Modeling Causal Relationship Between Brain Regions Within the Drug-Cue Processing Network in Chronic Cocaine Smokers (original) (raw)
INTRODUCTION
An important factor contributing to drug-seeking behavior and continuation of drug use is exposure to stimuli associated with drugs of abuse (Childress et al, 1993; O’Brien et al, 1992). Neuroimaging studies demonstrate that the areas activated by exposure to drug cues in chronic drug users include the prefrontal cortex (medial prefrontal cortex, orbital frontal cortex, dorsolateral prefrontal cortex), ventral tegmental area, anterior cingulate cortex, insula, striatum, amygdala, and hippocampus (see Jasinska et al, 2014 for a review). These areas comprise the mesocorticolimbic system of the brain, which is implicated in reward, motivation, and goal-directed behavior.
Recently, there has been a growing interest in understanding how brain regions interact, as opposed to focusing on the functionality of individual regions of interest (ROIs) in isolation. A few fMRI studies involving cocaine users have used functional connectivity analysis, which involves correlating activity in spatially remote brain regions (Friston, 1995a), but the results are mixed. Relative to controls, cocaine users have shown enhanced as well as reduced functional connectivity between brain areas during resting state (Cisler et al, 2013; Gu et al, 2010; Wilcox et al, 2011), as well as during an attention and a motor task (Hanlon et al, 2011; Tomasi et al, 2010). According to Cisler et al (2013), cocaine users had an enhanced functional connectivity of the insular cortex with prefrontal networks compared with controls. Wilcox et al (2011) showed that cocaine users had an increased activation in comparison to controls in dorsolateral prefrontal cortex in response to cocaine cues but not to neutral cues. Also, cocaine users exhibited increased resting-state functional connectivity between cue processing regions such as orbital frontal cortex and ventral striatum. A reduced functional connectivity between regions within the mesocorticolimbic system has been shown during resting state in cocaine users compared with controls (Gu et al, 2010). Furthermore, Hanlon et al (2011) found a reduced functional connectivity between frontal and striatal regions during a finger-tapping task, and Tomasi et al (2010) found a reduced functional connectivity between midbrain and thalamus, cerebellum, and rostral cingulate regions during an attention task in cocaine users compared with controls. These inconsistent findings involving functional connectivity analysis in cocaine users may be due, in part, to acute cocaine effects, as not all studies confirmed that the research volunteers had abstained from cocaine prior to the fMRI session (eg, Gu et al, 2010; Hanlon et al, 2011; Tomasi et al, 2010). Regardless, functional connectivity studies are limited in that although they provide information about the interaction of brain ROIs, these studies do not assess how one region influences another.
Yet critical to our understanding of the neurobiology of chronic drug use is defining effective connectivity, which measures the causal effect that one region’s activity has on another region (Friston, 1995a). To our knowledge, only one effective connectivity study in cocaine users has been conducted (Ma et al, 2012). This study showed that cocaine subjects differed from controls in that effective connectivity from inferior frontal cortex (left) to striatum (left) was less affected by the immediate working memory task in the cocaine compared with the control group, and the effective connectivity from middle frontal gyrus (left) to the striatum (left) was less affected by the delayed working memory task in the cocaine compared with the control group.
The objective of this fMRI study was to assess how cocaine users process cocaine cues in order to provide insight into the mechanisms that may underlie continued cocaine use. Specifically, we investigated the causal interaction between the brain regions within the drug-cue processing network in chronic cocaine users during cocaine-cue exposure. Data were collected from non-treatment-seeking cocaine smokers abstinent from cocaine for 72 h and similarly aged healthy controls with no cocaine experience while they were presented with cocaine and neutral picture cues. We used a graph theoretic approach (IMaGES: Independent Multisample Greedy Equivalence Search; Ramsey et al, 2010; Ramsey et al, 2011) utilizing a Bayesian search algorithm to model effective connectivity. We further examined whether the activation in individual ROIs and the effective connectivity strength between the brain regions in the drug-cue processing network were positively correlated with cocaine smokers’ subjective craving ratings. Note: IMaGES offers a considerable methodological strength to the earlier effective connectivity study (Ma et al, 2012), as rather than utilizing an a priori specification of an anatomical network model, IMaGES uses an exploratory search method that does not require a model to be prescribed in advance but rather can accommodate any number of brain regions (Ramsey et al, 2011).
According to earlier drug cue reactivity studies, drug users compared with controls showed an enhanced activation in response to drug cues in the regions within the drug-cue processing network, which is a component of the mesocorticolimbic system (see Jasinska et al, 2014 for a review). Also, Wilcox et al (2011) showed an increased resting-state functional connectivity between drug-cue processing regions such as orbital frontal cortex and ventral striatum in cocaine users. Based on these studies, we hypothesized that the cocaine users compared with controls would show a different causal interaction pattern among the ROIs of the drug-cue processing network during cocaine-cue exposure.
MATERIALS AND METHODS
Participants
Twenty (15M; 5F) non-treatment-seeking chronic cocaine smokers abstinent from cocaine for 72 h, and 17 (13M; 4F) healthy volunteers matched for age, education, and ethnic background took part in the study (Table 1; For participants’ inclusion and exclusion criteria, see Supplementary Material A). Participants were recruited from the Substance Use Research Center at Columbia University Medical Center and by advertising in New Jersey and New York newspapers and word-of-mouth. Groups did not differ in alcohol use quantity, cigarette use frequency and quantity, and caffeine use frequency and quantity. Of the 20 cocaine smokers, 13 met a DSM-IV-R diagnosis of either abuse or dependence for cocaine, whereas 7 did not meet any such criterion as confirmed by SCID (First et al, 1997). Although these seven individuals were heavy cocaine users, they were non-treatment seeking and thus reported no distress from their use, a defining feature of the diagnosis.
Table 1 Demographic and Substance Use Information for Cocaine Users and Controls
On the day of scanning, all participants provided written informed consent approved by the Rutgers University Institutional Review Board, and were administered a urine screen to rule out pregnancy in women, and to ensure negative urine toxicology for cocaine, methamphetamine, THC, opiate, and benzodiazepines (One Step Multi-Drug Screen Test Panel). They were also assessed for recent alcohol use with a breathalyzer. At the end of the day, participants were paid for their transportation and received a gift certificate worth $100 for their participation.
Stimuli
Participants viewed 30 cocaine-related visual stimuli (15 unique stimuli presented twice) and 30 neutral visual stimuli (15 unique stimuli presented twice). Cocaine stimuli comprised selections generously provided by Dr Rita Goldstein (Icahn School of Medicine, Mount Sinai) and Dr Robert Hester (The University of Melbourne) as well as from the Cognitive Neuroscience Laboratory at the Rutgers Center of Alcohol Studies. The cocaine stimuli included pictures of smokeable cocaine, paraphernalia, and people smoking cocaine. The neutral stimuli included nature scenes (Childress et al, 1999; Volkow et al, 2010), and were selected from non-copyrighted images on the internet.
Procedure
Experimental task
During the cue exposure task, participants viewed two blocks of cocaine cues and two blocks of neutral cues presented in a counterbalanced manner across participants. For example, if one participant viewed the blocks of cues in one sequence (ie, cocaine, neutral, cocaine, neutral), the next participant viewed the blocks in a counterbalanced sequence (ie, neutral, cocaine, neutral, cocaine). Stimuli (either cocaine or neutral) presented in the first block were repeated in the second block. Each block (cocaine or neutral) lasted for 90 s and consisted of 15 stimuli. Stimuli within the blocks were presented randomly and each stimulus was presented for 4 s followed by a fixation cross that lasted for 2 s. A trigger pulse from the MRI console was used to synchronize stimulus presentation with fMRI acquisition. The task was developed using E-prime (Psychology Software Tools, Inc., Pittsburgh, PA). After the presentation of the first cocaine cue and neutral cue blocks, all participants were administered a three-item version of the cocaine-craving questionnaire (CCQ-Brief; Sussner et al, 2006). The items appeared one at a time on the screen, and participants had to press a button on a MRI-compatible button-box to rate their craving for cocaine on a seven-point scale (1=Strongly Disagree, 7=Strongly Agree). The cue exposure task took 7 min to complete. Before participants left the laboratory, their craving ratings were collected for the second time using the 10-item CCQ-Brief (Sussner et al, 2006).
Image acquisition
A 3 T Siemens Trio scanner and Siemens 12 channel head coil were used to acquire the fMRI data. Functional imaging was done using a single-shot gradient echo-planar EPI sequence (TR=2000 ms, TE=25 ms, flip angle=90°, matrix=64 × 64, FOV=192 mm). Thirty-five contiguous oblique axial slices (1-mm gap; 3 × 3 × 3 mm3 voxels) parallel to the AC-PC line were obtained. Anatomical images were acquired using a T1-weighted protocol (TR=1900 ms, TE=2.52 ms, matrix=256 × 256, FOV=256 mm, 176 1-mm sagittal slices with a 5-mm gap).
Image analysis
Image preprocessing and data analysis were performed using the FSL 6.00 software (FMIRB’s Software Library, www.fmirb.ox.ac.uk/fsl). (For specific FSL commands used during image preprocessing and analysis, see Supplementary Material B) To model the cocaine cue and neutral cue blocks, a Gaussian hemodynamic response function and its temporal derivatives were applied to the basic waveform. BOLD scans for each participant were registered first to his or her high-resolution anatomical (mprage) scans and then registered to standard space using the FSL’s MNI (Montreal Neurologic Institute) template.
A two-level statistical analysis approach was used. The first-level analysis was directed at brain activity related to cue-type effect (cocaine cues versus neutral cues) during cue exposure. At first level, two predictors were coded ‘cocaine cues’ and ‘neutral cues’, respectively, representing mean activation during presentation of cocaine cues and neutral cues. Mean brain activation was analyzed by a GLM for each predictor in individual participants using FEAT (FMRI Expert Analysis Tool). Moreover, the cocaine cue predictor was contrasted to the neutral cue predictor: cocaine cues>neutral cues. The results were then entered into a higher (ie, group) level analysis using FLAME 1 mixed-effects (Beckman et al, 2003). In the higher-level whole-brain analysis, average activation was determined for each group (cocaine users and controls) as well as the difference between the groups (cocaine users>controls) for a total of 37 participants. Group-level statistic images were thresholded using clusters determined by _z_>1.65 and a (corrected) cluster significance threshold of _p_=0.001 (Worsley, 2001).
Two criteria were used to select ROIs for this study. First, the ROIs had to show a significantly enhanced activation in cocaine users compared with controls during cocaine cue presentation relative to neutral cue presentation as determined through our GLM analysis. Second, the ROIs had to be reported in earlier drug-cue reactivity studies. A mean voxel-based time series was extracted from each of these ROIs for each participant and used as input to the IMaGES graph analysis in modeling the causal interactions between the ROIs. A mean voxel-based time series data extracted from the same ROIs while control participants viewed cocaine stimuli were used as input to IMaGES to generate an additional IMaGES analysis output.
IMaGES was primarily designed to extract feed-forward causal structure from fMRI time series by exploring the possible decision space and constraining the search to connections that carry the greatest predictive power (Perez et al, 2010). The algorithm begins with an empty graph for a set of ROIs. It then chooses all possible models with one directed link and rates the models based on residuals computed in each time series data set. The model with the highest average Bayesian Information Criterion (BIC) score is selected. Next, models with two links are considered. At each stage, the algorithm attempts to maximize the BIC score. When additional links no longer improve the BIC score, a backward procedure is initiated. The backward procedure removes links using an analogous method (Ramsey et al, 2011). It exploits the strength of association between variables and cross-subject redundancy to eliminate spurious connections that result from indirect measurement of brain activity. IMaGES produces reliable and stable estimates of interactions between different ROIs and was recently validated on 28 benchmark simulations (Smith et al, 2011) where it performed ⩾90% accuracy of detecting connections and on recall of orientations (Ramsey et al, 2011).
To examine whether the effective connectivity strength, that is, the strength of causal influence between the ROIs of the drug-cue processing network and the activation in individual ROIs were positively correlated with cocaine smokers’ subjective craving ratings, correlation was computed between connectivity strengths and craving ratings and activation in individual ROIs and craving ratings in chronic cocaine smokers.
RESULTS
Craving Results
For each participant, two craving scores were obtained (Sussner et al, 2006): one after the presentation of the first cocaine cue block during the fMRI session (ie, during the cue exposure task), and the other after the fMRI session and before they left the laboratory. Participants who failed to respond on all the items of the craving questionnaire either during the fMRI session or during the post-fMRI session were excluded from the craving analysis but not from the GLM analysis. One cocaine user and 10 controls were excluded from the fMRI session craving rating analysis, and one control participant was excluded from the post-fMRI session craving analysis. During the cue exposure task, cocaine users had significantly higher craving ratings to the cocaine cues compared with controls (t (24)=2.81, _p_=0.01; 3.83 (SD=2.31) vs 1 (SD=0)). Also, craving ratings collected post-fMRI session showed that cocaine users had a higher craving rating compared with controls (t (34)=6.09, p<0.001; 3.27 (SD=1.49) vs 1 (SD=0)).
Imaging Results
As mentioned earlier, the ROIs for this study were selected based on two criteria: (1) a significantly enhanced activation in cocaine users compared with controls during cocaine cue presentation relative to neutral cue presentation as determined through our GLM analysis, and (2) the ROIs had to be reported in published drug cue reactivity studies. Regarding the first criterion, the group-level analysis revealed that cocaine users compared with controls showed significantly enhanced activation in response to cocaine cues in 25 brain areas relative to neutral cues (1 significant cluster; cluster size=10471 voxels): frontal pole, insula (bilateral), dorsolateral prefrontal cortex (left), inferior frontal gyrus (bilateral), precentral gyrus (left), temporal pole (right), superior parietal lobule (left), supramarginal gyrus (left), medial frontal cortex (bilateral), supplementary motor cortex (left), subcallosal cortex (bilateral), paracingulate gyrus (bilateral), anterior cingulate cortex, posterior cingulate cortex, orbital frontal cortex (bilateral), parahippocampal gyrus (right; both anterior and posterior parts), frontal operculum (bilateral), central opercular cortex (left), parietal operculum cortex (left), thalamus (bilateral), dorsal striatum (caudate (bilateral) and putamen (bilateral)), brain stem, hippocampus (right), amygdala (bilateral), and ventral striatum (accumbens (right)). The anatomical ROI masks from the Harvard–Oxford Cortical and the Harvard–Oxford Subcortical Structural Atlases implemented in FSLView were used to identify the 25 brain areas.
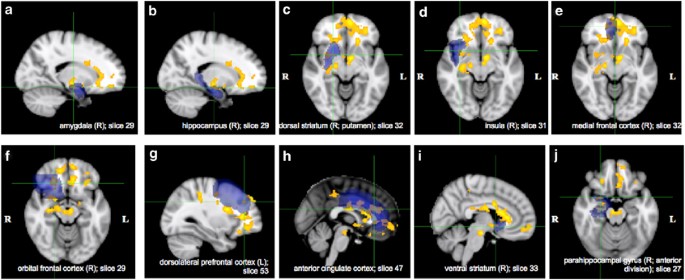
Regarding the second criterion, 10 of these 25 ROIs were selected for IMaGES analysis based on earlier studies on drug cue reactivity. Nine of these 10 ROIs were selected from Jasinska et al (2014) (see sections 3.1–3.2) and they included amygdala (bilateral; Figure 1a (right)), hippocampus ((right) (Figure 1b)), dorsal striatum (bilateral; Figure 1c (right; putamen)), insula (bilateral; Figure 1d (right)), medial frontal cortex (bilateral; Figure 1e (right)), orbital frontal cortex (bilateral; Figure 1f (right)), dorsolateral prefrontal cortex ((left) (Figure 1g)), anterior cingulate cortex (Figure 1h), and ventral striatum ((right) Figure 1i) (Table 2). As hippocampus and parahippocampal gyrus are a part of the hippocampal complex (Nadel et al, 2003), and activation in both of these regions has been reported in earlier drug cue reactivity studies (Charboneau et al, 2013; Janes et al, 2010b; Langleben et al, 2008), we decided to include the parahippocampal gyrus ((right; anterior division); Figure 1j) as one of the ROIs in our analysis. Altogether, these 10 ROIs encompass the major areas of the brain’s mesocorticolimbic and nigrostriatal systems (Jasinska et al, 2014). For bilateral activation, the mean voxel-based time series for the right brain area (for example, right amygdala) and the left brain area (for example, left amygdala) were averaged to create the mean voxel-based time series for that brain area (amygdala).
Figure 1
Brain areas that showed a significantly enhanced activation in cocaine users compared with controls during cocaine cue presentation relative to neutral cue presentation: (a) Amygdala (right); (b) hippocampus (right); (c) dorsal striatum (right; putamen); (d) insula (right); (e) medial frontal cortex (right); (f) orbital frontal cortex (right); (g) dorsolateral prefrontal cortex (left); (h) anterior cingulate cortex; (i) ventral striatum (right); and (j) parahippocampal gyrus (right; anterior division). Note: Blue areas indicate anatomical ROI masks from the Harvard–Oxford Cortical and the Harvard–Oxford Subcortical Structural Atlases implemented in FSLView that were used to identify the brain areas. Anatomical ROI masks are overlaid onto the activation. Group-level statistic images were thresholded using clusters determined by _z_>1.65 and a (corrected) cluster significance threshold of p<0.001.
Table 2 Ten Brain Areas that Showed a Significantly Enhanced Activation in Cocaine Users Compared with Controls During Cocaine Cues Presentation Relative to Neutral Cues Presentation and were used as the ROIs in the IMaGES Analysis
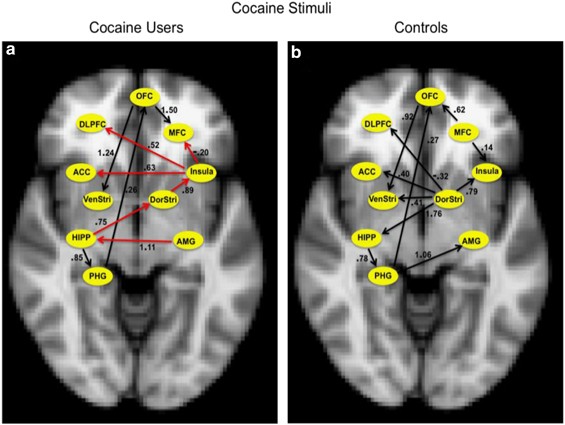
As illustrated in Figure 2, the direction of arrows represents a direct causal influence of one ROI on another. The numbers on the arrows are regression coefficients and they denote the strength of causal influence of one ROI on another. IMaGES analysis revealed that while the cocaine users viewed cocaine cues (Figure 2a), the amygdala had a feed-forward connection. That is, the amygdala had a direct causal influence on the hippocampus and the activation of the hippocampus initiated two causal pathways. In one pathway, hippocampus causally influenced the dorsal striatum. And activation of the dorsal striatum resulted in activation in the insula and it subsequently sent its causal influence on the medial frontal cortex, anterior cingulate cortex, and the dorsolateral prefrontal cortex. This particular causal brain interaction pattern between the ROIs of the drug-cue processing network (ie, amygdala→hippocampus→dorsal striatum→insula→medial frontal cortex, dorsolateral prefrontal cortex, anterior cingulate cortex) observed in cocaine users was not present while the controls viewed the cocaine cues (Figure 2b).
Figure 2
IMaGES analysis outputs that reveal causal interactions between the ROIs of the drug-cue processing network (among 10 ROIs) while the cocaine users viewed cocaine stimuli (a) and control participants viewed cocaine stimuli (b). The direction of arrows represents a direct causal influence of one ROI on another. The numbers on the arrows are regression coefficients and they denote the strength of causal influence of one ROI on another. The red arrows represent a particular feed-forward effective connectivity pattern between the ROIs of the drug-cue processing network (amygdala→hippocampus→dorsal striatum→insula→medial frontal cortex, dorsolateral prefrontal cortex, anterior cingulate cortex) during exposure to cocaine cues in cocaine smokers that was not present when the controls viewed the cocaine cues. ACC, anterior cingulate cortex; AMG, amygdala; DLPFC, dorsolateral prefrontal cortex; DorStri, dorsal striatum; HIPP, hippocampus; MFC, medial frontal cortex; OFC, orbital frontal cortex; PHG, parahippocampal gyrus; VenStri, ventral striatum.
In the other pathway (Figure 2a), hippocampus caused activation in the parahippocampal gyrus, and the activation of the parahippocampal gyrus had a causal influence on the orbital frontal cortex, which then had its causal influence on the ventral striatum (hippocampus→parahippocampal gyrus→orbital frontal cortex→ventral striatum). This same pathway (ie, hippocampus→parahippocampal gyrus→orbital frontal cortex→ventral striatum) was also activated in controls while they looked at cocaine cues (Figure 2b). In controls, the hippocampus was not involved in creating a feed-forward connection to the dorsal striatum. On the contrary, the dorsal striatum had a causal influence on the hippocampus. In controls, the dorsal striatum had a causal influence on multiple areas such as on insula, dorsolateral prefrontal cortex, anterior cingulate cortex, ventral striatum, and hippocampus. Activation of the hippocampus by the dorsal striatum resulted in a causal chain of connection between certain ROIs (hippocampus→parahippocampal gyrus→amygdala) as well as in the pathway as mentioned above (hippocampus→parahippocampal gyrus→orbital frontal cortex→ventral striatum). In cocaine users, both insula and orbital frontal cortex causally influenced the medial frontal cortex, whereas in controls the medial frontal cortex had a causal influence on both insula and orbital frontal cortex.
Craving Correlation Results
There was a significant positive correlation between the strength of causal influence of insula on dorsolateral prefrontal cortex and the craving rating in cocaine users’ collected during cocaine-cue exposure, r (19)=0.47, p<0.05. No other correlations tested were significant.
DISCUSSION
The overall objective of this study was to examine the causal interactions between the brain ROIs within the drug-cue processing network. Chronic cocaine smokers and control participants were scanned while they took part in a visual cue exposure task that involved cocaine and neutral cues. We utilized a graph theoretical approach (Ramsey et al, 2010; Ramsey et al, 2011) to model the effective connectivity between 10 ROIs implicated in drug-cue processing. Consistent with our hypothesis, we found that, during exposure to cocaine cues, cocaine smokers showed a feed-forward effective connectivity pattern between the ROIs of the drug-cue processing network (amygdala→hippocampus→dorsal striatum→insula→medial frontal cortex, dorsolateral prefrontal cortex, anterior cingulate cortex) that was not present when the controls viewed the cocaine cues. This study is the first to demonstrate a causal interaction between ROIs within the drug-cue processing network in chronic cocaine users.
IMaGES was used to produce two graphs: cocaine users and controls viewing cocaine stimuli (Figure 2a and b, respectively). The activation of the amygdala (Figure 2a) initiated a causal interaction among the seven ROIs within the drug-cue processing network. More specifically, the amygdala and hippocampus causally influenced the dorsal striatum. And activation of the dorsal striatum resulted in activation in the insula, which subsequently influenced prefrontal ROIs. The involvement of dorsal striatum in this pathway is important as it has been long implicated in chronic drug use in both human and animal studies (Jasinska et al, 2014; Vollstädt-Klein et al, 2010b). To propose a theoretical model based on our results, we speculate that the early stage of drug-cue processing may reflect activation of the cocaine users’ long-term memory of drug cues and contexts (Jasinska et al, 2014; Spaniol et al, 2009; Tiffany, 1990; Volkow et al, 2002), consistent with the activation of amygdala and hippocampus and the causal influence of amygdala on hippocampus. We further speculate that the later stage of the drug-cue processing may reflect activation of decision making/craving/motivated behavior related to continued drug use (Balleine et al, 2007; Jasinska et al, 2014). This theoretical model incorporates the considerable overlap between certain regions within the long-term memory network and the drug-cue processing network (Jasinska et al, 2014; Spaniol et al, 2009).
The present study extends the previous research by establishing for the first time that exposure to cocaine cues initiates a causal interaction between the ROIs within the drug-cue processing network, which is a component of the brain’s mesocorticolimbic and nigrostriatal systems.
In contrast to cocaine users, control participants did not show a feed-forward connectivity pattern between the ROIs of the drug-cue processing network involving dorsal striatum during cocaine-cue exposure (Figure 2b). In controls, dorsal striatum served as an information hub and was driving the activation of hippocampus. Perhaps the activation of the dorsal striatum and its causal influence on hippocampus, and the subsequent activation of parahippocampal gyrus and amygdala, in controls may indicate their conscious effort to learn (ie, encode) the cocaine cues that were novel to them, as well as to retrieve any information relating to these cues from their long-term memory (Cohn et al, 2010; Han et al, 2010; Scimeca and Badre, 2012). Although speculative, we propose that the common causal pathway activated in both cocaine users and controls (ie, hippocampus→parahippocampal gyrus→orbital frontal cortex→ventral striatum) may indicate two different types of cognitive processing. In cocaine users, it may have indicated reward/motivational processing related to their chronic cocaine use, whereas in controls it may have indicated their effort to learn and retrieve any information relating to the novel cocaine stimuli (Cohn et al, 2010). Our finding of the causal influence of orbital frontal cortex on ventral striatum extends the study by Wilcox et al (2011), which showed an increased resting-state functional connectivity between orbital frontal cortex and ventral striatum in cocaine users compared with controls.
As predicted, the cocaine users had significantly higher ratings of cocaine craving during exposure to the cocaine cues while in the scanner compared with individuals with no experience with cocaine. Importantly, the craving ratings positively correlated with the strength of causal influence of insula on dorsolateral prefrontal cortex in cocaine users. This finding advances earlier cocaine cue reactivity studies that showed that activation in drug-cue processing-related brain regions, such as insula and dorsolateral prefrontal cortex, was positively correlated with cocaine users’ subjective craving rating collected postscan (Grant et al, 1996; Maas et al, 1998; Wang et al, 1999). The present finding supports the model by Jasinska et al (2014) that predicts that activation of the mesocorticolimbic and nigrostriatal systems in drug users result in a subjective experience of craving that may lead to continued drug use.
Next, we would like to address a number of challenges to this study. First, although we matched the cocaine and control groups in terms of their age, educational, and ethnic/racial background, controls drank significantly more alcohol than the cocaine-using group (Table 1). However, importantly, alcohol use was still very low for both groups (<1 drink/day) so was unlikely to impact our findings. Second, although a ‘pure’ cocaine-using group may have been a strength of our study, our study findings may not be generalizable to cocaine users who abuse alcohol as our cocaine group had a very low alcohol use history. Third, there were not enough female cocaine smokers (_n_=5) to investigate potential gender differences in the study outcome. Fourth, although we speculated a theoretical model based on our results, the model was not empirically tested in the current study. Fifth, we speculated that the common causal pathway between the groups may have indicated two different types of cognitive processing. In cocaine users, it may have indicated reward/motivational processing related to their chronic cocaine use, whereas in controls it may have indicated their conscious effort to learn and retrieve any information relating to the cocaine cues that were novel to them. We acknowledge that the participants did not perform any novelty detection task and any other encoding and retrieval tasks to confirm this speculation. Despite these limitations, the results of the present study demonstrate a causal interconnectivity between the ROIs of the drug-cue processing network.
To conclude, this study has contributed two novel findings: (1) during exposure to cocaine cues, cocaine smokers showed a feed-forward effective connectivity pattern between the ROIs of the drug-cue processing network (amygdala→hippocampus→dorsal striatum→insula→medial frontal cortex, dorsolateral prefrontal cortex, anterior cingulate cortex) that was not present when the controls viewed the cocaine cues, and (2) craving ratings among cocaine smokers were positively correlated with the strength of causal influence of insula on dorsolateral prefrontal cortex. Future research will include using both fMRI and diffusion tensor imaging techniques to look for a relationship between effective and anatomical connectivity (Skudlarski et al, 2008) between the ROIs of the drug-cue processing network in cocaine users. Another future research will examine the influence of gender on the causal relationship between the ROIs of the drug-cue processing network during cocaine-cue exposure and empirically test the theoretical model as we propose in this study. Graph analysis of individual brains makes it relatively easy to monitor treatment response through changes in network connectivity. An additional future study would be to determine the effects of effective therapeutic interventions on activation of the drug-cue processing network in chronic users of cocaine.
Funding and Disclosure
This research was supported by a National Institute on Drug Abuse grant to Dr Suchismita Ray (NIDA K01DA029047). The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The authors declare no conflict of interest.
References
- Balleine BW, Delgado MR, Hikosaka O (2007). The role of the dorsal striatum in reward and decision-making. J Neurosci 27: 8161–8165.
Article CAS Google Scholar - Beckman CF, Jenkinson M, Smith S (2003). General multi-level linear modeling for group analysis in fMRI. Neuroimage 20: 1052–1063.
Article Google Scholar - Charboneau EJ, Dietrich MS, Park S, Cao A, Watkins TJ, Blackford JU et al (2013). Cannabis cue-induced brain activation correlates with drug craving in limbic and visual salience regions: preliminary results. Psychiatry Res 214: 122–131.
Article CAS Google Scholar - Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP (1999). Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156: 11–18.
Article CAS Google Scholar - Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP (1993). Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr 137: 73–95.
CAS PubMed Google Scholar - Cisler JM, Elton A, Kennedy AP, Young J, Smitherman S, James GA et al (2013). Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Res 213: 39–46.
Article CAS Google Scholar - Cohn M, Moscovitch M, Davidson PS (2010). Double dissociation between familiarity and recollection in Parkinson's disease as a function of encoding tasks. Neuropsychologia 48: 4142–4147.
Article Google Scholar - First MB, Spitzer RL, Gibbon M, Williams JBW (1997). Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0, 4/97 revision).
- Friston KJ (1995a). Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp 2: 56–78.
Article Google Scholar - Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C et al (1996). Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci 93: 12040–12045.
Article CAS Google Scholar - Gu H, Salmeron JB, Ross JT, Geng X, Zhan W, Stein EA et al (2010). Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage 53: 593–601.
Article CAS Google Scholar - Han S, Huettel S, Raposo A, Adcock R, Dobbins I (2010). Functional significance of striatal responses during episodic decisions: recovery or goal attainment? J Neurosci 30: 4767–4775.
Article CAS Google Scholar - Hanlon CA, Wesley MJ, Stapleton JR, Laurienti PJ, Porrino LJ (2011). The association between frontal-striatal connectivity and sensorimotor control in cocaine users. Drug Alcohol Depend 115: 240–243.
Article Google Scholar - Janes AC, Pizzagalli DA, Richardt S, Frederick B, Holmes AJ, Sousa J et al (2010b). Neural substrates of attentional bias for smoking-related cues: an FMRI study. Neuropsychopharmacology 35: 2339–2345.
Article Google Scholar - Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y (2014). Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev 38: 1–16.
Article Google Scholar - Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J et al (2008). Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry 165: 390–394.
Article Google Scholar - Ma L, Steinberg JL, Hasan KM, Narayana PA, Kramer LA, Moeller FG (2012). Stochastic dynamic causal modeling of working memory connections in cocaine dependence. Hum Brain Mapp 35: 760–778.
Article Google Scholar - Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW et al (1998). Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry 155: 124–126.
Article CAS Google Scholar - Nadel L, Pran L, Hayes SM, Gilboa A, Moscovitch M (2003). The role of the hippocampal complex in long-term episodic memory. Int Congr Ser 1250: 215–234.
Article Google Scholar - O’Brien C, Childress A, McLellan A, Ehrman R (1992). Classical conditioning in drug-dependent humans. Ann NY Acad Sci 65: 4400–4415.
Google Scholar - Perez CA, El-Sheikh EM, Glymour C (2010). Discovering effective connectivity among brain regions from functional MRI data. Int J Comput Healthc 1: 86–102.
Article Google Scholar - Ramsey JD, Hanson SJ, Glymour C (2011). Multi-subject search correctly identifies causal connections and most causal directions in the DCM models of the Smith et al simulation study. Neuroimage 58: 838–848.
Article Google Scholar - Ramsey JD, Hanson SJ, Hanson C, Halchenko YO, Poldrack RA, Glymour C (2010). Six problems for causal inference from fMRI. Neuroimage 49: 1545–1558.
Article CAS Google Scholar - Scimeca JM, Badre D (2012). Striatal contributions to declarative memory retrieval. Neuron 75: 380–392.
Article CAS Google Scholar - Skudlarski P, Jagannathan K, Calhoun VD, Hampson M, Skudlarska BA, Pearlson G (2008). Measuring brain connectivity: diffusion tensor imaging validates resting state temporal correlations. Neuroimage 43: 554–561.
Article Google Scholar - Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann C, Nichols T et al (2011). Network modeling methods for fMRI. Neuroimage 54: 875–891.
Article Google Scholar - Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL (2009). Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia 47: 1765–1779.
Article Google Scholar - Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D (2006). The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend 83: 233–237.
Article Google Scholar - Tiffany ST (1990). A cognitive model of drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev 97: 147–168.
Article CAS Google Scholar - Tomasi D, Volkow DN, Wang R, Carrillo HJ, Maloney T, Alia-Klien N et al (2010). Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One 5: e10815.
Article Google Scholar - Volkow ND, Fowler JS, Wang GJ, Goldstein RZ (2002). Role of dopamine, the frontal cortex and memory circuits in drug addiction: Insights from imaging studies. Neurobiol Learn Mem 78: 610–624.
Article CAS Google Scholar - Volkow ND, Wang G-J, Tomasi D, Telang F, Fowler JS et al (2010). Methylphenidate attenuates limbic brain inhibition after cocaine-cues exposure in cocaine abusers. PLoS One 5: e11509.
Article Google Scholar - Vollstädt-Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G et al (2010b). Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction 105: 1741–1749.
Article Google Scholar - Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ et al (1999). Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci 64: 775–784.
Article CAS Google Scholar - Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR (2011). Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend 115: 137–144.
Article CAS Google Scholar - Worsley KJ (2001) Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM (eds). Functional MRI: an Introduction to Methods. Oxford University Press: New York, NY, USA, pp 251–270.
Google Scholar
Acknowledgements
We thank Aradhana Srinagesh, Ashley Aya, and Brian Foster for assistance with subject recruitment, data collection, and behavioral data coding; Dr Rita Goldstein and Dr Robert Hester for sharing cocaine picture cues; and Suril Gohel for helpful comments on the data.
Author Contributions
Suchismita Ray, PhD, conducted the literature searches and summaries of previous related work, designed and conducted the study, wrote the first draft of the manuscript, conducted the group-level image analysis, the IMaGES analysis, and interpreted the results. Margaret Haney, PhD, helped with selection of cocaine stimuli for the study, subjects screening and recruitment, and edited the manuscript. Catherine Hanson, PhD, helped with the IMaGES analysis and the study design and also edited the manuscript. Bharat Biswal helped with the study design and edited the manuscript. Stephen J Hanson, PhD, helped with creating imaging protocol, interpretation of IMaGES analysis, and edited the manuscript. All authors contributed to and have approved the final manuscript.
Author information
Authors and Affiliations
- Center of Alcohol Studies, Rutgers University, Piscataway, NJ, USA
Suchismita Ray - Columbia University Medical Center, New York, NY, USA
Margaret Haney - Rutgers University Brain Imaging Center, Newark, NJ, USA
Catherine Hanson & Stephen José Hanson - New Jersey Institute of Technology, Newark, NJ, USA
Bharat Biswal - Psychology Department, Rutgers University, Newark, NJ, USA
Stephen José Hanson
Authors
- Suchismita Ray
- Margaret Haney
- Catherine Hanson
- Bharat Biswal
- Stephen José Hanson
Corresponding author
Correspondence toSuchismita Ray.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Ray, S., Haney, M., Hanson, C. et al. Modeling Causal Relationship Between Brain Regions Within the Drug-Cue Processing Network in Chronic Cocaine Smokers.Neuropsychopharmacol 40, 2960–2968 (2015). https://doi.org/10.1038/npp.2015.150
- Received: 22 August 2014
- Revised: 27 May 2015
- Accepted: 28 May 2015
- Published: 03 June 2015
- Issue Date: December 2015
- DOI: https://doi.org/10.1038/npp.2015.150