Structure and activity of an aminoacyl-tRNA synthetase that charges tRNA with nitro-tryptophan (original) (raw)
- Brief Communication
- Published: 20 February 2005
Nature Structural & Molecular Biology volume 12, pages 274–275 (2005)Cite this article
- 669 Accesses
- 30 Citations
- Metrics details
Abstract
The most divergent of two tryptophanyl tRNA synthetases (TrpRS II) found in Deinococcus radiodurans interacts with a nitric oxide synthase protein that produces 4-nitro-tryptophan (4-NRP). TrpRS II efficiently charges transfer RNATrp with 4-NRP and 5-hydroxy-tryptophan (5-HRP). The crystal structures of TrpRS II bound to tryptophan and 5-HRP reveal residue substitutions that accommodate modified indoles. A class of auxiliary bacterial TrpRSs conserve this capacity to charge tRNA with nonstandard amino acids.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
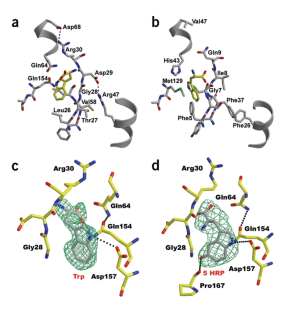
Figure 1: Comparison of tryptophan and 5-HRP recognition by drTrpRS II and bsTrpRS (PDB entry 1MB2).
Similar content being viewed by others
Accession codes
Accessions
Protein Data Bank
References
- Ibba, M. & Soll, D. Genes Dev. 18, 731–738 (2004).
Article CAS Google Scholar - Blight, S.K. et al. Nature 431, 333–335 (2004).
Article CAS Google Scholar - Lovato, M.A., Chihade, J.W. & Schimmel, P. EMBO J. 20, 4846–4853 (2001).
Article CAS Google Scholar - Buddha, M.R., Keery, K.M. & Crane, B.R. Proc. Natl. Acad. Sci. USA 101, 15881–15886 (2004).
Article CAS Google Scholar - Buddha, M.R., Tao, T. Parry, R.J. & Crane, B.R. J. Biol. Chem. 279, 49567–49570 (2004).
Article CAS Google Scholar - Kers, J.A. et al. Nature 429, 79–82 (2004).
Article CAS Google Scholar - Retailleau, P. et al. J. Mol. Biol. 325, 39–63 (2003).
Article CAS Google Scholar - Yadong, Y. et al. J. Biol. Chem. 279, 8378–8388 (2004).
Article Google Scholar - Yang, X.L. et al. Proc. Natl. Acad. Sci. USA 100, 15376–15380 (2003).
Article CAS Google Scholar - Praetorius-Ibba, M. et al. Biochemistry 39, 13136–13143 (2000).
Article CAS Google Scholar - Zhang, Z. et al. Proc. Natl. Acad. Sci. USA 101, 8882–8887 (2004).
Article CAS Google Scholar - Senear, D.F. et al. Anal. Biochem. 300, 77–86 (2002).
Article CAS Google Scholar - Nomanbhoy, T.K., Hendrickson, T.L. & Schimmel, P. Mol. Cell 4, 519–528 (1999).
Article CAS Google Scholar
Acknowledgements
We thank R.J. Parry and T. Tao for providing synthetic 4-NRP, A.M. Bilwes for help with refinement, and Cornell High Energy Synchrotron Source and the National Synchrotron Light Source for access to data collection facilities. Support from the US National Science Foundation and the US National Institutes of Health is gratefully achnowledged.
Author information
Authors and Affiliations
- Department of Chemistry and Chemical Biology, Cornell University, Ithaca, 14850, New York, USA
Madhavan R Buddha & Brian R Crane
Authors
- Madhavan R Buddha
- Brian R Crane
Corresponding author
Correspondence toBrian R Crane.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Buddha, M., Crane, B. Structure and activity of an aminoacyl-tRNA synthetase that charges tRNA with nitro-tryptophan.Nat Struct Mol Biol 12, 274–275 (2005). https://doi.org/10.1038/nsmb907
- Received: 13 December 2004
- Accepted: 01 February 2005
- Published: 20 February 2005
- Issue Date: 01 March 2005
- DOI: https://doi.org/10.1038/nsmb907