Immune responses to RNA-virus infections of the CNS (original) (raw)
Key Points
- Successful clearance of virus infection from the central nervous system (CNS) requires the elimination of virus from its intracellular location without damage to essential non-renewable cells, such as neurons.
- Several different types of RNA virus are important causes of acute encephalomyelitis worldwide, and mechanisms of disease recovery can be studied in mouse models.
- Normally, the CNS is maintained in an immunologically quiescent state through interactions between neurons and microglia, and the constitutive production of immunomodulatory factors.
- After virus infection, innate responses are quickly activated with local expression of MHC molecules, chemokines and cytokines.
- The adaptive immune response is initiated outside the CNS, and circulating activated natural killer cells, T cells, B cells and macrophages enter sites of virus infection in the CNS.
- Immune-mediated virus clearance from neurons occurs mainly through non-cytolytic processes that are mediated by antibody specific for the virus and interferon-γ, which allow neurons to survive, although virus RNA is not completely eliminated.
- Immune-mediated virus clearance from glial cells is more likely to occur through cytolytic processes, but long-term control is dependent on antibody.
Abstract
A successful outcome for the host of virus infection of the central nervous system (CNS) requires the elimination of the virus without damage to essential non-renewable cells, such as neurons. As a result, inflammatory responses must be tightly controlled, and many unique mechanisms seem to contribute to this control. In addition to being important causes of human disease, RNA viruses that infect the CNS provide useful models in which to study immune responses in the CNS. Recent work has shown the importance of innate immune responses in the CNS in controlling virus infection. And advances have been made in assessing the relative roles of cytotoxic T cells, antibodies and cytokines in the clearance of viruses from neurons, glial cells and meningeal cells.
Similar content being viewed by others
Main
Virus infections of the central nervous system (CNS) are relatively uncommon, but potentially devastating. The longevity of many cells in the CNS and the relative inaccessibility of this tissue to components of the immune system make the brain and spinal cord particularly susceptible to persistent virus infection. Because of the potential for neurological damage by inflammatory mediators and cytotoxic cells, the brain has intrinsic mechanisms for controlling immune responses that are different from other organs. Nevertheless, many virus infections are cleared from the CNS through virus-specific immune mechanisms without marked neurological damage. Clearance of virus from non-neural tissues often involves cytolytic elimination of the infected cells. However, immune responses to virus infection of the CNS can also clear the virus from the tissue or sustain prolonged inhibition of virus replication without damage to the structure or function of the nervous system. Non-cytolytic clearance mechanisms are advantageous to the host when the infected cells cannot be replaced, but they might provide less complete elimination of the virus than cytolytic processes. Mechanisms for non-cytolytic clearance are beginning to be elucidated and new roles have been identified for lymphocytes, local antibody production and cytokines. The degree to which clearance is successful differs with the type of virus that causes the infection and with the target cells that the virus infects.
Types of virus that infect the CNS
Several RNA and DNA viruses from different virus families can infect the nervous system and cause human disease (Table 1). DNA viruses that infect the CNS have nuclear phases in their replication cycles and often have an inherent capacity to remain latent in the nucleus (and undetectable to the immune system), either by integrating a copy of the virus genome into host DNA or by maintaining an episomal form of the virus genome in the cell nucleus. These viruses are common causes of disease in immunocompromised hosts because of their ability to reactivate when host immune responses fail. Most RNA viruses replicate only in the cytoplasm and do not establish true latency and, therefore, are susceptible to different mechanisms of immune control than are DNA viruses. This review focuses on the immune response to RNA viruses in the CNS.
Table 1 Examples of important virus infections of the CNS
The viruses and their target cells. The most common virus infections of the CNS cause meningitis by targeting the cells of the leptomeninges that cover the surface of the brain. These viruses do not usually cause serious disease or persistent infection because they infect renewable cells in a part of the nervous system that is accessible to the immune system. Less common, but more likely to cause fatal disease, are viruses that infect neurons. Neurons are long-lived, terminally differentiated cells that are important target cells for viruses causing encephalomyelitis (Box 1). Not all types of neuron are equally susceptible or likely to become infected; this depends on the virus tropism, route of entry of the virus into the CNS and the mechanism of spread. Many neuronotropic viruses spread within the nervous system by axonal transport and move from neuron to neuron through connecting synapses1. If infection is induced experimentally by intracerebral inoculation, there might also be rapid spread through the cerebrospinal fluid2. Other viruses target the supporting glial cells of the CNS — oligodendrocytes, astrocytes and microglia — alone or in addition to neurons. Oligodendrocytes form and maintain the myelin that surrounds the axons of neurons, and infection of these cells might lead to acute or chronic demyelinating disease. Astrocytes are the 'supporting' cells for neurons. Among other functions, they produce neurotrophic factors, remove toxic materials and maintain the BLOOD–BRAIN BARRIER. Infection of these cells might also indirectly damage neurons. Microglia are the resident macrophages of the CNS, can express MHC class I and class II molecules, have an important role in local immune responses and can produce various factors that are toxic to neurons.
Mouse models of infection. Most of our knowledge about immune responses to virus infection of the CNS comes from studying a few well-characterized mouse models of infection that characterize these types of virus-induced disease (Table 2). The most detailed studies of immune responses to neuronal infection have been with rabies virus and mosquito-borne alphaviruses, such as Sindbis virus, Venezuelan equine encephalitis virus and Semliki Forest virus, that can cause acute encephalomyelitis in mice. The most extensive studies of immune responses to viruses that infect glial cells, often in addition to neurons, have involved the natural mouse pathogens Theiler's mouse encephalomyelitis virus (TMEV), which is a picornavirus, and mouse hepatitis virus (MHV), which is a coronavirus. Another natural mouse pathogen, lymphocytic choriomeningitis virus (LCMV), which is an arena virus that can infect ependymal cells, choroid plexus cells or neurons, has also been studied extensively. LCMV, as well as Borna disease virus, can cause immune-mediated disease and persistent infection of the CNS. For all of these RNA viruses, the outcome of infection depends on the age and genetic background of the mouse, and on the strain of virus that is studied. For this review, the focus is on immune responses of adult animals to the most commonly studied strains of each virus. These infections have many common, as well as distinctive, features. Immune responses to each infection progress through several phases that start with intrinsic responses in the CNS as the presence of the pathogen is recognized, followed by entry of lymphocytes and monocytes from the periphery as part of the adaptive inflammatory response, and then complete or partial silencing of immune-mediated inflammation as the virus is cleared or its replication is controlled.
Table 2 Mouse models of CNS infection and immune-mediated clearance
Innate immune responses to CNS virus infection
The normal, uninfected CNS lacks immunological activity. Endothelial cells have intact tight junctions and express low levels of adhesion molecules that promote interaction with circulating leukocytes. Microglia, which express the CD200 receptor (CD200R), are kept in a quiescent state through interaction with electrically active, healthy neurons, which express CD200 (Ref. 3), and by the local production of neurotrophins4. Production of the anti-inflammatory cytokine transforming growth factor-β (TGF-β) by astrocytes and meningeal cells suppresses activation of endothelial and other cells by cytokines5,6(Fig. 1a).
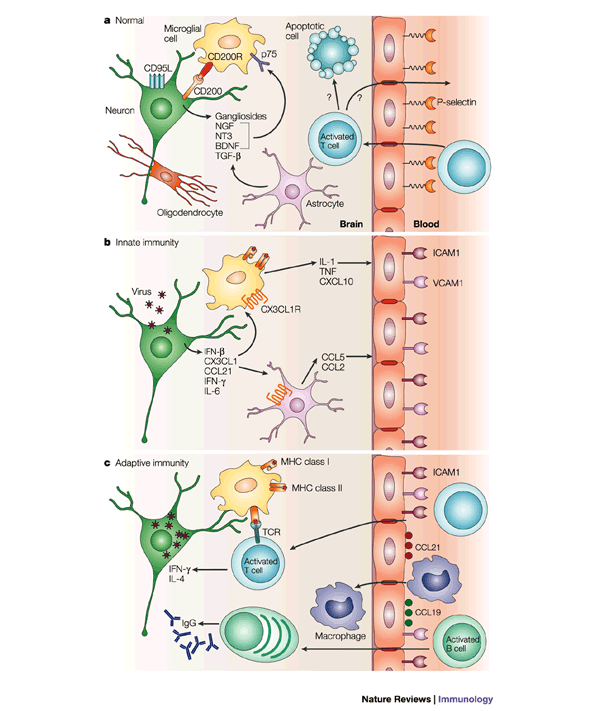
Figure 1: Changes that occur in the CNS during the immune response to a neuronal virus infection.
a | In the normal uninfected central nervous system (CNS), endothelial cells express few adhesion molecules. Neurons maintain microglia in a quiescent state through CD200–CD200 receptor (CD200R) interactions and astrocytes contribute by the production of neurotrophins, such as brain-derived neurotrophic factor (BDNF), nerve-growth factor (NGF) and neurotrophin 3 (NT3), that interact with p75, a low-affinity neurotrophin receptor. Gangliosides are abundant and transforming growth factor-β (TGF-β) is constitutively produced by neurons and astrocytes. Activated T cells enter the CNS routinely through interaction with low levels of P-selectin expressed by endothelial cells, but leave or die soon thereafter. b | Virus infection of neurons initiates the early production of interferon-β (IFN-β), chemokines and pro-inflammatory cytokines, which results in further activation of microglia and increased expression of adhesion molecules, such as vascular-cell adhesion molecule 1 (VCAM1) and intercellular adhesion molecule 1 (ICAM1), by endothelial cells. c | By three to four days after infection, inflammatory cells — such as natural killer (NK) cells, macrophages and lymphocytes — that are activated in secondary lymphoid tissue begin to enter the CNS at regions where virus replication is occurring and chemokines, such as CCL19 and CCL21, are expressed by endothelial cells. T cells produce additional cytokines, such as interleukin-4 (IL-4) and IFN-γ, and the B cells produce antibody, which initiates clearance of virus from infected cells. TNF, tumour-necrosis factor.
When neurons or other CNS cells become infected, changes quickly occur to initiate protective responses (Fig. 1b). Rapid production of TYPE 1 INTERFERON (IFN) is important for host survival and, in most experimental virus infections of the CNS, susceptibility to fatal disease is markedly increased in mice that lack the receptor for IFN-α/β7,8,9,10. IFN-β, an immediate early IFN, which is produced by neurons as well as glial cells, is the main type 1 IFN that is produced by the CNS and is required for efficient induction of IFN-α in vitro11,12. Preferential production of IFN-β rather than IFN-α in the CNS might be neuroprotective, as IFN-α has marked CNS toxicity compared with IFN-β13,14, and IFN-β might induce the production of neurotrophic factors by astrocytes15 and the local production of the anti-inflammatory cytokine interleukin-10 (IL-10)16. Rapid local production of type 1 IFN after infection slows virus spread and constrains virus replication before the induction of virus-specific adaptive immune responses. Virus inhibition of type 1 IFN production or activity — for example, by the leader protein of TMEV — increases the probability that the virus will not be cleared17.
Damaged or stressed neurons signal to astrocytes and microglia in the vicinity of the neuronal cell body, even when the neuronal damage occurs to the axon at a distance18. The factors that are produced by neurons in response to injury — which includes injury caused by virus infection — are not completely catalogued, but they probably include the cytokines IFN-γ and IL-6, and the chemokines fractalkine (CX3CL1) and secondary lymphoid tissue chemokine (SLC, CCL21), in addition to IFN-β19,20,21. CX3CL1 is a membrane-bound chemokine that is constitutively expressed by subsets of neurons and is rapidly cleaved from the cell surface after neurotoxic damage22. Macrophages and glial cells express the CX3CL1 receptor (CX3CR1) and so become activated in early phases of the response to neuronal infection23,24, in which they have an important role. Activated microglia and astrocytes can rapidly produce an array of cytokines and chemokines. These vary with the specific virus infection, but frequently include IL-1, IL-6, IL-12, tumour-necrosis factor (TNF), macrophage inflammatory protein-1β (MIP1β, CCL4), monocyte chemo attractant protein 1 (MCP1, CCL2), MCP3 (CCL7), regulated on activation normal T-cell expressed and secreted (RANTES, CCL5) and IFN-inducible 10 kDa protein (IP10, CXCL10)25,26,27,28,29,30. Production of these factors results in the upregulation of expression of MHC molecules on the cell surface of microglia and in increased expression of adhesion molecules by capillary endothelial cells. Chemokines are transcytosed to the endothelial luminal surface and presented to circulating leukocytes on the tips of microvillous processes31.
Induction of the adaptive immune response
The normal brain is relatively deficient in expression of MHC molecules, and the structure of the blood–brain barrier inhibits the entry of circulating proteins and cells from the blood to the brain parenchyma. It is generally agreed that, although there are cells that can present antigen to primed T cells in the CNS, the activation of naive T cells and B cells occurs in secondary lymphoid tissues outside the CNS32,33,34. The parenchyma of the normal CNS does not contain dendritic cells, although they are present in the meninges and cerebro–spinal fluid (CSF)35. Some of the CSF and CNS interstitial fluid drains to the deep cervical lymph nodes through the nasal submucosa32,36. Experimental inoculation of virus directly into the CSF elicits a potent immune response, whereas inoculation directly into the brain parenchyma elicits little or no response37. Responses that are initiated by inoculation of antigen into the CSF tend to be characterized by marked antibody responses, priming of cytotoxic CD8+ T cells and no DELAYED-TYPE HYPERSENSITIVITY32. However, essentially all neurotropic viruses naturally initiate infection in the periphery either through the gastrointestinal tract, the respiratory tract or the bite of an infected mosquito or animal. Therefore, induction of the virus-specific immune response occurs through virus infection, or uptake by dendritic cells in peripheral tissue and antigen presentation in lymph nodes that drain those initial sites of replication, rather than in the CNS.
The entry of circulating leukocytes into the CNS is generally restricted by the tight junctions and by the relative nonreactivity of cerebral capillary endothelial cells, which have limited endocytosis and expression of adhesion molecules38,39. However, several studies have shown that activated T cells routinely cross the blood–brain barrier as part of the normal immunological surveillance of all tissues39,40,41 (Fig. 1a). Transendothelial migration of activated CD4+ T cells occurs, in part, through the interaction of P-selectin glycoprotein ligand, which is expressed by activated T cells, with P-selectin, which is expressed at low levels by normal cerebrovascular endothelial cells38,42. However, one side effect of the induction of a systemic immune response, even in the absence of infection in the CNS, is the upregulation of expression of adhesion molecules by CNS endothelial cells and increased surveillance of the CNS by activated T cells43,44. Activated T cells that enter the CNS are not retained in the CNS in the absence of antigen, and either leave or die in situ (Fig. 1a). However, activated T cells are retained in the CNS when the relevant antigen is present and associated with appropriate MHC molecules39. During inflammation, local production of chemokines and induction of expression of adhesion molecules by endothelial cells further enhance the entry of activated cells into the CNS45. Expression of intercellular adhesion molecule 1 (ICAM1, CD54) and vascular-cell adhesion molecule 1 (VCAM1) is upregulated by cerebral capillary endothelial cells during virus infection, and antibody specific for the integrin leukocyte function-associated antigen 1 (LFA1), which is the ligand for ICAM1, blocks lymphocyte entry into the brain during the acute inflammatory response to Sindbis virus39.
MHC class I molecules are not expressed by cells in the normal CNS, but are abundantly expressed by endothelial, meningeal and microglial cells during the early phases of the immune response to most CNS virus infections46, and are occasionally expressed by astrocytes and oligodendrocytes47. However, neurons rarely express MHC class I molecules. Neurons respond to IFN-β with induction of antiviral proteins, such as 2′–5′ oligoadenylate synthetase, but not with expression of MHC class I molecules48. Suppression of expression of MHC class I molecules by neurons seems to be both transcriptional and post transcriptional. There is little translocation from the cytoplasm to the nucleus of nuclear factor-κB (NF-κB) — a key transcription factor for MHC class I expression — in neurons in response to pro-inflammatory mediators for example, TNF and IL-1 or virus infection, despite the presence of these receptors. By contrast, astrocytes readily respond in this manner. Lack of NF-κB translocation in neurons seems to be due to a failure to induce degradation of the inhibitor of NF-κB (IκBα) in response to cytokines48,49. However, neurons that are damaged or electrically silenced by treatment with a neurotoxin can be induced to express MHC class I molecules by treatment with IFN-γ50. In vivo, the levels of mRNAs that encode MHC class I heavy chain, β2-microglobulin (β2m), transporter associated with antigen processing 1 (TAP1) and TAP2 are increased in neurons during infection46. However, there is controversy over whether mature MHC class I molecules are expressed on the neuronal cell surface46,51. The β2m molecules that are sometimes detected on the neuronal cell surface might be associated with expression of non-classical rather than classical MHC class I molecules. Differences in sensitivity of detection might also account for some of the controversy that surrounds this issue — when expression of MHC class I molecules by neurons is detected, it is at markedly lower levels than expression by glial cells in the same region51.
MHC class II molecules are not constitutively expressed by cells in the brain parenchyma, but are induced quickly after initiation of infection and also by trafficking of activated T cells through the CNS52. The cells that express MHC class II molecules most abundantly are microglia and perivascular macrophages34. Astrocytes can be induced to express MHC class II molecules in vitro, but this is rarely observed in vivo34.
Infiltration of mononuclear inflammatory cells into the CNS begins three to four days after infection. Cells first accumulate in perivascular areas and then infiltrate the parenchyma in the regions of virus infection. Essentially all components of the cellular immune response are detected in the infiltrate: natural killer (NK) cells, antigen-specific CD4+ and CD8+ T cells, B cells and monocyte/macrophages53,54,55,56,57. NK cells are detected first, followed by CD8+ and CD4+ T cells, then B cells and monocytes/macrophages58. In addition, dendritic cells are detected in the CNS during inflammation and either enter the CNS from the circulation or develop from a subpopulation of activated microglia33,59. The number of inflammatory cells peaks within one to two weeks after infection and then these cells are gradually eliminated, unless the virus persists60. Cytokines that are produced by these infiltrating cells include IFN-γ, IL-4 and IL-10, but rarely IL-2 (Refs 27–29,54,61,62). B cells in the CNS usually produce antibody, mainly of the immunoglobulin A and IgG subclasses63, although IgA is not produced in response to all infections.
Regulation of immune responses in the CNS
Several observations indicate that there is marked regulation of immune responses in the CNS. Active regulation includes functional control of the immune activation of intrinsic cells of the CNS and of cells entering the CNS. In addition, there is induced death of T cells that infiltrate the brain. Several regulatory mechanisms are involved and these tend to inhibit T-cell proliferation and favour the production of T HELPER 2 (TH2)-type cytokines, although these cytokines might not have an important role in clearance per se. CNS GANGLIOSIDES inhibit the expression of MHC class I and class II molecules by astrocytes and inhibit IL-2 production and proliferation of T cells in the brain parenchyma without affecting the production of IL-4 or IL-5. The immunoregulatory activity of brain gangliosides on T cells is due, in part, to suppression of NF-κB activity and phosphorylation of retinoblastoma protein64,65,66. T cells from strains of mice that are susceptible to TH1-mediated autoimmune disease of the CNS are less responsive to regulation by brain gangliosides than T cells from disease-resistant strains67.
However, ganglioside production by neurons does not inhibit the expression of MHC class II molecules by microglia4,68. Antigen presentation by microglia stimulates CD4+ T cells to produce IFN-γ, but not IL-2 and does not stimulate T-cell proliferation. By contrast, macrophages that infiltrate the CNS from the periphery, which tend to accumulate near vessels, can support complete T-cell activation and survival69. Activity of these macrophages might be controlled by the production of TGF-β in the CNS, which suppresses the development of T-cell cytotoxicity and delayed-type hypersensitivity, and which deactivates infiltrating antigen-presenting cells (APCs)70,71.
Activated T cells that leave the perivascular area often undergo apoptosis after exposure to APCs in the parenchyma72,73. In addition, constitutive expression of FAS ligand (CD95L) by cells of the CNS induces apoptosis of FAS (CD95)-expressing activated T cells. Neuronal expression of CD95L might further protect neurons from T-cell cytotoxicity74,75,76.
Mechanisms of immune-mediated virus clearance
Clearance strategies. Clearance of virus from cells in the brain parenchyma is a multistep process. Each of the several phases of virus clearance are assessed in different ways. First, there is inhibition of virus spread to new cells, then elimination of cell-free infectious virus. Subsequently, virus-infected cells must be eliminated or intracellular virus replication permanently suppressed. The most frequently measured parameter of virus clearance is the amount of infectious virus that is present in CNS tissue. However, as antibody specific for the virus is produced, the ability to detect infectious virus by standard assays, which depend on the detection of viral cytopathic effects in susceptible indicator cells, is compromised by the presence of neutralizing antibody in the tissue homogenates. During this phase of clearance, virus can often be detected by cocultivation of cells from the infected tissue with susceptible tissue culture cells. As replication in cells is suppressed or infected cells are eliminated, quantitative measurement of virus nucleic acid provides the best indicator of whether the virus has truly been eliminated from the relevant cells and tissue.
There is no dou bt that the most reliable way to completely eliminate a virus from tissue is to eliminate all cells in which the virus is replicating, either by virus-induced cytolysis or by immune-mediated cytolysis. However, mature CNS neurons and many glial cells are relatively resistant to both processes. The fact that neurons are non-renewable cells might underly their resistance to apoptosis. If infected cells are allowed to survive, the clearance of virus must include mechanisms for inhibiting intracellular synthesis of virus nucleic acid and protein, and for removing virus genomes from cells. If the clearance process is not complete, then mechanisms for preventing resumption of virus replication must be in place to avoid progressive or relapsing disease58,60,77.
The immunological processes that are required for clearance are cell-type specific. Therefore, control might be achieved in one target-cell population (for example, neurons), but not another (for example, microglia or oligodendrocytes). In general, antibody responses have an important role in the clearance of viruses from neurons, whereas T-cell-mediated responses are more important for clearance from glial cells, for reasons that are not yet understood. However, as with any important biological process, mechanisms for the control of virus replication are redundant and overlapping. Experimental approaches to define these clearance mechanisms are dependent on the transient depletion of specific immune-cell populations and on the use of mice that have selective deficiencies in various components of the immune system. Because of the interdependent relationships of components of the immune system in the development of an immune response, deficiencies of one type of cell or molecule might affect several facets of the immune response, making it difficult to identify specific effectors that are crucial for in vivo clearance.
Clearance from neurons. For most RNA viruses, clearance of virus from neurons occurs through a non-cytolytic, immunologically specific process. Anti-viral antibodies are important for the clearance of rabies virus, Sindbis virus, MHV and TMEV from neurons78,79,80,81. B cells, including virus-specific antibody-secreting cells, infiltrate the brain as a part of the inflammatory response and are retained in the CNS57,82,83(Fig. 1c). Many of the specific antibodies that are associated with protection or clearance are directed against virus surface proteins and inhibit intracellular virus replication in vitro80,84. The mechanism by which antibody specific for a virus surface glycoprotein inhibits virus replication is not completely clear, but has been studied in greatest detail for Sindbis virus.
The anti-viral effect of antibody specific for the Sindbis virus E2 glycoprotein does not require complement or phagocytic cells, but does require that the antibody be bivalent80,84. This implies that crosslinking of virus proteins on the surface of the infected cell is necessary for the control of intracellular virus replication. Treatment of persistently infected cells with antibody leads to the restoration of sodium-pump function and synthesis of host proteins, both of which are inhibited by Sindbis-virus replication85. Type 1 IFN synergizes with antibody to further inhibit virus replication, and it is probable that this interaction is important for the clearance of virus in vivo86. Antibody is also necessary for the clearance of rabies virus87 and MHV from neurons79. The 'clearance' of Sindbis virus and other viruses that infect neurons is incomplete, as virus RNA can be detected throughout the life of the animal82,88. Long-term secretion of antibody in the brains of mice that have recovered from infection indicates that there is a continued requirement for immune-mediated control of virus replication57,82.
Although studies of immunodeficient mice show that T cells are not necessary for the clearance of Sindbis virus from neurons, the level of intracellular virus RNA is reduced more rapidly when CD8+ T cells are present46. CD8+ T cells are also important for rapid control of rabies virus and herpes simplex virus replication in neurons87,89. In general, CD8+ T cells can exert anti-viral effector functions either through a non-lytic cytokine-mediated pathway or by a cytotoxic pathway, which involves either the cytotoxic effector molecules perforin and granzymes or CD95–CD95L. It is not known whether CD8+ T cells in the CNS are activated by the virus-peptide–MHC class I complexes expressed by virus-infected neurons or by surrounding glial cells that have taken up antigen released by infected cells — a process known as cross-priming. Neurons are severely restricted in their expression of MHC class I molecules, but MHC class I expression has been observed in damaged neurons that were exposed to IFN-γ and occasionally on virus-infected neurons51,90. CD8+ T-cell-mediated killing of neurons through a CD95–CD95L-dependent pathway has also been documented74,91. Although neuronal death mediated by CD8+ T cells has been reported in some virus infections of the CNS92, the main contributions of CD8+ T cells to the clearance of virus from neurons are not mediated through cytolysis of infected cells, and do not require perforin or CD95 (Ref. 93).
Recent studies of antibody-deficient mice have shown that both CD4+ and CD8+ T cells infiltrating the brain contribute to the clearance of Sindbis virus through the production of IFN-γ94. An important role for CD4+ and CD8+ T cells in virus clearance that is associated with the production of IFN-γ has also been shown for a neuronotropic strain of MHV61. IFN-γ also has a role in the clearance of vesicular stomatitis virus, a virus that is related to rabies virus, from neurons, perhaps through the production of nitric oxide95. CD8+ T cells alone can clear LCMV from persistently infected neurons after a prolonged period of time96 and these cells might produce anti-viral factors in addition to IFN-γ. For example, the cytotoxic effector molecule granzyme A seems to have an additional role in restricting the replication of herpes simplex virus in neurons97. However, in contrast to its essential role in non-cytolytic clearance of hepatitis B virus from hepatocytes, there is no evidence of a role for TNF in clearing virus from neurons94,98.
Interestingly, neurons in different regions of the CNS differ in their ability to respond to the anti-viral effects of IFN-γ. Motor neurons in the spinal cord show complete resolution of infection in the absence of antibody, whereas cortical neurons require antibody for the clearance of Sindbis virus94. Whether this is due to differences in the ability to bind IFN-γ or to differences in the ability to respond to IFN-γ is not known. The IFN-γ receptor is widely expressed by neurons and constitutive expression by neurons is markedly higher than it is by glial cells99. It is interesting that some neurons can produce IFN-γ19, and the autocrine effects of IFN-γ might also have a role in virus clearance100. In all of these examples of virus clearance, no infectious virus can be recovered, but virus RNA in the CNS remains detectable by sensitive techniques — such as PCR after reverse transcription of RNA (RT-PCR) — and this RNA is presumed to reside in infected neurons that survived both infection and immune-mediated virus clearance77,82.
Clearance from glial cells. Clearance of infectious virus from glial cells seems to be more complicated than clearance from neurons and is probably dependent on T cells (Fig. 2), perhaps because these cells are more likely to be induced by inflammatory mediators to express MHC molecules. In the best-studied examples of virus infection of glial cells — TMEV and MHV — clearance mechanisms often fail, which results in persistent infection and immune-mediated demyelinating disease in susceptible strains of mice. TMEV has an early encephalitic phase, which mainly involves infection of neurons, followed, in genetically susceptible strains of mice, by persistent infection of glial cells. Clearance of TMEV from strains of mice that do not develop persistent infection or demyelinating disease is dependent on a rapid CD8+ T-cell response101. In strains of mice that are susceptible to persistent infection, infectious virus is cleared from neurons, but virus is not completely cleared from oligodendrocytes, astrocytes and microglia, although it is reduced to low levels, and infectious virus can be cultured from the CNS.
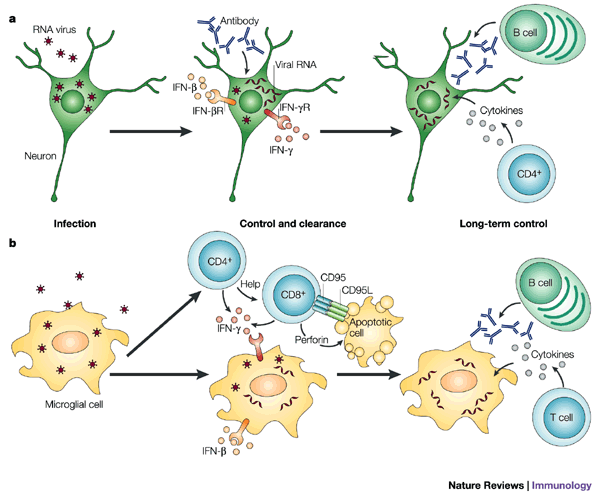
Figure 2: Mechanisms of virus clearance from neurons and microglia.
a | In neurons that survive infection with RNA viruses, infection is controlled initially by interferon-β (IFN-β) and subsequently by virus-specific antibody and IFN-γ produced by T cells and possibly neurons. This effectively inhibits the production of new virus, but does not necessarily eliminate virus RNA from the cell. CD4+ T cells that secrete cytokines, such as IFN-γ, and antibody-secreting B cells are resident for prolonged periods of time in the nervous system and probably have a role in long-term control of virus replication. b | Initial control of virus replication in infected glial cells involves IFN-β. Clearance involves cytolytic CD8+ T cells. CD4+ T cells supply help for CD8+ T cells and both subsets of T cells produce IFN-γ, which has an independent role in virus clearance from cells that survive. Long-term control of virus replication requires the local production of virus-specific antibody and possibly T cells.
The JHM strain of MHV (MHV4) mainly infects glial cells. Although passive antibody can protect mice from fatal encephalomyelitis, in strains of mice that clear the infection, antibody is not detectable until after the virus is cleared, which indicates that antibody is not the main mechanism of clearance28,102,103. A reduction in virus replication can be mediated by CD4+ or CD8+ T cells104,105, and transfer of virus-specific CD4+ or CD8+ T cells can protect from fatal MHV-induced encephalitis104, which further indicates a role for T cells in control of virus replication.
The mechanism of T-cell-mediated clearance of MHV is glial-cell-type specific102. IFN-γ is particularly important for the clearance of MHV from oligodendrocytes, whereas perforin, and presumably CD8+ T-cell-mediated cytolysis, is important for the clearance of virus from astrocytes and microglia103,105,106. Clearance occurs in perforin-deficient mice but is delayed, indicating that many mechanisms are involved103. Auxiliary roles have been identified for CD4+ T cells, CD95 and IFN-γ in virus clearance98,107. CD4+ T-cell help is required for the prevention of apoptosis and expression of the cytotoxic effector function of CD8+ T cells in the MHV-infected CNS56. Virus RNA that persists can resume replication and, therefore, long-term local immune control of virus replication is required60. IFN-γ-secreting CD8+ T cells that are specific for virus antigen persist in the CNS and might contribute to the demyelinating process that follows resolution of the acute virus infection108,109,110. CD4+ T cells also persist in the CNS for several weeks60. Despite a lack of evidence for a role for antibody in the initial clearance of MHV, antibody-secreting B cells are essential for the prevention of renewed virus replication111. T-cell numbers eventually decline, but antibody-secreting B cells persist57. Both B cells and the virus-specific antibody they produce seem to have independent roles in preventing the renewed replication of MHV in astrocytes and oligodendrocytes111,112. Although B cells can be cytotoxic in vitro, it is not clear whether this mechanism is relevant to the role of B cells in vivo.
Concluding remarks
Although there is an increasing understanding of many of the unique aspects of the immune responses to virus infections of the CNS, many questions remain unanswered. The adhesion molecules expressed by endothelial cells and lymphocytes that are responsible for entry of the earliest T and B cells involved in immune surveillance and initiation of inflammation are unclear. The molecular mechanisms involved in non-cytolytic virus clearance from neurons are not defined for any of the model infections that are being studied. Knowledge of these mechanisms would allow the identification of cell-type-dependent differences — that is, between different neuronal populations, oligodendroglia, astrocytes and microglia — in response to various immune mediators, such as IFNs and antibodies. Another important area of investigation will be the use of new genetic information to identify host determinants that affect inflammation, virus persistence in the CNS, and host susceptibility to fatal virus and immune-mediated disease. This information will help the development of new treatments for infections of the CNS caused by RNA viruses. For example, knowing the target virus proteins and contributions of specific components of the immune response will help in rational vaccine design. In addition, the ability of passively transferred immune serum that contains polyclonal antibody to aid the clearance of virus indicates that therapeutic monoclonal antibodies can be developed for post-exposure prophylaxis. This could potentially be combined with the appropriate use of cytokines that are involved in virus clearance. Whether such approaches would be therapeutically useful after the development of symptoms that are associated with neuronal loss or dysfunction is less clear.
References
- Ehrengruber, M. U., Ehler, E., Billeter, M. A. & Naim, H. Y. Measles virus spreads in rat hippocampal neurons by cell-to-cell contact and in a polarized fashion. J. Virol. 76, 5720–5728 (2002).
Article CAS PubMed PubMed Central Google Scholar - Stevenson, P. G., Freeman, S., Bangham, C. R. & Hawke, S. Virus dissemination through the brain parenchyma without immunologic control. J. Immunol. 159, 1876–1884 (1997).
CAS PubMed Google Scholar - Hoek, R. M. et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290, 1768–1771 (2000). CD200–CD200R-mediated interactions between neurons and microglia are essential for maintenance of the anti-inflammatory state in the central nervous system (CNS).
Article CAS PubMed Google Scholar - Neumann, H., Misgeld, T., Matsumuro, K. & Wekerle, H. Neurotrophins inhibit major histocompatibility class II inducibility of microglia: involvement of the p75 neurotrophin receptor. Proc. Natl Acad. Sci. USA 95, 5779–5784 (1998).
Article CAS PubMed PubMed Central Google Scholar - Johnson, M. D., Gold, L. I. & Moses, H. L. Evidence for transforming growth factor-β expression in human leptomeningeal cells and transforming growth factor-β-like activity in human cerebrospinal fluid. Lab. Invest. 67, 360–368 (1992).
CAS PubMed Google Scholar - Fabry, Z. et al. TGF-β2 decreases migration of lymphocytes in vitro and homing of cells into the central nervous system in vivo. J. Immunol. 155, 325–332 (1995).
CAS PubMed Google Scholar - Muller, U. et al. Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921 (1994). This paper reports the development of mice that are deficient in receptors for interferon-α/β (IFN-α/β) and IFN-γ and indicates the essential role of these receptors in the control of virus replication.
Article CAS PubMed Google Scholar - Fiette, L. et al. Theilers virus infection of 129SV mice that lack the interferon-α/β or interferon-γ receptors. J. Exp. Med. 181, 2069–2076 (1995).
Article CAS PubMed Google Scholar - Ryman, K., Klimstra, W., Nguyen, K., Biron, C. & Johnston, R. α/β interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J. Virol. 74, 3366–3378 (2000).
Article CAS PubMed PubMed Central Google Scholar - Byrnes, A. P., Durbin, J. E. & Griffin, D. E. Control of Sindbis virus infection by antibody in interferon-deficient mice. J. Virol. 74, 3905–3908 (2000).
Article CAS PubMed PubMed Central Google Scholar - Sandberg, K., Eloranta, M. L. & Campbell, I. L. Expression of α/β interferons (IFN-α/β) and their relationship to IFN-α/β-induced genes in lymphocytic choriomeningitis. J. Virol. 68, 7358–7366 (1994).
Article CAS PubMed PubMed Central Google Scholar - Erlandsson, L. et al. Interferon-β is required for interferon-α production in mouse fibroblasts. Curr. Biol. 8, 223–226 (1998).
Article CAS PubMed Google Scholar - Akwa, Y. et al. Transgenic expression of IFN-α in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J. Immunol. 161, 5016–5026 (1998).
CAS PubMed Google Scholar - McLaurin, J., Antel, J. P. & Yong, V. W. Immune and non-immune actions of interferon-β-1b on primary human neural cells. Multiple Sclerosis 1, 10–19 (1995).
Article CAS PubMed Google Scholar - Boutros, T., Croze, E. & Yong, V. W. Interferon-β is a potent promoter of nerve growth factor production by astrocytes. J. Neurochem. 69, 939–946 (1997).
Article CAS PubMed Google Scholar - Chabot, S. & Yong, V. W. Interferon-β-1b increases interleukin-10 in a model of T cell–microglia interaction: relevance to MS. Neurology 55, 1497–1505 (2000).
Article CAS PubMed Google Scholar - van Pesch, V., van Eyll, O. & Michiels, T. The leader protein of Theiler's virus inhibits immediate-early α/β interferon production. J. Virol. 75, 7811–7817 (2001).
Article CAS PubMed PubMed Central Google Scholar - Neumann, H. Control of glial immune function by neurons. Glia 36, 191–199 (2001).
Article CAS PubMed Google Scholar - Neumann, H., Schmidt, H., Wilharm, E., Behrens, L. & Wekerle, H. Interferon γ gene expression in sensory neurons: evidence for autocrine gene regulation. J. Exp. Med. 186, 2023–2031 (1997). This paper describes for the first time that neurons produce IFN-γ.
Article CAS PubMed PubMed Central Google Scholar - Harrison, J. K. et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl Acad. Sci. USA 95, 10896–10901 (1998).
Article CAS PubMed PubMed Central Google Scholar - Rappert, A. et al. Secondary lymphoid tissue chemokine (CCL21) activates CXCR3 to trigger a Cl(-) current and chemotaxis in murine microglia. J. Immunol. 168, 3221–3226 (2002).
Article CAS PubMed Google Scholar - Chapman, G. A. et al. Fractalkine cleavage from neuronal membranes represents an acute event in the inflammatory response to excitotoxic brain damage. J. Neurosci. 20, 1–5 (2000). The authors describe that neurons use the chemokine fractalkine (CX3CL1) to signal the presence of injury to microglia.
Article Google Scholar - Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell Biol. 20, 4106–4114 (2000).
Article CAS PubMed PubMed Central Google Scholar - Maciejewski-Lenoir, D., Chen, S., Feng, L., Maki, R. & Bacon, K. B. Characterization of fractalkine in rat brain cells: migratory and activation signals for CX3CR-1-expressing microglia. J. Immunol. 163, 1628–1635 (1999).
CAS PubMed Google Scholar - Asensio, V. C. & Campbell, I. L. Chemokine gene expression in the brains of mice with lymphocytic choriomeningitis. J. Virol. 71, 7832–7840 (1997).
Article CAS PubMed PubMed Central Google Scholar - Lane, T. E. et al. Dynamic regulation of α- and β-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J. Immunol. 160, 970–978 (1998).
CAS PubMed Google Scholar - Wesselingh, S. L., Levine, B., Fox, R. J., Choi, S. & Griffin, D. E. Intracerebral cytokine mRNA expression during fatal and nonfatal alphavirus encephalitis suggests a predominant type 2 T cell response. J. Immunol. 152, 1289–1297 (1994).
CAS PubMed Google Scholar - Parra, B., Hinton, D. R., Lin, M. T., Cua, D. J. & Stohlman, S. A. Kinetics of cytokine mRNA expression in the central nervous system following lethal and nonlethal coronavirus-induced acute encephalomyelitis. Virology 233, 260–270 (1997).
Article CAS PubMed Google Scholar - Chang, J. R., Zaczynska, E., Katsetos, C. D., Platsoucas, C. D. & Oleszak, E. L. Differential expression of TGF-β, IL-2, and other cytokines in the CNS of Theiler's murine encephalomyelitis virus-infected susceptible and resistant strains of mice. Virology 278, 346–360 (2000).
Article CAS PubMed Google Scholar - Galelli, A., Baloul, L. & Lafon, M. Abortive rabies virus central nervous infection is controlled by T lymphocyte local recruitment and induction of apoptosis. J. Neurovirol. 6, 359–372 (2000).
Article CAS PubMed Google Scholar - Middleton, J., Patterson, A. M., Gardner, L., Schmutz, C. & Ashton, B. A. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood 100, 3853–3860 (2002).
Article CAS PubMed Google Scholar - Harling-Berg, C. J., Park, T. J. & Knopf, P. M. Role of the cervical lymphatics in the TH2-type hierarchy of CNS immune regulation. J. Neuroimmunol. 101, 111–127 (1999).
Article CAS PubMed Google Scholar - Fischer, H. G. & Reichmann, G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J. Immunol. 166, 2717–2726 (2001).
Article CAS PubMed Google Scholar - Pope, J. G., Vanderlugt, C. L., Rahbe, S. M., Lipton, H. L. & Miller, S. D. Characterization of and functional antigen presentation by central nervous system mononuclear cells from mice infected with Theiler's murine encephalomyelitis virus. J. Virol. 72, 7762–7771 (1998).
Article CAS PubMed PubMed Central Google Scholar - McMenamin, P. G. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J. Comp. Neurol. 405, 553–562 (1999).
Article CAS PubMed Google Scholar - Cserr, H. F. & Knopf, P. M. Cervical lymphatics, the blood–brain barrier and the immunoreactivity of the brain: a new view. Immunol. Today 13, 507–512 (1992).
Article CAS PubMed Google Scholar - Stevenson, P. G., Hawke, S., Sloan, D. J. & Bangham, C. R. The immunogenicity of intracerebral virus infection depends on anatomical site. J. Virol. 71, 145–151 (1997).
Article CAS PubMed PubMed Central Google Scholar - Carrithers, M. D., Visintin, I., Kang, S. J. & Janeway, C. A. Jr. Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain 123, 1092–1101 (2000).
Article PubMed Google Scholar - Irani, D. N. & Griffin, D. E. Regulation of lymphocyte homing into the brain during viral encephalitis at various states of infection. J. Immunol. 156, 3850–3857 (1996).
CAS PubMed Google Scholar - Wekerle, H., Linington, C., Lassmann, H. & Meyermann, R. Cellular immune reactivity within the CNS. Trends Neurosci. 9, 271–277 (1986). The authors show that activated T cells can enter the normal CNS.
Article Google Scholar - Hickey, W. F., Hsu, B. L. & Kimura, H. T-lymphocyte entry into the central nervous system. J. Neurosci. Res. 28, 254–260 (1991).
Article CAS PubMed Google Scholar - Piccio, L. et al. Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric G(i)-linked receptors. J. Immunol. 168, 1940–1949 (2002).
Article CAS PubMed Google Scholar - Hickey, W. F. Basic principles of immunological surveillance of the normal central nervous system. Glia 36, 118–124 (2001).
Article CAS PubMed Google Scholar - Licinio, J. & Wong, M. L. Pathways and mechanisms for cytokine signaling of the central nervous system. J. Clin. Invest. 100, 2941–2947 (1997).
Article CAS PubMed PubMed Central Google Scholar - Alt, C., Laschinger, M. & Engelhardt, B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL21 (SLC) at the blood–brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur. J. Immunol. 32, 2133–2144 (2002).
Article CAS PubMed Google Scholar - Kimura, T. & Griffin, D. E. The role of CD8+ T cells and major histocompatibility complex class I expression in the central nervous system of mice infected with neurovirulent Sindbis virus. J. Virol. 74, 6117–6125 (2000).
Article CAS PubMed PubMed Central Google Scholar - Suzumura, A. et al. Induction of glial cell MHC antigen expression in neurotropic coronavirus infections. Characterization of the H-2-inducing soluble factor elaborated by infected brain cells. J. Immunol. 140, 2068–2072 (1988).
CAS PubMed Google Scholar - Massa, P. T., Whitney, L. W., Wu, C., Ropka, S. L. & Jarosinski, K. W. A mechanism for selective induction of 2′–5′ oligoadenylate synthetase, anti-viral state, but not MHC class I genes by interferon-β in neurons. J. Neurovirol. 5, 161–171 (1999).
Article CAS PubMed Google Scholar - Jarosinski, K. W., Whitney, L. W. & Massa, P. T. Specific deficiency in nuclear factor-κB activation in neurons of the central nervous system. Lab. Invest. 81, 1275–1288 (2001).
Article CAS PubMed Google Scholar - Neumann, H., Schmidt, H., Cavalie, A., Jenne, D. & Wekerle, H. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-γ and tumor necrosis factor (TNF)-α. J. Exp. Med. 185, 305–316 (1997).
Article CAS PubMed PubMed Central Google Scholar - Pereira, R. A. & Simmons, A. Cell surface expression of H2 antigens on primary sensory neurons in response to acute but not latent herpes simplex virus infection in vivo. J. Virol. 73, 6484–6489 (1999).
Article CAS PubMed PubMed Central Google Scholar - Sedgwick, J. D., Ford, A. L., Foulcher, E. & Airriess, R. Central nervous system microglial cell activation and proliferation follows direct interaction with tissue-infiltrating T cell blasts. J. Immunol. 160, 5320–5330 (1998).
CAS PubMed Google Scholar - Irani, D. N. & Griffin, D. E. Isolation of brain parenchymal lymphocytes for flow cytometric analysis. Application to acute viral encephalitis. J. Immunol. Methods 139, 223–231 (1991).
Article CAS PubMed Google Scholar - Hatalski, C. G., Hickey, W. F. & Lipkin, W. I. Evolution of the immune response in the central nervous system following infection with Borna disease virus. J. Neuroimmunol. 90, 137–142 (1998).
Article CAS PubMed Google Scholar - Haring, J. S., Pewe, L. L. & Perlman, S. High-magnitude, virus-specific CD4 T-cell response in the central nervous system of coronavirus-infected mice. J. Virol. 75, 3043–3047 (2001).
Article CAS PubMed PubMed Central Google Scholar - Stohlman, S. A., Bergmann, C. C., Lin, M. T., Cua, D. J. & Hinton, D. R. CTL effector function within the central nervous system requires CD4+ T cells. J. Immunol. 160, 2896–2904 (1998).
CAS PubMed Google Scholar - Tschen, S. I. et al. Recruitment kinetics and composition of antibody-secreting cells within the central nervous system following viral encephalomyelitis. J. Immunol. 168, 2922–2929 (2002).
Article CAS PubMed Google Scholar - Dorries, R. The role of T-cell-mediated mechanisms in virus infections of the nervous system. Curr. Top. Microbiol. Immunol. 253, 219–245 (2001).
CAS PubMed Google Scholar - Pashenkov, M. & Link, H. Dendritic cells and immune responses in the central nervous system. Trends Immunol. 23, 69–70 (2002).
Article CAS PubMed Google Scholar - Marten, N. W., Stohlman, S. A. & Bergmann, C. C. Role of viral persistence in retaining CD8+ T cells within the central nervous system. J. Virol. 74, 7903–7910 (2000).
Article CAS PubMed PubMed Central Google Scholar - Pearce, B. D., Hobbs, M. V., McGraw, T. S. & Buchmeier, M. J. Cytokine induction during T-cell-mediated clearance of mouse hepatitis virus from neurons in vivo. J. Virol. 68, 5483–5495 (1994).
Article CAS PubMed PubMed Central Google Scholar - Rowell, J. F. & Griffin, D. E. The inflammatory response to nonfatal Sindbis virus infection of the nervous system is more severe in SJL than in BALB/c mice and is associated with low levels of IL-4 mRNA and high levels of IL-10-producing CD4+ T cells. J. Immunol. 162, 1624–1632 (1999).
CAS PubMed Google Scholar - Griffin, D. E. Immunoglobulins in the cerebrospinal fluid: changes during acute viral encephalitis in mice. J. Immunol. 126, 27–31 (1981).
CAS PubMed Google Scholar - Irani, D. N., Lin, K.-I. & Griffin, D. E. Brain-derived gangliosides regulate the cytokine production and proliferation of activated T cells. J. Immunol. 157, 4333–4340 (1996).
CAS PubMed Google Scholar - Irani, D. N., Lin, K.-I. & Griffin, D. E. Regulation of brain-derived T cells during acute central nervous system inflammation. J. Immunol. 158, 2318–2326 (1997).
CAS PubMed Google Scholar - Massa, P. T. Specific suppression of major histocompatibility complex class I and class II genes in astrocytes by brain-enriched gangliosides. J. Exp. Med. 178, 1357–1363 (1993).
Article CAS PubMed Google Scholar - Irani, D. N. The susceptibility of mice to immune-mediated neurologic disease correlates with the degree to which their lymphocytes resist the effects of brain-derived gangliosides. J. Immunol. 161, 2746–2752 (1998). This paper shows that brain gangliosides regulate T-cell responses in the CNS.
CAS PubMed Google Scholar - Tontsch, U. & Rott, O. Cortical neurons selectively inhibit MHC class II induction in astrocytes but not in microglial cells. Int. Immunol. 5, 249–254 (1993).
Article CAS PubMed Google Scholar - Ford, A. L., Foulcher, E., Lemckert, F. A. & Sedgwick, J. D. Microglia induce CD4 T lymphocyte final effector function and death. J. Exp. Med. 184, 1737–1745 (1996).
Article CAS PubMed Google Scholar - Gordon, L. B., Nolan, S. C., Ksander, B. R., Knopf, P. M. & Harling-Berg, C. J. Normal cerebrospinal fluid suppresses the in vitro development of cytotoxic T cells: role of the brain microenvironment in CNS immune regulation. J. Neuroimmunol. 88, 77–84 (1998).
Article CAS PubMed Google Scholar - Hailer, N. P., Heppner, F. L., Haas, D. & Nitsch, R. Astrocytic factors deactivate antigen presenting cells that invade the central nervous system. Brain Pathol. 8, 459–474 (1998).
Article CAS PubMed Google Scholar - Bauer, J. et al. T-cell apoptosis in inflammatory brain lesions: destruction of T cells does not depend on antigen recognition. Am. J. Pathol. 153, 715–724 (1998).
Article CAS PubMed PubMed Central Google Scholar - Gold, R. et al. Antigen presentation by astrocytes primes rat T lymphocytes for apoptotic cell death. A model for T-cell apoptosis in vivo. Brain 119, 651–659 (1996).
Article PubMed Google Scholar - Medana, I. et al. Fas ligand (CD95L) protects neurons against perforin-mediated T lymphocyte cytotoxicity. J. Immunol. 167, 674–681 (2001).
Article CAS PubMed Google Scholar - Flugel, A. et al. Neuronal FasL induces cell death of encephalitogenic T lymphocytes. Brain Pathol. 10, 353–364 (2000).
Article CAS PubMed Google Scholar - Shin, D. H. et al. Fas ligand mRNA expression in the mouse central nervous system. J. Neuroimmunol. 123, 50–57 (2002).
Article CAS PubMed Google Scholar - Hawke, S., Stevenson, P. G., Freeman, S. & Bangham, C. R. Long-term persistence of activated cytotoxic T lymphocytes after viral infection of the central nervous system. J. Exp. Med. 187, 1575–1582 (1998).
Article CAS PubMed PubMed Central Google Scholar - Fujinami, R. S., Rosenthal, A., Lampert, P. W., Zurbriggen, A. & Yamada, M. Survival of athymic (nu/nu) mice after Theiler's murine encephalomyelitis virus infection by passive administration of neutralizing monoclonal antibody. J. Virol. 63, 2081–2087 (1989).
Article CAS PubMed PubMed Central Google Scholar - Fleming, J. O., Shubin, R. A., Sussman, M. A., Casteel, N. & Stohlman, S. A. Monoclonal antibodies to the matrix (E1) glycoprotein of mouse hepatitis virus protect mice from encephalitis. Virology 168, 162–167 (1989).
Article CAS PubMed Google Scholar - Levine, B. et al. Antibody-mediated clearance of alphavirus infection from neurons. Science 254, 856–860 (1991). This was the first paper to show that antibody can mediate non-cytolytic clearance of virus from neurons.
Article CAS PubMed Google Scholar - Perry, L. L. & Lodmell, D. L. Role of CD4+ and CD8+ T cells in murine resistance to street rabies virus. J. Virol. 65, 3429–3434 (1991).
Article CAS PubMed PubMed Central Google Scholar - Tyor, W. R., Wesselingh, S., Levine, B. & Griffin, D. E. Long term intraparenchymal Ig secretion after acute viral encephalitis in mice. J. Immunol. 149, 4016–4020 (1992).
CAS PubMed Google Scholar - Hatalski, C. G., Hickey, W. F. & Lipkin, W. I. Humoral immunity in the central nervous system of Lewis rats infected with Borna disease virus. J. Neuroimmunol. 90, 128–136 (1998).
Article CAS PubMed Google Scholar - Ubol, S., Levine, B., Lee, S.-H., Greenspan, N. S. & Griffin, D. E. Roles of immunoglobulin valency and the heavy-chain constant domain in antibody-mediated downregulation of Sindbis virus replication in persistently infected neurons. J. Virol. 69, 1990–1993 (1995).
Article CAS PubMed PubMed Central Google Scholar - Despres, P., Griffin, J. W. & Griffin, D. E. Effects of anti-E2 monoclonal antibody on Sindbis virus replication in AT3 cells expressing Bcl-2. J. Virol. 69, 7006–7014 (1995).
Article CAS PubMed PubMed Central Google Scholar - Despres, P., Griffin, J. W. & Griffin, D. E. Antiviral activity of α-interferon in Sindbis virus-infected cells is restored by anti-E2 monoclonal antibody treatment. J. Virol. 69, 7345–7348 (1995).
Article CAS PubMed PubMed Central Google Scholar - Hooper, D. C. et al. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J. Virol. 72, 3711–3719 (1998).
Article CAS PubMed PubMed Central Google Scholar - Destombes, J. et al. Persistent poliovirus infection in mouse motoneurons. J. Virol. 71, 1621–1628 (1997).
Article CAS PubMed PubMed Central Google Scholar - Liu, T., Khanna, K. M., Carriere, B. N. & Hendricks, R. L. γ-interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 75, 11178–11184 (2001).
Article CAS PubMed PubMed Central Google Scholar - Neumann, H., Cavalie, A., Jenne, D. E. & Wekerle, H. Induction of MHC class 1 genes in neurons. Science 269, 549–552 (1995). This paper describes that damaged neurons can express MHC class I molecules in response to IFN-γ.
Article CAS PubMed Google Scholar - Medana, I. M. et al. MHC class I-restricted killing of neurons by virus-specific CD8+ T lymphocytes is effected through the Fas–FasL, but not the perforin pathway. Eur. J. Immunol. 30, 3623–3633 (2000).
Article CAS PubMed Google Scholar - Bilzer, T. & Stitz, L. Immune-mediated brain atrophy. CD8+ T cells contribute to tissue destruction during borna disease. J. Immunol. 153, 818–823 (1994). This study provides evidence that populations of neurons differ in their susceptibility to IFN-γ-mediated virus clearance.
CAS PubMed Google Scholar - Patterson, C. E., Lawrence, D. M., Echols, L. A. & Rall, G. F. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and γ-interferon dependent. J. Virol. 76, 4497–4506 (2002).
Article CAS PubMed PubMed Central Google Scholar - Binder, G. & Griffin, D. Interferon-γ-mediated site specific clearance of alphavirus from CNS neurons. Science 293, 303–306 (2001).
Article CAS PubMed Google Scholar - Komatsu, T., Ireland, D. D., Chen, N. & Reiss, C. S. Neuronal expression of NOS-1 is required for host recovery from viral encephalitis. Virology 258, 389–395 (1999).
Article CAS PubMed Google Scholar - Oldstone, M. B., Blount, P., Southern, P. J. & Lampert, P. W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature 321, 239–243 (1986).
Article CAS PubMed Google Scholar - Pereira, R. A., Simon, M. M. & Simmons, A. Granzyme A, a noncytolytic component of CD8+ cell granules, restricts the spread of herpes simplex virus in the peripheral nervous systems of experimentally infected mice. J. Virol. 74, 1029–1032 (2000).
Article CAS PubMed PubMed Central Google Scholar - Lin, M. T., Hinton, D. R. & Stohlman, S. A. Mechanisms of viral clearance in perforin-deficient mice. Adv. Exp. Med. Biol. 440, 431–436 (1998).
Article CAS PubMed Google Scholar - Robertson, B., Kong, G., Peng, Z., Bentivoglio, M. & Kristensson, K. Interferon-γ-responsive neuronal sites in the normal rat brain: receptor protein distribution and cell activation revealed by Fos induction. Brain Res. Bull. 52, 61–74 (2000).
Article CAS PubMed Google Scholar - Eneroth, A. et al. Interferon-γ-like immunoreactivity in sensory neurons may influence the replication of Sendai and mumps viruses. J. Neurosci. Res. 31, 487–493 (1992).
Article CAS PubMed Google Scholar - Lindsley, M. D. & Rodriguez, M. Characterization of the inflammatory response in the central nervous system of mice susceptible or resistant to demyelination by Theiler's virus. J. Immunol. 142, 2677–2682 (1989).
CAS PubMed Google Scholar - Korner, H. et al. Nucleocapsid or spike protein-specific CD4+ T lymphocytes protect against coronavirus-induced encephalomyelitis in the absence of CD8+ T cells. J. Immunol. 147, 2317–2323 (1991).
CAS PubMed Google Scholar - Lin, M. T., Stohlman, S. A. & Hinton, D. R. Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. J. Virol. 71, 383–391 (1997).
Article CAS PubMed PubMed Central Google Scholar - Yamaguchi, K., Goto, N., Kyuwa, S., Hayami, M. & Toyoda, Y. Protection of mice from a lethal coronavirus infection in the central nervous system by adoptive transfer of virus-specific T cell clones. J. Neuroimmunol. 32, 1–9 (1991).
Article CAS PubMed PubMed Central Google Scholar - Stohlman, S. A., Bergmann, C. C., Van der Veen, R. C. & Hinton, D. R. Mouse hepatits virus-specific cytotoxic T lymphocytes protect from lethal infection without eliminating virus from the central nervous system. J. Virol. 69, 684–694 (1995).
Article CAS PubMed PubMed Central Google Scholar - Parra, B. et al. IFN-γ is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 162, 1641–1647 (1999).
CAS PubMed Google Scholar - Parra, B. et al. Contributions of Fas–Fas ligand interactions to the pathogenesis of mouse hepatitis virus in the central nervous system. J. Virol. 74, 2447–2450 (2000).
Article CAS PubMed PubMed Central Google Scholar - Marten, N. W. et al. Contributions of CD8+ T cells and viral spread to demyelinating disease. J. Immunol. 164, 4080–4088 (2000).
Article CAS PubMed Google Scholar - Marten, N. W. et al. Selection of CD8+ T cells with highly focused specificity during viral persistence in the central nervous system. J. Immunol. 162, 3905–3914 (1999).
CAS PubMed Google Scholar - Bergmann, C. C., Altman, J. D., Hinton, D. & Stohlman, S. A. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 163, 3379–3387 (1999).
CAS PubMed Google Scholar - Ramakrishna, C., Stohlman, S. A., Atkinson, R. D., Shlomchik, M. J. & Bergmann, C. C. Mechanisms of central nervous system viral persistence: the critical role of antibody and B cells. J. Immunol. 168, 1204–1211 (2002). A report showing that B cells are required to prevent renewed replication of coronavirus in glial cells after initial T-cell-mediated control of replication.
Article CAS PubMed Google Scholar - Morales, S., Parra, B., Ramakrishna, C., Blau, D. M. & Stohlman, S. A. B-cell-mediated lysis of cells infected with the neurotropic JHM strain of mouse hepatitis virus. Virology 286, 160–167 (2001).
Article CAS PubMed Google Scholar
Acknowledgements
Work in my laboratory was supported by a research grant from the National Institutes of Health.
Author information
Authors and Affiliations
- W. Harry Feinstone Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Baltimore, 21205, Maryland, USA
Diane E. Griffin
Authors
- Diane E. Griffin
You can also search for this author inPubMed Google Scholar
Related links
Glossary
BLOOD–BRAIN BARRIER
This is formed by endothelial tight junctions that limit entry of leukocytes, immunoglobulins, cytokines and complement proteins into the central nervous system.
TYPE 1 INTERFERON
(Type 1 IFN). IFN-α and IFN-β are induced by virus replication and are important early inducers of anti-virus immune responses. They limit virus replication before the antigen-specific immune response.
DELAYED-TYPE HYPERSENSITIVITY
A cellular immune response to antigen that develops over 24–72 hours with the infiltration of T cells and monocytes, and is dependent on the production of T helper 1-type cytokines.
T HELPER 1/2 CELLS
(TH1/2). At least two distinct subsets of activated CD4+ T cells have been described. TH1 cells produce interferon-γ, lymphotoxin and tumour-necrosis factor, and support cell-mediated immunity. TH2 cells produce interleukin-4 (IL-4), IL-5 and IL-13, support humoral immunity and downregulate TH1-cell responses.
GANGLIOSIDES
Sialic acid-containing glycosphingolipids that are constituents of plasma membranes. They are enriched in the brain and can be shed from the cell surface as regulatory molecules.
Rights and permissions
About this article
Cite this article
Griffin, D. Immune responses to RNA-virus infections of the CNS.Nat Rev Immunol 3, 493–502 (2003). https://doi.org/10.1038/nri1105
- Issue Date: 01 June 2003
- DOI: https://doi.org/10.1038/nri1105