M (2007). Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors. J Neurosci 27: 158–166.
Article CAS Google Scholar
Acknowledgements
We thank Drew D Kiraly and Drs Jennifer Quinn and Natalie Tronson for excellent technical support and advice. This study was supported by PHS MH 25642 and 66172 (JRT) and 79680 (SLG) and the State of Connecticut Department of Mental Health and Addiction Services.
Author information
Authors and Affiliations
- Interdepartmental Neuroscience Program, Yale University, New Haven, CT, USA
Shannon L Gourley & Jane R Taylor - Division of Molecular Psychiatry, Department of Psychiatry, Connecticut Mental Health Center,Yale University, New Haven, CT, USA
Shannon L Gourley, Alexia T Kedves, Peter Olausson & Jane R Taylor - Department of Psychology, Yale University, New Haven, CT, USA
Jane R Taylor
Authors
- Shannon L Gourley
You can also search for this author inPubMed Google Scholar - Alexia T Kedves
You can also search for this author inPubMed Google Scholar - Peter Olausson
You can also search for this author inPubMed Google Scholar - Jane R Taylor
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toJane R Taylor.
Additional information
DISCLOSURE/CONFLICT OF INTEREST
SL Gourley, AT Kedves, P Olausson, and JR Taylor declare they have no competing financial interests regarding the methods, results, clinical implications, or any other aspect of this paper, and no personal financial holdings that could be perceived as constituting a potential conflict of interest. Further, the authors declare that, except for income received from their primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service.
Rights and permissions
About this article
Cite this article
Gourley, S., Kedves, A., Olausson, P. et al. A History of Corticosterone Exposure Regulates Fear Extinction and Cortical NR2B, GluR2/3, and BDNF.Neuropsychopharmacol 34, 707–716 (2009). https://doi.org/10.1038/npp.2008.123
- Received: 02 May 2008
- Revised: 02 July 2008
- Accepted: 11 July 2008
- Published: 20 August 2008
- Issue Date: February 2009
- DOI: https://doi.org/10.1038/npp.2008.123