Modulation of Total Sleep Time by Transcranial Direct Current Stimulation (tDCS) (original) (raw)
INTRODUCTION
The regulation of arousal and sleep represents a basic brain process across species and its modulation in humans, either to promote arousal or sleep, is of great clinical importance. The current study follows the concept that arousal and sleep can be modulated by non-invasive transcranial direct current stimulation (tDCS) targeting a ‘top-down’ cortico-thalamic pathway of sleep-wake regulation.
Classic models of sleep-wake regulation in mammals highlight the importance of a ‘bottom-up’ pathway. This evolutionary ancient pathway, the ascending reticular activating system (ARAS), originates in the brain stem, comprises aminergic and cholinergic cell groups, and activates the thalamus and cerebral cortex during wakefulness (Moruzzi and Magoun, 1949; Saper et al, 2005; Steriade, 1995). For sleep onset and maintenance, GABAergic sleep-promoting neurons in the ventro-lateral preoptic area (VLPO) inhibit the wake-promoting cell groups of the ARAS (Berridge et al, 2012; España and Scammell, 2011; Nelson et al, 2002). The ARAS and the VLPO form a functional ‘flip-flop switch’ creating distinct behavioral states (wake or sleep) that are stabilized by orexinergic neurons in the lateral hypothalamus (Saper et al, 2005). This sleep-wake regulation is governed by an interplay of two processes, a circadian process C emerging from pacemaker cells in the suprachiasmatic nucleus and a sleep-wake dependent (homeostatic) process S that increases as a function of prior waking time and declines during sleep (Borbély, 1982, 2009). Current treatments for clinical conditions of disturbed arousal or sleep primarily target the aminergic, cholinergic, or GABAergic neurotransmission of the ‘bottom-up’ pathway pharmacologically. This pharmacological approach is widely used with some success, but with important side effects and limited treatment efficiency (Riemann and Nissen, 2012).
More recently, a ‘top-down’ pathway of sleep-wake regulation has been identified. Here, cortical neurons serve as the primary oscillators of a cortico-thalamo-cortical feedback loop (Chauvette et al, 2010; Le Bon-Jego and Yuste, 2007; Steriade et al, 1993), with synchronized slow activity underlying the emergence of consolidated sleep (Steriade, 2006). Particularly, regional synchronization of neural activity (Riedner et al, 2007) and reductions in metabolism in the prefrontal cortex (Nofzinger et al, 2006) have been identified as a hallmark of sleep. High-density EEG studies demonstrate the specific importance of frontal areas for the onset and maintenance of sleep (Marzano et al, 2013). In turn, elevated metabolism in the prefrontal cortex correlated with increased EEG beta activity during NREM sleep, a marker of arousal and the subjective perception of poor sleep (Nofzinger et al, 2004, 2006). Targeting the ‘top-down’ pathway with non-invasive brain stimulation may provide novel inroads into the treatment for clinical conditions of disturbed arousal or sleep. Given that respective disturbances represent highly prevalent transdiagnostic syndromes across a variety of neuropsychiatric disorders, the identification and implementation of novel treatments would be of high clinical importance (Riemann et al, 2015).
The current study used bi-frontal tDCS to induce changes of neural excitability in the cerebral cortex (Nitsche and Paulus, 2000) and to target the ‘top-down’ pathway of sleep-wake regulation. Particularly, anodal stimulation was used to increase cortical excitability (‘activation’) and cathodal stimulation to diminish cortical excitability (‘deactivation’, Nitsche et al, 2008). It was hypothesized that bi-frontal tDCS-induced functional connectivity alterations would involve also subcortical arousal networks (Polanía et al, 2012). Repetitive tDCS protocols were applied before sleep that induce after-effects on cortical excitability that last for several hours (Monte-Silva et al, 2013), most probably by induction of synaptic long-term plasticity (Ranieri et al, 2012). Different transcranial current stimulation protocols are currently explored in various neuropsychiatric disorders (for overview, eg, Kuo et al, 2014) and have shown effects on distinct characteristics of sleep, such as EEG slow waves during NREM sleep (Marshall et al, 2006) or EEG gamma activity during REM sleep (Voss et al, 2014). Yet to date, studies on the modulation of tonic arousal processes and total sleep time (TST) are lacking.
The current study was designed to provide proof-of-concept that excitability changes in the cerebral cortex induced by tDCS can modulate TST in humans. Specifically, we tested the hypotheses that bi-frontal anodal tDCS results in a decrease in TST and that bi-frontal cathodal tDCS results in an increase in TST, compared with sham stimulation in healthy humans.
MATERIALS AND METHODS
Participants
Nineteen healthy participants (13 females, 6 males, age 53.7±6.9 years, age range 40–65 years) were included in the analysis. Three additional participants did not complete the protocol due to technical problems. All participants underwent an extensive screening to rule out any relevant mental (World Health Organization's Composite International Diagnostic Interview; Robins et al, 1988), physical or sleep disorder (polysomnography), or any tDCS-specific exclusion criteria (Nitsche et al, 2003). All participants maintained a regular sleep-wake schedule before and during the study, as monitored by actigraphy (Actiwatches, Cambridge Neurotechnology) and sleep diaries (Carney et al, 2012), and were free of any CNS-active medication. All participants were right handed, non-smokers, and did not consume any caffeine or alcohol during the study. All participants provided written informed consent before the study. The study was conducted in accordance with the Declaration of Helsinki, approved by the Ethics Committee of the University Medical Center Freiburg (271/12-130471), and registered in the German Register for Clinical Studies (www.germanctr.de, DRKS00004299).
Study Design
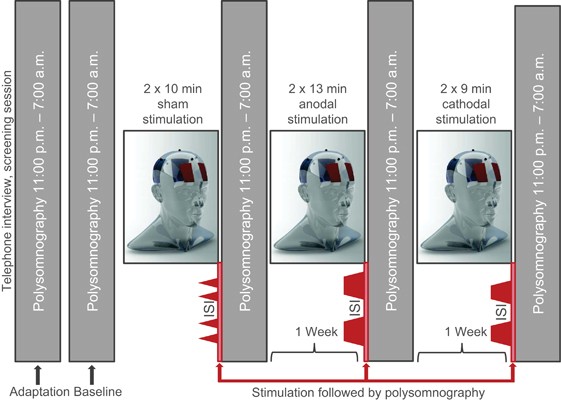
All participants underwent a within-subject, repeated-measures protocol across five nights in the sleep laboratory (Figure 1). One adaptation night was followed by a baseline night and three experimental nights with polysomnographic recordings 2300–0700 h. tDCS was applied between 2200 and 2246 h before sleep according to the experimental protocol (anodal, cathodal, and sham stimulation). Resting-state EEG was recorded before (T0) and after (T1) the stimulation protocol in the evening and in the following morning (T2). Participants completed self-reports and tests for alertness in the morning. Experimental nights were alternated in a quasi-randomized and counterbalanced order to exclude sequence effects and were separated by 1 week to prevent carry-over effects.
Figure 1
Study design. Adaption, baseline and three trial nights with tDCS immediately before polysomnography; stimulation protocols with electrode positioning over the prefrontal and parieto-occipital cortex: I. Sham stimulation: 2 blocks of 11 min tDCS with the stimulator fading in and out at the beginning and end of each 11 min period with 20 min interval between sham stimulation blocks (inter-stimulation interval, ISI). II. Anodal stimulation: 2 blocks of 13 min with fade-in/fade-out and 20 min ISI. III. Cathodal stimulation: 2 blocks of 9 min with fade-in/fade-out and 20 min ISI.
Transcranial Direct Current Stimulation
tDCS was delivered by a battery-driven, micro-processor-controlled CE-certified constant current stimulator (neuroConn GmbH, Illmenau, Germany) and comprises bi-frontal target electrodes (5 × 7 cm, FP1/FP2) and bi-parietal return electrodes (10 × 10 cm, P3/P4) covered with electrode cream (Ten20 Conductive EEG Paste, Weaver, Aurora, Colorado). Bi-frontal stimulation was selected to target the proposed ‘top-down’ pathway of sleep-wake regulation. Target electrodes used the standard size for effective stimulation (Peterchev et al, 2012). Return electrodes were larger to reduce current density to a level previously shown to be functionally inert to the cerebral cortex (Nitsche et al, 2007). For robust effects within the safety recommendations, a constant current of 1 mA over each electrode was applied (2 mA stimulator output, Y-cable split for stimulation and reference electrodes). A fade-in/fade-out design (30 s each) was used to decrease potential skin sensations during the beginning and end of the stimulation (Nitsche et al, 2008). To induce prolonged after-effects for the modulation of sleep continuity, optimized repetitive stimulation protocols were employed for each condition (Monte-Silva et al, 2010, 2013) with 13 min of anodal and 9 min of cathodal stimulation with 20 min inter-stimulation intervals (Figure 1). The duration of the sham stimulation blocks was 11 min (30 s fade-in followed by 30 s fade-out at the beginning and end of each block, no active stimulation). This sham procedure has repeatedly been reported to keep the participants blinded for stimulation conditions (Gandiga et al, 2006). In accordance with these studies, the participants of the current study were not able to discern between the tDCS conditions when asked in the mornings following the experimental nights. As listed in Supplementary Table S1 (supplements), most participants described skin sensations during the stimulation and some headache and unspecific somatic reactions, without any differences between the tDCS conditions. During one cathodal stimulation session, a superficial skin lesion of the right ear occurred due to unintended direct contact with the anode.
Sleep Recordings
Polysomnography was recorded from 2300 to 0700 h according to standard procedures (eg, Nissen et al, 2011). All recordings included an EEG (C3-A2) (analog filter setting 0.53–70 Hz, sampling rate 200 Hz), electrooculogram, submental electromyogram, and an electrocardiogram. Polysomnographic recordings were visually scored off-line by experienced raters according to the standard criteria (Iber, 2007). The raters were blinded for the experimental conditions. The following polysomnographic parameters of sleep continuity and architecture were assessed: sleep-onset latency (SOL), defined as the period between turning the lights off and the first 30-s epoch of stage 2 sleep (N2), slow wave sleep (SWS/N3) or rapid eye movement (REM) sleep; TST, defined as the time spent in stage 1 or 2 sleep, slow wave sleep (SWS), or REM sleep after sleep onset; sleep efficiency (SE), defined as the ratio of TST to time in bed × 100%; wake time, defined as the time spent awake during bed time; number of sleep stage changes; number of wake periods; arousal index (AI), defined as the number of arousals per hour of sleep for TST; percentages of sleep stage 2, SWS and REM sleep referred to TST; REM sleep latency (REML), defined as the period between sleep onset and the occurrence of the first 30-s epoch of REM sleep; number of REM sleep cycles per night; EOGS, defined as the number of 3-s mini-epochs including REMs during REM sleep; REM density, defined as the ratio of 3-s REM sleep mini-epochs including REMs to the total number of REM sleep mini-epochs × 100%.
Sleep EEG spectral analysis was carried out to assess power spectra as described previously (eg, Holz et al, 2012; Nissen et al, 2001). The analysis was performed on the C3-A2 derivation in 30-s epochs for which sleep stages had been determined. Spectral estimates for each epoch were obtained by averaging of 22 overlapping FFT windows (512 data points, 2.56 s) covering a 30-s epoch to obtain the spectral power within that epoch, resulting in a spectral resolution of 0.39 Hz. A Welch taper was applied to each FFT window after demeaning and detrending the data in that window. The spectral power values were then log-transformed (base e) and continuously stored on disk. All subsequent steps including statistical analysis were performed on these logarithmic values, which have a more symmetrical distribution of errors as compared with raw spectral power. Artifact rejection was conducted by an automatic method discarding epochs due to abnormal total or gamma-band power values relative to a 10-min moving window. The log spectra of the remaining epochs were averaged across all NREM sleep epochs. Spectral band power was calculated for the following frequency ranges: delta 0.1–3.5 Hz (delta1 0.1–1.5 Hz; delta2 1.5–3.5 Hz); theta 3.5–8 Hz; alpha 8–12 Hz; sigma 12–16 Hz (sigma1 12–14 Hz; sigma2 14–16 Hz); beta 16–24 Hz; and gamma 24–50 Hz.
Wake EEG Recordings
To further assess indices of cortical arousal during wakefulness, we conducted 5 min resting-state wake EEGs (C3-A2) before stimulation (T0), immediately after the stimulation (T1), and on the following morning (T2). Participants were seated in a quiet sleep laboratory with eyes closed, using a standardized resting state and muscle relaxation instruction. EEG recordings were visually scored off-line for possible sleep stages by experienced raters according to the standard criteria (C3-A2 derivation, 30 s epochs). EEG spectral power was calculated for single frequency bins for each EEG measurement according to the procedures described for polysomnographic recordings, using 2.56 s Welch-tapered FFTs. In each 5 min EEG trace, technical or movement artifacts were marked. Then, data were segmented into windows of 2.56 s overlapping by half (ie, steps of 1.28 s) avoiding a region from 5 s before the start to 5 s after the end of each marked artifact as well as any 30 s epoch scored as non-wake.
Neuropsychological Testing
Cognitive performance, including alertness (Test for Attentional Performance, Zimmermann and Fimm, 2007), was assessed at 2030 h and at 0745 h. In addition, subjective sleep parameters, tiredness, and dream recall were recorded in the morning using self-report questionnaires (see Supplementary Table S2).
Statistical Analyses
Descriptive values are given as means and standard deviations. To test for polysomnographic differences, repeated-measures analyses of variance (ANOVAs) with the within-subject factor Condition (anodal stimulation, cathodal stimulation, sham stimulation) were conducted. TST was used as the primary outcome parameter. Other analyses were secondary analyses. For resting-state EEG analyses, the repeated-measures factor Testsection (T0, T1, T2) was added. For the estimation of effect sizes, partial ETA square (η p 2) values were calculated (low: <0.06; medium: ⩾0.06; and<0.14; large: ⩾0.14). Post hoc contrasts were calculated for significant effects. The level of significance was set at p<0.05 (two-tailed). All analyses were conducted with the statistical software R (R version 3.1.2, The R Foundation for Statistical Computing).
RESULTS
Polysomnography
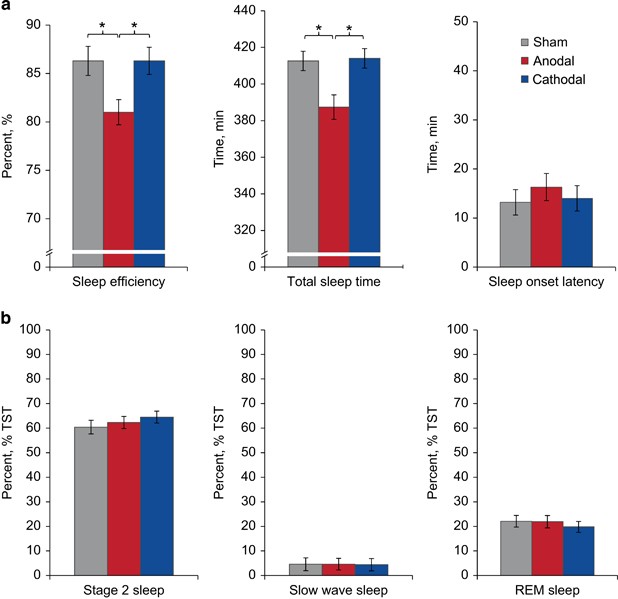
Polysomnographic parameters and statistics are shown in Table 1 and Figure 2. As the main result of the current study, bi-frontal cortical anodal stimulation resulted in a significant decrease in TST of about 25 min with a corresponding increase in wake time and a decrease in sleep efficiency (primary analysis). Post hoc tests confirmed this difference for both contrasts, anodal vs sham stimulation and anodal vs cathodal stimulation. Further exploratory analyses on single quarters of the night (2 h intervals) revealed a significant difference in TST for the second quarter with significant post hoc contrasts for anodal vs sham stimulation and anodal vs cathodal stimulation. No differences in other sleep continuity parameters were observed. Particularly, SOL showed highly similar values between the conditions. In addition, no difference was detected for the number of wake periods, AI, or sleep architecture variables.
Table 1 Polysomnography
Figure 2
Polysomnographic results. (a) Sleep continuity. Sleep efficiency in percent and total sleep time in minutes were decreased following anodal stimulation compared with sham and cathodal stimulation. Sleep-onset latency in minutes remained unchanged. (b) Sleep architecture. Percentage TST of S2, Slow wave sleep and REM sleep showed no differences. Bars indicate standard errors of the mean. *Significant post hoc contrasts (p<0.05).
In contrast to our primary hypothesis, no increase in TST after cathodal stimulation was observed in the investigated sample of good sleepers. To unmask potential ceiling effects, we explored whether a short TST in the sham stimulation night would predict a higher increase in TST in the cathodal stimulation night. An exploratory correlation analysis between the TST in the sham stimulation night and the increase in TST from the sham to the cathodal stimulation night revealed a trend toward a negative correlation (Pearson’s _r_=−0.5, _p_=0.053), indicative for a potential ceiling effect.
tDCS did not significantly alter REM latency or the number of REM cycles. Exploratory analyses showed a significant difference in REM density, with cathodal stimulation increasing REM density compared with sham, but not with anodal stimulation.
Sleep EEG Spectral Analysis
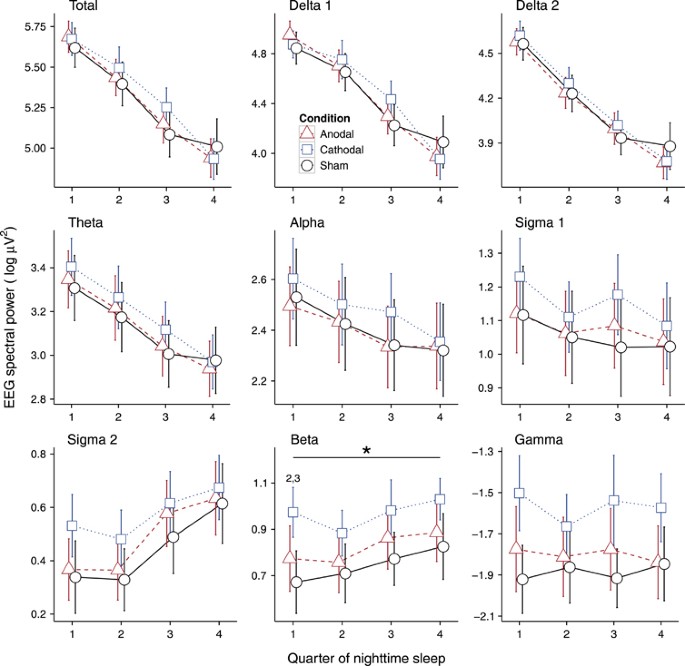
To further determine sleep alterations, EEG spectral power during NREM sleep was analyzed (Figure 3). Total EEG power did not differ between the conditions. Further analyses of single frequency bands demonstrated a significant Condition effect for EEG beta power (F=3.4, _p_=0.045, pETA2=0.16), with cathodal stimulation leading to higher power levels compared with sham (_t_=2.8, df=18, _p_=0.012) and anodal stimulation (_t_=2.5, df=18, _p_=0.021) during the first quarter of the night. No other significant effects were detected.
Figure 3
EEG spectral power values of NREM sleep for single quarters of nighttime sleep. A significant main effect for the factor Condition was observed in the beta frequency band. Post hoc tests revealed higher EEG power values in the first quarter of the night following cathodal stimulation compared with both anodal and sham stimulation. Bars indicate the standard error of the mean. *Significant main effect for Condition. 2Significant contrast sham vs cathodal stimulation; 3Significant contrast anodal vs cathodal stimulation.
Resting State EEG Spectral Analysis
To further assess indices of cortical arousal during wakefulness, we conducted resting-state wake EEGs before stimulation (T0), immediately after the stimulation (T1), and on the following morning (T2). Visual staging of a total of 1710 30-s EEG epochs across participants and conditions detected 24 epochs of stage 1 sleep and 9 epochs of stage 2 sleep that were excluded from further resting-state EEG analyses. No SWS or REM sleep epochs were detected. The distribution of the sleep stages did not differ between the conditions (_p_>0.2).
In a first step of analysis for EEG power differences between conditions at a single frequency, we calculated false discovery rate (FDR) corrected significances, obtained using the Benjamini–Hochberg step-up procedure as a correct assessment of the significance of the multiple tests. This approach did not reveal any significant condition effect.
In a second exploratory step, we used uncorrected ANOVAs to further explore tDCS effects on EEG power spectra. The results are visualized in Figure 4a and b.
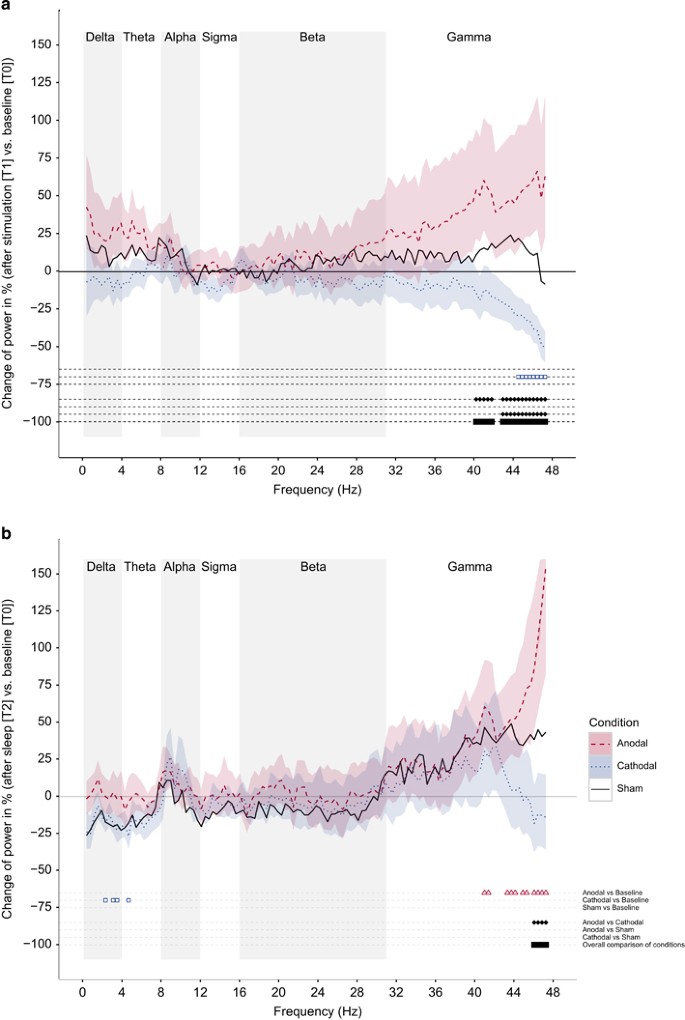
Figure 4
Wake EEG spectral power changes in percent. We used uncorrected ANOVAs to explore tDCS effects on EEG power spectra. For display purposes, spectral power comparisons were performed bin-wise, denoting uncorrected significance with a black bar. (a) After stimulation (T1) vs baseline (T0). ANOVAs demonstrated significant changes in EEG gamma power, with a decrease of 45–48 Hz after cathodal stimulation. A significant main effect for the factor Condition was observed for frequencies between 40 and 48 Hz (gamma range). (b) After nighttime sleep (T2) vs baseline (T0). ANOVAs demonstrated a significant increase in EEG gamma power (40–48 Hz) after anodal stimulation referred to T0 and a significant main effect for the factor Condition for frequencies >46 Hz (gamma band). Highlighted areas indicate the standard error of the mean.
We first analyzed short-term effects of tDCS from T0 to T1 for all three conditions separately (min/mean/max number of segments obtained from the 5-min wake EEG traces for anodal stimulation 43/134/203, cathodal stimulation 77/125/231, and sham stimulation 106/142/204). ANOVAs with the repeated measures factor Testsection (T0, T1) demonstrated significant changes in EEG gamma power, with a decrease in 44–48 Hz after cathodal stimulation referred to T0. In a second step, we conducted ANOVAs with the repeated-measures factors Testsection (T0, T1) and Condition (anodal, cathodal and sham stimulation). A significant main effect for the factor Condition was observed for almost all frequency bins between 40 and 48 Hz (gamma range). Post hoc analyses demonstrated significant contrasts between anodal stimulation and cathodal stimulation for frequencies >40 Hz and between cathodal and sham stimulation for frequencies >43 Hz.
We further analyzed tDCS long-term effects from T0 to T2 for all three conditions separately (min/mean/max number of segments obtained from the 5-min wake EEG traces for anodal stimulation 32/106/167, cathodal stimulation 57/110/169, and sham stimulation 20/107/156). ANOVAs with the repeated measures factor Testsection (T0, T2) demonstrated a significant increase in EEG gamma power (40–47 Hz) after anodal stimulation and a significant decrease in some frequency bins in a 3- to 5-Hz range after cathodal stimulation referred to T0. We then again conducted ANOVAs with the repeated-measures factors Testsection (T0, T2) and Condition (anodal stimulation, cathodal stimulation, and sham stimulation) showing a significant difference between anodal and cathodal stimulation in a 45–48 Hz frequency range.
Subjective Sleep Parameters and Neuropsychology
As listed in Supplementary Table S2, the participants showed no differences in subjective sleep parameters or tiredness (VIS-M) and alertness (TAP). Some participants mentioned vivid dreams, but without differences between the conditions (Chi-square test, _p_>0.2).
Control for Localization Specificity
To control for localization specificity, we recruited 10 additional participants (7 females, 3 males; age 47.8±6.2 years; age range 40–65 years). All 10 participants completed the described study protocol with a reverse electrode configuration, comprising bi-parietal target electrodes (5 × 7 cm, P3/P4) and bi-frontal return electrodes (10 × 10 cm, FP1/FP2). The control group showed no Condition effects on polysomnographic or spectral EEG parameters (all _p_>0.1, data shown in Supplementary Table S3). Small effect sizes suggest that the lack of significant findings was not due to the smaller sample size of the control group.
DISCUSSION
This is the first report that non-invasive tDCS can modulate TST. Particularly, the study provides proof-of-concept that TST can be decreased by bi-frontal anodal tDCS in healthy humans. Exploratory analyses suggest polarity-specific tDCS effects on cortical arousal as indexed by resting-state EEG power. These findings bear interesting theoretical and clinical implications.
Anodal stimulation led, in line with our first hypothesis, to a polarity-specific reduction of TST of about 25 min relative to cathodal and sham stimulation. This effect was location specific for bi-frontal stimulation, ie, it was not observed in a control group with reversed electrode montage. In contrast to our second hypothesis, cathodal stimulation did not increase TST. These findings corroborate previous studies showing more robust effects for anodal than for cathodal stimulation protocols (Jacobson et al, 2012). Specifically in our study, a potential effect of cathodal stimulation might have been missed since it might be difficult to further prolong sleep in good sleepers (ceiling effect). Exploratory analyses revealed a trend that participants with a short TST in the sham stimulation night showed a higher increase in TST from the sham to the cathodal stimulation night, indicative for a potential ceiling effect in the sample of good sleepers.
It is to note that the idea of modifying sleep continuity through electrical stimulation of the brain is an old one. After anecdotal reports on the use of electro-fishes in the ancient Greece (Sconocchia, 1983) and early reports in the 19th century on various effects of electrical brain stimulation (Duchenne de Boulogne, 1876; Finger and Piccolino, 2011), there have been several attempts to induce ‘electrosleep’ in the 1970s and early 1980s. These studies usually applied 30 min of tDCS with a fronto-mastoidal (cathodal-anodal) electrode position during the daytime on 5–10 days over a period of 2–3 weeks (Feighner et al, 1973). In summary, these studies were not effective in inducing sleep. Even though there are single reports on the improvement of self-reported insomnia symptoms (eg, Cartwright and Weiss, 1975), the majority of studies did not corroborate these findings (von Richthofen and Mellor, 1979). Major limitations of these studies include small and inhomogeneous samples of patients with various disorders and the reliance on self-report measures (eg, Hearst et al, 1974). The few polysomnographic studies conducted in patients with insomnia also reported negative results, which—from a today’s perspective—most likely relate to poorly refined tDCS protocols without reliable after-effects applied several hours before sleep (eg, Coursey et al, 1980; Frankel et al, 1973).
Some recent studies demonstrated effects of different protocols of transcranial current stimulation on distinct characteristics of sleep, including EEG slow wave activity during NREM sleep (Marshall et al, 2006) and EEG gamma activity during REM sleep (Voss et al, 2014). These effects were limited to brief periods of the stimulation and short after-effects (seconds) and did not alter sleep continuity. Other studies used transcranial magnetic stimulation (TMS) and demonstrated an increase in current density changes in the alpha2 band after intermittent theta burst stimulation of the left dorsolateral prefrontal cortex (Grossheinrich et al, 2009). To our knowledge, our study is the first to show relevant effects of non-invasive brain stimulation on sleep continuity.
Exploratory EEG analyses during wakefulness suggested polarity-specific changes in cortical arousal, indexed by resting-state EEG power in the gamma frequency range, as a potential neural mechanism of the tDCS effect on sleep continuity. Cortical gamma activity is considered to emerge from synchronous activity of fast-spiking inhibitory neurons in the cortex (Cardin et al, 2009) and has been linked to the integration of temporally correlated neural events (Wang, 2010) as a prerequisite for higher-level cognitive processing and attentive wakefulness (Clayton et al, 2015). It is plausible that depolarization of cortical structures after anodal stimulation (Medeiros et al, 2012) facilitates fast-spiking and EEG gamma activity, with reverse effects after cathodal stimulation. Following models of ‘top-down’ control of sleep regulation and functional connectivity effects of tDCS, these modulatory effects might extend to subcortical arousal networks via cortico-thalamic feedback loops.
The observed time course of the effect and microstructure of sleep might be explained by the two-process model and the flip-flop model of sleep-wake regulation, respectively. First, bi-frontal anodal stimulation reduced TST, but, counter-intuitively, did not prolong the latency to sleep onset. Of note, the observed reduction of TST showed its peak effect during the second quarter of the night. This pattern of results might be explained by an interaction of the time courses of the wake-promoting stimulation effect and the physiological sleep pressure (process S; Borbély, 1982). More specifically, the wake-promoting effect of anodal tDCS might have been overdriven by physiologically high sleep pressure at sleep onset and thus only have emerged with the dissipation of sleep pressure in the second quarter of the night (threshold function). Subsequently, the stimulation effect might diminish to a level that is not sufficient to alter sleep continuity. Second, TST was decreased after anodal stimulation, but no effect on the number of microarousals or wake periods was observed. This suggests that the stimulation does not destabilize the basic flip-flop switch of sleep-wake regulation. Rather, anodal tDCS appeared to increase the duration of wake periods without affecting the frequency of awakenings. No effects on sleep architecture were observed.
Several limitations need to be addressed. First, cathodal stimulation did not increase TST possibly, as discussed, due to ceiling effects. Future studies are needed to test whether cathodal tDCS can improve sleep continuity in clinical conditions with disruptions of sleep, eg, insomnia disorder as a human model of hyperarousal (Riemann et al, 2010), that frequently co-occur with other mental, neurological, and somatic disorders (Riemann et al, 2015). Second, potential tDCS effects on REM sleep, eg, the observed increase in REM density in our study and a previous premotor cortex study (Nitsche et al, 2010), remain to be further examined. Third, our study was conducted in middle-aged participants; studies across other developmental periods are warranted. Future work is also needed to better determine the neural mechanisms of tDCS on arousal and sleep.
Our results might have relevant clinical implications. Alterations in arousal or sleep are among the most prevalent health problems worldwide (Riemann et al, 2015). Future work could translate the non-invasive brain stimulation concept to patients with hypersomnia, such as in narcolepsy, idiopathic hypersomnia, or secondary forms after brain lesion, such as inflammation, trauma, or stroke. We recently provided preliminary evidence that the described anodal tDCS protocol improved vigilance and reduced daytime sleepiness in a patient with organic hypersomnia following reanimation (Frase et al, 2015). Future studies are needed to investigate the clinical potential of stimulation protocols for conditions of altered arousal or sleep—an important domain of neuropsychiatric disorders (Insel, 2014). The application of the current tDCS protocol during wakefulness would even allow for home treatment.
Together, our study provides proof-of-concept that TST can be decreased in healthy humans by non-invasive bi-frontal anodal tDCS. Further elucidating and targeting the ‘top-down’ pathway of sleep-wake regulation is expected to increase knowledge on the fundamentals of sleep-wake regulation and to contribute to the development of novel treatments for clinical conditions of decreased arousal/hypersomnia and increased arousal/insomnia that are among the most prevalent health problems worldwide.
FUNDING AND DISCLOSURE
ClN has received speaker honoraria from Servier and Roche. He is an investigator in multicenter clinical trials sponsored by Otsuka, Lundbeck, Roche, and Forum Pharmaceuticals. He has received research support from Lundbeck. DR has received a consulting fee from Abbvie Germany. MAN is in the Advisory Board of Neuroelectrics. ChN has received speaker honoraria from Servier.
References
- Berridge CW, Schmeichel BE, España RA (2012). Noradrenergic modulation of wakefulness/arousal. Sleep Med Rev 16: 187–197.
Article PubMed PubMed Central Google Scholar - Borbély AA (1982). A two process model of sleep regulation. Hum Neurobiol 1: 195–204.
PubMed Google Scholar - Borbély AA (2009). Refining sleep homeostasis in the two-process model. J Sleep Res 18: 1–2.
Article PubMed Google Scholar - Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K et al (2009). Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459: 663–667.
Article CAS PubMed PubMed Central Google Scholar - Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL et al (2012). The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep 35: 287–302.
Article PubMed PubMed Central Google Scholar - Cartwright RD, Weiss M (1975). The effects of electrosleep on insomnia revisited. J Nerv Ment Dis 161: 134–137.
Article CAS PubMed Google Scholar - Chauvette S, Volgushev M, Timofeev I (2010). Origin of active states in local neocortical networks during slow sleep oscillation. Cereb Cortex 20: 2660–2674.
Article PubMed PubMed Central Google Scholar - Clayton MS, Yeung N, Cohen Kadosh R (2015). The roles of cortical oscillations in sustained attention. Trends Cogn Sci 19: 188–195.
Article PubMed Google Scholar - Coursey RD, Frankel BL, Gaarder KR, Mott DE (1980). A comparison of relaxation techniques with electrosleep therapy for chronic, sleep-onset insomnia a sleep-EEG study. Biofeedback Self Regul 5: 57–73.
Article CAS PubMed Google Scholar - Duchenne de Boulogne GBA (1876) Mécanisme de la Physionomie Humaine ou Analyse Électro-Physiologique de l'Expression des Passions. 2nd edn. Librairie J.-B. Bailliere et Fils: Paris.
Google Scholar - España RA, Scammell TE (2011). Sleep neurobiology from a clinical perspective. Sleep 34: 845–858.
PubMed PubMed Central Google Scholar - Feighner JP, Brown SL, Olivier JE (1973). Electrosleep therapy: a controlled double blind study. J Nerv Ment Dis 157: 121–128.
Article CAS PubMed Google Scholar - Finger S, Piccolino M (2011) The Shocking History of Electric Fishes. Oxford University Press: New York.
Book Google Scholar - Frankel BL, Buchbinder R, Snyder F (1973). Ineffectiveness of electrosleep in chronic primary insomnia. Arch Gen Psychiatry 29: 563–568.
Article CAS PubMed Google Scholar - Frase L, Maier JG, Zittel S, Freyer T, Riemann D, Normann C et al (2015). Bifrontal anodal transcranial direct current stimulation (tDCS) improves daytime vigilance and sleepiness in a patient with organic hypersomnia following reanimation. Brain Stimul 8: 844–846.
Article PubMed Google Scholar - Gandiga PC, Hummel FC, Cohen LG (2006). Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 117: 845–850.
Article PubMed Google Scholar - Grossheinrich N, Rau A, Pogarell O, Hennig-Fast K, Reinl M, Karch S et al (2009). Theta burst stimulation of the prefrontal cortex: safety and impact on cognition, mood, and resting electroencephalogram. Biol Psychiatry 65: 778–784.
Article PubMed Google Scholar - Hearst ED, Cloninger CR, Crews EL, Cadoret RJ (1974). Electrosleep therapy—a double-blind trial. Arch Gen Psychiatry 30: 463–466.
Article CAS PubMed Google Scholar - Holz J, Piosczyk H, Feige B, Spiegelhalder K, Baglioni C, Riemann D et al (2012). EEG Σ and slow-wave activity during NREM sleep correlate with overnight declarative and procedural memory consolidation. J Sleep Res 21: 612–619.
Article PubMed Google Scholar - Iber C (2007) The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine: Westchester, IL.
Google Scholar - Insel TR (2014). The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry 171: 395–397.
Article PubMed Google Scholar - Jacobson L, Koslowsky M, Lavidor M (2012). tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res 216: 1–10.
Article PubMed Google Scholar - Kuo M, Paulus W, Nitsche MA (2014). Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage 85 (Pt 3): 948–960.
Article PubMed Google Scholar - Le Bon-Jego M, Yuste R (2007). Persistently active, pacemaker-like neurons in neocortex. Front Neurosci 1: 123–129.
Article PubMed PubMed Central Google Scholar - Marshall L, Helgadóttir H, Mölle M, Born J (2006). Boosting slow oscillations during sleep potentiates memory. Nature 444: 610–613.
Article CAS PubMed Google Scholar - Marzano C, Moroni F, Gorgoni M, Nobili L, Ferrara M, de Gennaro L (2013). How we fall asleep: Regional and temporal differences in electroencephalographic synchronization at sleep onset. Sleep Med 14: 1112–1122.
Article PubMed Google Scholar - Medeiros LF, de Souza IC, Vidor LP, de Souza A, Deitos A, Volz MS et al (2012). Neurobiological effects of transcranial direct current stimulation: a review. Front Psychiatry 3: 110.
Article PubMed PubMed Central Google Scholar - Monte-Silva K, Kuo M, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W et al (2013). Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul 6: 424–432.
Article PubMed Google Scholar - Monte-Silva K, Kuo M, Liebetanz D, Paulus W, Nitsche MA (2010). Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS). J Neurophysiol 103: 1735–1740.
Article PubMed Google Scholar - Moruzzi G, Magoun HW (1949). Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol 1: 455–473.
Article CAS PubMed Google Scholar - Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M (2002). The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci 5: 979–984.
Article CAS PubMed Google Scholar - Nissen C, Feige B, König A, Voderholzer U, Berger M, Riemann D (2001). Delta sleep ratio as a predictor of sleep deprivation response in major depression. J Psychiatr Res 35: 155–163.
Article CAS PubMed Google Scholar - Nissen C, Kloepfer C, Feige B, Piosczyk H, Spiegelhalder K, Voderholzer U et al (2011). Sleep-related memory consolidation in primary insomnia. J Sleep Res 20: 129–136.
Article PubMed Google Scholar - Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A et al (2008). Transcranial direct current stimulation: state of the art 2008. Brain Stimul 1: 206–223.
Article PubMed Google Scholar - Nitsche MA, Doemkes S, Karaköse T, Antal A, Liebetanz D, Lang N et al (2007). Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol 97: 3109–3117.
Article CAS PubMed Google Scholar - Nitsche MA, Jakoubkova M, Thirugnanasambandam N, Schmalfuss L, Hullemann S, Sonka K et al (2010). Contribution of the premotor cortex to consolidation of motor sequence learning in humans during sleep. J Neurophysiol 104: 2603–2614.
Article PubMed Google Scholar - Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W (2003). Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol 114: 2220–2222, author reply 2222-3.
Article PubMed Google Scholar - Nitsche MA, Paulus W (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol (Lond) 527 (Pt 3): 633–639.
Article CAS Google Scholar - Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ (2004). Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry 161: 2126–2128.
Article PubMed Google Scholar - Nofzinger EA, Nissen C, Germain A, Moul D, Hall M, Price JC et al (2006). Regional cerebral metabolic correlates of WASO during NREM sleep in insomnia. J Clin Sleep Med 2: 316–322.
PubMed Google Scholar - Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH et al (2012). Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimul 5: 435–453.
Article PubMed Google Scholar - Polanía R, Paulus W, Nitsche MA (2012). Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp 33: 2499–2508.
Article PubMed Google Scholar - Ranieri F, Podda MV, Riccardi E, Frisullo G, Dileone M, Profice P et al (2012). Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. J Neurophysiol 107: 1868–1880.
Article CAS PubMed Google Scholar - Riedner BA, Vyazovskiy VV, Huber R, Massimini M, Esser S, Murphy M et al (2007). Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep 30: 1643–1657.
Article PubMed PubMed Central Google Scholar - Riemann D, Nissen C . Sleep and Psychotropic Drugs. In: Morin CM, Espie CA (eds). Oxford Handbook of Sleep and Sleep Disorders. Oxford University Press: Oxford; (2012), pp 190–222.
Google Scholar - Riemann D, Nissen C, Palagini L, Otte A, Perlis ML, Spiegelhalder K (2015). The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol 14: 547–558.
Article PubMed Google Scholar - Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M et al (2010). The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev 14: 19–31.
Article PubMed Google Scholar - Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J et al (1988). The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry 45: 1069–1077.
Article CAS PubMed Google Scholar - Saper CB, Scammell TE, Lu J (2005). Hypothalamic regulation of sleep and circadian rhythms. Nature 437: 1257–1263.
Article CAS PubMed Google Scholar - Sconocchia S (ed) (1983) Sribonii Largi Compositiones. B. G. Teubner: Leipzig.
Google Scholar - Steriade M (1995). Neuromodulatory systems of thalamus and neocortex. Sem Neurosci 7: 361–370.
Article Google Scholar - Steriade M (2006). Grouping of brain rhythms in corticothalamic systems. Neuroscience 137: 1087–1106.
Article CAS PubMed Google Scholar - Steriade M, Nuñez A, Amzica F (1993). A novel slow (1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci 13: 3252–3265.
Article CAS PubMed PubMed Central Google Scholar - von Richthofen CL, Mellor CS (1979). Cerebral electrotherapy: Methodological problems in assessing its therapeutic effectiveness. Psychol Bull 86: 1264–1271.
Article CAS PubMed Google Scholar - Voss U, Holzmann R, Hobson A, Paulus W, Koppehele-Gossel J, Klimke A et al (2014). Induction of self awareness in dreams through frontal low current stimulation of gamma activity. Nat Neurosci 17: 810–812.
Article CAS PubMed Google Scholar - Wang X (2010). Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev 90: 1195–1268.
Article PubMed Google Scholar - Zimmermann P, Fimm B (2007) Testbatterie zur Aufmerksamkeitsleistung (TAP). Psytest: Würselen, Germany.
Google Scholar
Acknowledgements
This work has been supported by intramural funds of the University Medical Center Freiburg. MK and JGM have received PhD grants provided by the FAZIT foundation. MAN receives research support within the framework of the EU Research and Innovation programme Horizon 2020 (grant 686764, LUMINOUS).
Author information
Authors and Affiliations
- Department of Psychiatry and Psychotherapy, University Medical Center Freiburg, Freiburg, Germany
Lukas Frase, Hannah Piosczyk, Sulamith Zittel, Friederike Jahn, Peter Selhausen, Lukas Krone, Bernd Feige, Florian Mainberger, Jonathan G Maier, Marion Kuhn, Stefan Klöppel, Claus Normann & Christoph Nissen - Department of Clinical Psychology and Psychophysiology, University Medical Center Freiburg, Freiburg, Germany
Lukas Frase, Hannah Piosczyk, Sulamith Zittel, Friederike Jahn, Peter Selhausen, Lukas Krone, Bernd Feige, Jonathan G Maier, Marion Kuhn, Kai Spiegelhalder, Dieter Riemann & Christoph Nissen - Department of Neurology, University Medical Center Freiburg, Freiburg, Germany
Stefan Klöppel - Department of Psychology, University of Surrey, Guildford, UK
Annette Sterr - Department of Clinical Neurophysiology, University Medical Center Göttingen, Göttingen, Germany
Michael A Nitsche - Leibniz Research Centre for Working Environment and Human Factors, Dortmund, Germany
Michael A Nitsche - Department of Neurology, University Medical Hospital Bergmannsheil, Bochum, Germany
Michael A Nitsche
Authors
- Lukas Frase
You can also search for this author inPubMed Google Scholar - Hannah Piosczyk
You can also search for this author inPubMed Google Scholar - Sulamith Zittel
You can also search for this author inPubMed Google Scholar - Friederike Jahn
You can also search for this author inPubMed Google Scholar - Peter Selhausen
You can also search for this author inPubMed Google Scholar - Lukas Krone
You can also search for this author inPubMed Google Scholar - Bernd Feige
You can also search for this author inPubMed Google Scholar - Florian Mainberger
You can also search for this author inPubMed Google Scholar - Jonathan G Maier
You can also search for this author inPubMed Google Scholar - Marion Kuhn
You can also search for this author inPubMed Google Scholar - Stefan Klöppel
You can also search for this author inPubMed Google Scholar - Claus Normann
You can also search for this author inPubMed Google Scholar - Annette Sterr
You can also search for this author inPubMed Google Scholar - Kai Spiegelhalder
You can also search for this author inPubMed Google Scholar - Dieter Riemann
You can also search for this author inPubMed Google Scholar - Michael A Nitsche
You can also search for this author inPubMed Google Scholar - Christoph Nissen
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toChristoph Nissen.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Frase, L., Piosczyk, H., Zittel, S. et al. Modulation of Total Sleep Time by Transcranial Direct Current Stimulation (tDCS).Neuropsychopharmacol 41, 2577–2586 (2016). https://doi.org/10.1038/npp.2016.65
- Received: 15 June 2015
- Revised: 03 March 2016
- Accepted: 11 April 2016
- Published: 04 May 2016
- Issue Date: September 2016
- DOI: https://doi.org/10.1038/npp.2016.65