Dietary constraints can preclude the expression of an honest chemical sexual signal (original) (raw)
Introduction
The ‘handicap paradigm’ proposes that individuals that are able to afford the costs associated with the elaboration and maintenance of secondary sexual characters are favoured by selection because these individuals display honest signals that truly convey useful information on the quality of bearers to the receivers (competitors and potential mates)1, 2. In this context, the way in which ecological factors contribute to the expression of these honest signals is a subject of continued research. However, despite the fundamental role that chemical signalling plays in sexual selection and the diversification of many species3, 4, this question has mainly been examined in visual traits5, 6.
The foregoing reviews highlight lizards and their chemical signalling as an insightful model system to improve the knowledge on social and sexual animal interactions7,[8](#ref-CR8 "Martín, J. & López, P. Condition-dependent chemosignals in reproductive behavior of lizards. Horm. Behav. 68, 14–24, doi: 10.1016/j.yhbeh.2014.06.009
(2015)."),[9](/articles/s41598-017-06323-8#ref-CR9 "Weldon, P. J., Flachsbarth, B. & Schulz, S. Natural products from the integument of nonavian reptiles. Nat. Prod. Rep.
25, 738–756 (2008).") because the different structures (_i.e_., composition) of scents usually determines the response of receivers and also the reproductive and survivorship success of the emitter (see examples in ref. [8](/articles/s41598-017-06323-8#ref-CR8 "Martín, J. & López, P. Condition-dependent chemosignals in reproductive behavior of lizards. Horm. Behav.
68, 14–24, doi:
10.1016/j.yhbeh.2014.06.009
(2015).")). Most of the evidence in this regard comes from studies that analyse the scents produced by femoral and precloacal glands, typically denoted as ‘chemical secretions’[10](/articles/s41598-017-06323-8#ref-CR10 "Martín, J. & López, P. In Reproductive Biology and Phylogeny of Lizards and Tuatara (eds Rheubert, J.L. Siegel, D.S. & S.E. Trauth) 43-75 (CRC Press, Boca Raton, Florida, 2014)."), [11](/articles/s41598-017-06323-8#ref-CR11 "Mayerl, C., Baeckens, S. & Van Damme, R. Evolution and role of the follicular epidermal gland system in non-ophidian squamates. Amphibia-Reptilia
36, 185–206 (2015)."). The qualitative and quantitative composition of the lipophilic fraction of these chemical secretions differ among individuals, populations and species[12](#ref-CR12 "García-Roa, R., Cabido, C., López, P. & Martín, J. Interspecific differences in chemical composition of femoral gland secretions between two closely related wall lizard species, Podarcis bocagei and Podarcis carbonelli. Biochem. Syst. Ecol.
64, 105–110 (2016)."),[13](#ref-CR13 "García-Roa, R., Carreira, S., López, P. & Martín, J. Genders matters: Sexual differences in chemical signals of Liolaemus wiegmannii lizards (Iguania, Liolaemidae). Biochem. Syst. Ecol.
69, 108–114 (2016)."),[14](/articles/s41598-017-06323-8#ref-CR14 "Runemark, A., Gabirot, M. & Svensson, E. Population divergence in chemical signals and the potential for premating isolation between islet‐and mainland populations of the Skyros wall lizard (Podarcis gaigeae). J. Evol. Biol.
24, 795–809 (2011)."), and this variation is used by lizards in social and sexual interactions to assess individual differences in many traits[15](#ref-CR15 "López, P. & Martín, J. Male Iberian rock lizards may reduce the costs of fighting by scent matching of the resource holders. Behav. Ecol. Soc.
65, 1891–1898 (2011)."),[16](#ref-CR16 "Martín, J., Moreira, P. & López, P. Status‐signalling chemical badges in male Iberian rock lizards. Funct. Ecol.
21, 568–576 (2007)."),[17](/articles/s41598-017-06323-8#ref-CR17 "Carazo, P., Font, E. & Desfilis, E. Beyond ‘nasty neighbours’ and ‘dear enemies’? Individual recognition by scent marks in a lizard (Podarcis hispanica). Anim. Behav.
76, 1953–1963 (2008)."). The composition of the chemical secretions seems to be highly dependent on physiological processes and endocrine regulation[8](/articles/s41598-017-06323-8#ref-CR8 "Martín, J. & López, P. Condition-dependent chemosignals in reproductive behavior of lizards. Horm. Behav.
68, 14–24, doi:
10.1016/j.yhbeh.2014.06.009
(2015).") that occur while individuals are under biotic (_e.g_., trophic resources[18](/articles/s41598-017-06323-8#ref-CR18 "Henneken, J., Goodger, J. Q., Jones, T. M. & Elgar, M. A. Diet-Mediated Pheromones and Signature Mixtures can Enforce Signal Reliability. Front. Ecol. Evol.
4, 145 (2017).")) and abiotic (_e.g_., climatic conditions[19](/articles/s41598-017-06323-8#ref-CR19 "Heathcote, R. J., Bell, E., d’Ettorre, P., While, G. M. & Uller, T. The scent of sun worship: basking experience alters scent mark composition in male lizards. Behav. Ecol. Soc.
68, 861–870 (2014).")) pressures. However, identifying which factors underlie the intra- and interspecific divergences in chemical signalling is still a pending task.In this regard, diet is a major driver of differences in sexual ornamentation because diet is particularly important in the chemical signalling of many organisms18. However, it is still unclear how diet influences the expression of honest chemical signals in lizards. Several compounds with a dietary origin that can act as potentially honest chemical signals, such as vitamin E (α-tocopherol; VE), have been described in the chemical secretions of many lizard species but not in others10. This vitamin is only synthesised by plants or microorganisms and must be acquired by animals through their diet9. VE is a radical scavenger and antioxidant involved in cell membrane defence and with relevant roles in the immune response20, 21. VE deficiency produces neurological disorders and physiological diseases22, 23. However, although VE participates in many metabolic and physiological pathways, lizards of some species allocate large amounts of this vitamin in chemical secretions that are deposited outside of the body. The magnitude of such allocation has been correlated with the quality of males in some species, which may explain the preference of females for the scent of those males24,25,26. Only best-quality males can obtain enough VE in their diet to support physiological functions (for example, to maintain and enhance the immune response24, 26) and, at the same time, allocate high levels of VE to chemical signals. Nevertheless, in addition to a signalling function of male quality, a pre-existing sensory bias in females for a chemosensory search of VE in their food might simply explain why females also preferred chemical secretions of males with higher proportions of VE27, 28.
The Carpetan rock lizard (Iberolacerta cyreni) is a species often used as a model to study chemical ecology in lizards (see refs 7, 9, 11). Although previous analyses have not identified VE in chemical secretions of I. cyreni 16 nor in phylogenetically related species such as I. monticola and I. galani 29, 30, recent research revealed that other closely related species within the same genus (i.e., I. aurelioi, I. bonnali and I. martinezricae) divert large amounts of VE to chemical secretions30. In the present study, we investigated whether a low availability in the diet of VE explained the lack of expression of this vitamin as a chemical signal in male I. cyreni. We experimentally supplemented male lizards with VE in their diet and examined changes in the composition of chemical signals. We hypothesized that the allocation of a particular compound (e.g., VE) in the chemical signal could be restricted if this compound was scarce and the cost associated with its expression, such as the diversion from important metabolic functions, was too high. However, if the costs were relaxed (e.g., when the availability of VE in the diet increased) those compounds could be expressed and retained or acquired a signalling function. Thus, to further assess whether this potential signal might convey honest information, we examined the relationship between signal composition and immunity, as well as the chemosensory responses of females to the scent of males as a measure of the females’ interest.
Results
Analysis of chemical secretions
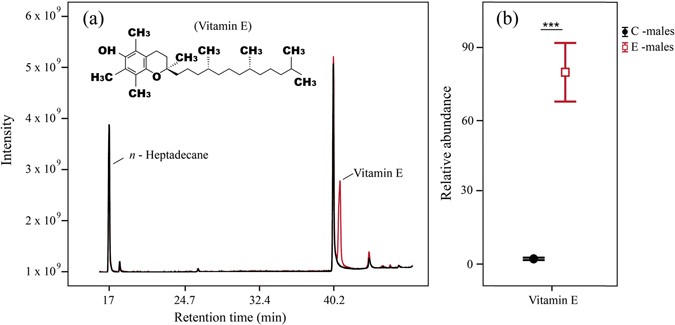
The chemical analyses revealed the presence of VE in secretions of I. cyreni males at the end of the dietary supplementation (Fig. 1a). In control males (C-males), the main classes of compounds found in secretions were steroids (91.76% of TIC), followed by carboxylic acids (2.56%), alcohols (1.67%), aldehydes (1.45%), waxy esters (1.23%), VE (1.21%) and squalene (0.12%). In vitamin E dietary supplemented males (E-males), we also found steroids as the main compounds (69% of TIC). However, we subsequently found VE (28.12%) as the second most abundant compound, followed by waxy esters (0.88%), aldehydes (0.81%), alcohols (0.80%), carboxylic acids and their esters (0.32%) and squalene (0.06%). A significant decrease in the relative TIC proportions of carboxylic acids and their esters (F1,36 = 5.33, P = 0.02) and squalene (F1,36 = 5.37, P = 0.02) was detected in supplemented E-males, whereas the proportion of VE was significantly higher in secretions of E-males than in C-males (F1,36 = 77.78, P < 0.001). However, we did not find significant differences between treatments in proportions of steroids (F1,36 = 1.04, P = 0.31), alcohols (F1,36 = 3.94, P = 0.054), waxy esters (F1,36 = 0.51, P = 0.47) or aldehydes (F1,36 = 1.68, P = 0.20).
Figure 1
Chromatograms from femoral secretions of Iberolacerta cyreni male lizards. (a) Comparison of chromatograms from two different treatments: ‘C-males’ (black) were supplemented with soybean oil, and ‘E-males’ (red) were supplemented with vitamin E. Peaks of interest in this study are the _n_-heptadecane (internal standard; IS) and vitamin E (α-tocopherol), which is especially visible in E-males. (b) Comparison of the relative abundances (mean ± SE) of vitamin E in secretions of C-males and E-males.
Our analyses, using the internal standard to explore potential differences in the relative abundance of VE, confirmed that E-males secreted significantly higher amounts of VE than C-males (79.42 ± 18.22 vs. 1.64 ± 0.36 respectively; F1,36 = 236.45, P < 0.001) (Fig. 1b). We also found small amounts of VE in C-males (1.21%), probably due to the VE traces contained in the oil used as a control for supplying these males.
Relationships between chemical signalling and the immune response
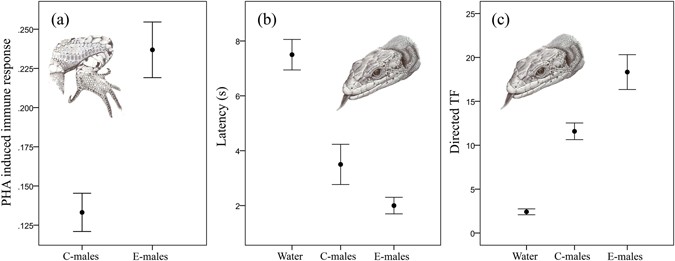
Vitamin E supplementation affected the immune response of lizards, with E-males having significantly higher immune responses than C-males (One-way ANOVA; F1,36 = 23.14, P < 0.001) (Fig. 2a). However, within each group of males, there were no significant relationships between the relative abundance of VE and the immune response of each individual (C-males: r2 = 0.05, F1,18 = 0.084, P = 0.77; E-males: r2 = 0.03, F1,18 = 0.45, P = 0.50).
Figure 2
Effect of vitamin E dietary supplementation on the immune response of Iberolacerta cyreni male lizards and chemosensory responses of females to scent of males. (a) Immune response measured in control (C-males) and supplemented (E-males) male lizards. (b) Latencies (mean ± SE in s) and (c) number (mean ± SE) of directed tongue-flicks (TF) elicited by female lizards to swabs with deionised water and scents from femoral secretions of C-males and E-males.
Chemosensory responses of females to the scent of males
In all of the cases, the females responded to the impregnated swabs by tongue flicking (TF). Our results showed significant differences in latency among scent stimuli (repeated measures ANOVA; F2,22 = 20.77, P < 0.001) (Fig. 2b). Responses to swabs with water had significantly longer latencies than those to swabs with males’ scent (Tukey´s test: P < 0.0001 in both cases), but there were no significant differences between latencies to secretions of E-males and C-males (P = 0.11). The overall rate of TF to swabs significantly differed among the types of stimuli (repeated measures ANOVA; F2,22 = 118.13, P < 0.001) (Fig. 2c). Swabs with water triggered significantly lower TF rates than those with secretions of any male (Tukey´s test: P < 0.0001 in both cases). Moreover, swabs with secretions of E-males received significantly higher TF responses than swabs with secretions of C-males (P = 0.01).
Discussion
The present study showed that the expression of an honest chemical sexual signal could be significantly affected by its dietary availability to the point of precluding its entire expression in all of the individuals of a population or species, which probably explained the absence of VE in femoral secretions of wild I. cyreni 10, 16. However, our experiment also revealed that increasing the availability of VE in the diet drove metabolic changes that enhanced the immune responses and allowed the expression of this vitamin in chemical secretions of I. cyreni. The dietary supplementation modified the composition of male chemical secretions, with a notable increase in VE, which probably explained why females showed a higher chemosensory response to chemical secretions of supplemented males, which could indicate a higher interest of females for the scent of these males9, 10.
The role of some chemical signals in social and sexual behaviour has been supported for several lizard species, especially for those signals believed to be honest, such as VE23,24,25,26. However, the reason why these compounds have been found in some species and not in others has been unclear. In this experiment, we detected VE in chemical secretions of I. cyreni for the first time, but only after males received a dietary supplementation of this vitamin. Therefore, a dietary constraint in the natural populations could explain why this vitamin had not been detected before in wild individuals10, 16. The absence of particular compounds in chemical secretions of some lizard species should not lead to the conclusion that these species are unable to express these compounds. As in the case of I. cyreni and VE, striking divergences in chemical profiles of scents might be unleashed by ecological factors rather than phylogenetic differences per se. Namely, due to the antioxidant and immunostimulatory properties of VE24, 31, lizards must trade-off between the associated costs and benefits of diverting this vitamin from metabolism to chemical secretions. The result of such trade-off is indicative of male quality25, 26, 31. In addition, lizards mainly thermoregulate by exposing themselves to solar radiation, whose ultraviolet fraction has oxidative effects. At high elevations, where thermal energy availability is low, lizards increase their basking activity32. Therefore, these lizards would need high amounts of this antioxidant vitamin in metabolism pathways21, 33, which might force many species such as I. cyreni to reduce VE in chemical secretions. The VE supplementation could have balanced this physiological trade-off, allowing individuals to allocate higher amounts of VE in chemical secretions without detrimental effects for their metabolism. This hypothesis may also be applicable to closely related species (i.e., I. galani and I. monticola) in which VE has not been found in chemical secretions. However, Iberolacerta lizard species from the Pyrenees (I. aranica, I. aurelioi and I. bonnali) do notably show this vitamin in their chemical secretions. This fact might be partially explained by interspecific potential differences in VE availability in their diets. In this respect, further research is needed to establish the relative role of biotic (e.g., diet, parasitism, predation) and abiotic (e.g., climate, microhabitat) factors in determining VE secretion patterns among species.
The levels of secretion of VE in chemical signals are determinant in intersexual interactions of several lizard species because such levels are correlated with male quality[8](/articles/s41598-017-06323-8#ref-CR8 "Martín, J. & López, P. Condition-dependent chemosignals in reproductive behavior of lizards. Horm. Behav. 68, 14–24, doi: 10.1016/j.yhbeh.2014.06.009
(2015)."). Our study supports the hypothesis that a higher availability of VE in the diet allows individuals to divert more amounts of this vitamin both for metabolism processes, which in turn increases the immune response, and for chemical signalling. Interestingly, although VE has not been previously detected in scents of wild male _I. cyreni_, females elicited higher TF rates to secretions of supplemented males. Although the proportion of other compounds (fatty acids and squalene) also changed after the supplementation, the already low proportions of these compounds decreased even more. Thus, it is unlikely that the enhanced responses of females were explained by compounds that decreased slightly in relative abundance, leaving the high proportions of VE as the most likely candidate to explain the increased chemosensory responses. This hypothesis is in line with the hypothesis that VE may function as an honest chemical sexual signal[24](#ref-CR24 "Kopena, R., López, P. & Martín, J. What are carotenoids signaling? Immunostimulatory effects of dietary vitamin E, but not of carotenoids, in Iberian green lizards. Naturwissenschaften
101, 1107–1114 (2014)."),[25](#ref-CR25 "Kopena, R., Martín, J., López, P. & Herczeg, G. Vitamin E supplementation increases the attractiveness of males’ scent for female European green lizards. PLoS One
6, e19410 (2011)."),[26](/articles/s41598-017-06323-8#ref-CR26 "Martín, J. & López, P. Multimodal sexual signals in male ocellated lizards Lacerta lepida: vitamin E in scent and green coloration may signal male quality in different sensory channels. Naturwissenschaften
97, 545–553 (2010).") in this species too. Nevertheless, because VE is needed in metabolism and must be acquired from dietary sources, female preferences could be sensory biased. Similarly, behavioural experiments performed with this species showed that hungry females increased chemosensory responses to stimuli from both invertebrate prey and femoral secretions of males, suggesting that cholesta-5,7-dien-3-ol (provitamin D3) may be one of the compounds potentially responsible for eliciting these higher chemosensory responses[34](/articles/s41598-017-06323-8#ref-CR34 "Martín, J. & López, P. Links between male quality, male chemical signals, and female mate choice in Iberian rock lizards. Funct. Ecol.
20, 1087–1096 (2006).").We conclude that if the costs associated with the allocation of some specific compounds in a chemical signal are too high, their presence in the signal could be restricted or even precluded entirely. Dietary constraints can contribute to increasing these costs. However, when those costs are relaxed, the compound may be expressed in the scent and even function as an honest sexual signal.
Material and Methods
Field work, lizard care and maintenance
The Carpetan rock lizard (I. cyreni) is a medium sized diurnal lacertid lizard that inhabits rocky highlands (above 1700 m elevation) along the Sistema Central Spanish Mountains, where this species is endemic. We collected live adult male (n = 38) and female (n = 13) lizards at ‘Alto del Telégrafo’ (40° 47ʹ N, 04° 00ʹ W, Sierra de Guadarrama, Madrid, Spain) during the first week of May 2015. Capture of lizards was carried out by noosing. Then, we transferred lizards to “El Ventorrillo” field station of the Museo Nacional de Ciencias Naturales (5 km from the capture area). During the study, we individually housed lizards in outdoor 51 × 36 × 28 cm PVC terraria with coconut fibre as substratum, rocks for cover and water ad libitum. We fed lizards with mealworm larvae (Tenebrio molitor) and house crickets (Acheta domesticus) dusted with calcium powder. We released all animals at their capture sites at the end of the experiment (approx. five weeks after capture). All experimental methods were performed in concordance of the Environmental Agency of Madrid Government (“Consejería de Medio Ambiente de la Comunidad de Madrid”, Spain), being reviewed and approved by the Animal Ethics Committee of the Museo Nacional de Ciencias Naturales (CSIC).
Vitamin E supplementation
We randomly assigned males to two treatments, C-males (n = 19) and E-males (n = 19). Males in both treatments were of similar body size (snout-to-vent length, mean ± SE, C-males: 66 ± 1 mm, E-males: 67 ± 1 mm; One-way ANOVA; F1,36 = 1.23, P = 0.27). We administered to E-males a dietary dose of 5 μL of vitamin E supplement (synthetic (±)-α-tocopherol; purchased from Sigma-Aldrich Chemicals Co.) every two days, during a period of 30 days. The vitamin supplement mainly contained synthetic vitamin E (97%; approx. 1014 IU mL−1) and soybean oil (3%; approx. 0.32 IU mL−1 of natural vitamin E, i.e., D-α-tocopherol). Therefore, we provided E-males with approximately 5.05 IU of vitamin E per dose, which is below the tolerable levels of ingestion35, 36. C-males were provided with 5 μL of soybean oil alone, once every two days, during an interval of 30 days. We used sterile plastic syringes with a cannula to slowly deliver the solution into the mouth of lizards to ensure that all of the individuals received the entire dose. After the supplementation period, the femoral gland secretions of each male were collected and preserved at −20 °C in glass vials closed with Teflon-lined stoppers. We also obtained blank control vials using the same procedure without collecting secretions. We conducted with these blank vials the same collection and analytical methodology to compare with vials that carried secretions to exclude potential contaminants.
Chemical analyses of chemical secretions
The secretion of each male was weighed using a XP2U ultra-microbalance (±0.1 μg) in a room at controlled temperature (20 °C). All of the laboratory supplies used for weighing were cleaned with _n_-hexane (95%) before and after the weighing of the secretions. We prepared a solution of 5 ppm of _n_-heptadecane in _n_-hexane to be used as an internal standard. Then, 1 μL of this solution was added per 20 μg of femoral secretion, and the mixture was preserved again in a new glass vial. The mixture was vortex-mixed for two minutes and left in the fridge for the precipitation of solid particles for five minutes. Subsequently, the liquid phase was collected and transferred to a total recovery glass vial. The final samples were kept at −20 °C until further analysis.
For sample extract analyses, we used a TRACE GC Ultra gas chromatograph coupled to a TSQ Quantum XLS mass spectrometer settled by a triple quadrupole analyser (Thermo Fisher Scientific Inc., Bremen, Germany) that operated in electron ionisation (EI, −70 eV of electron energy) and scan detection mode. We set the current of the filament to 150 μA. Then, we injected 2 μL of each sample extract in the gas chromatograph using a programmed temperature vaporization (PTV) injector in splitless mode. We used a split flow of 10 mL/min of helium and 2 min of splitless time. In addition, we kept the injector at 250 °C during the transfer phases with a constant septum purge. We also performed a cleaning phase of the injector after the transfer phase, increasing the injector temperature at 14.5 °C/s up to 350 °C and holding at 350 °C for 5 min. Additionally, the split flow was enlarged up to 50 mL/min in the cleaning phase. We used a capillary column HP-5MS (30 m × 0.25 mm i.d., 0.25 μm film thicknesses) purchased from Agilent Technologies (Palo Alto, CA, USA) for the separation with an initial oven temperature of 100 °C (3 min) and posterior increment of 5 °C/min to 300 °C. The final temperature was held for 15 min. We used helium as the carrier gas at a constant flow rate of 0.8 mL/min. The temperature of the transfer line and the MS source were set at 300 °C and 240 °C, respectively.
For the chromatogram analyses, we used the software XcaliburTM 2.1.0.1140 (Thermo Fischer Scientific Inc., San Jose, CA, USA). Then, to identify the chemicals, we first compared their mass spectra in the NIST/EPA/NIH (NIST 02) computerised mass spectral library. Subsequently, we compared the spectra and retention times of compounds with commercial standards (from Sigma– Aldrich Chemical Co.) when these were available. No relevant impurities were found in the solvent and/or the control vial samples. We determined the relative amount of each compound using the percentage of the total ion current (TIC). We studied potential differences in the relative abundances of chemicals between C- and E-males by using general linear models (GLMs) analyses. In addition, once α-tocopherol was identified, its area in the chromatogram was relativized to the area of the internal standard (i.e., _n_-heptadecane), whose concentration was known, as we mentioned above. This procedure allowed confirmation of potential variations in relative abundance of VE regardless of the other components in the chemical secretions.
Immune response
At the end of the dietary supplementation, we assessed the immune response of male lizards by conducting a delayed-type hypersensivity test (phytohemaglutinin injection test; PHA) by means of a subcutaneous injection of a mitogen (0.02 mg of PHA dissolved in 0.02 mL of phosphate-buffered saline, PBS) in the left hindlimb foot pad. We used a pressure-sensitive spessimeter to measure thickness (to the nearest 0.01 mm) at the point of injection before and 24 h after the injection at the marked point. For each point, we made five consecutive measurements and calculated an average value. Repeatability (see ref. 37) of these five measurements was very high (pre-injection: r = 0.9876, F37,152 = 269.30, P > 0.0001; post-injection: r = 0.9875, F37,152 = 274.00, P > 0.0001). Then, we calculated the lizard immune responses, subtracting pre- to post-injection average measures38,39,40,41. It has been suggested that physiological PHA reaction may be a nonspecific complex inflammation related to infiltration of cells, representing both adaptive and innate immunity39, 42. The final swelling may be result of a diverse index of cutaneous immune activity. Additionally, there is some debate on the meaning of a thicker swelling (see ref. 43). However, because of its simplicity, this test is often used in studies on lizards (e.g. refs 44,45,46). Therefore, with the PHA test, we aimed to assess a standardised index of immunocompetence, regardless of the types of immune cells concerned. To assess potential differences in immune responses between males of both treatments, we used a one-way analysis of variance (ANOVA) on log-transformed data.
Chemosensory responses of females to scent of males
Tongue-flicking behaviour has been widely associated with squamate chemoreception47. Lizards and snakes extrude tongues to sample chemicals from the environment and other individuals48, 49. It is thought that differential TF rates are the result of stimuli discrimination. Thus, an increase in the elicited number of TF towards a given scent can be interpreted as a higher ‘interest’ in this particular scent48. At the end of the dietary supplementation, we measured the responses of females to scents (i.e., femoral secretions) from males in the two treatments (C-males and E-males). We compared the TF rates of females to swabs impregnated with the secretions of the two groups of males. These swabs were imbued with approximately the same amount of secretion (2 × 1 mm of waxy secretion from each of two femoral pores of a male), which avoided the problem of females responding to variation in the amount of secretion instead of to differences in chemical composition. We also used water as an odourless control stimulus. All of the females were exposed to the three stimuli (water vs. C-male vs. E-male) in a randomized order. The TF experiments were conducted in outdoor conditions during an interval of three days. We only performed one trial per day with each female to avoid stressing the lizards. Each female could bask at least 2 h before trials. We exposed the secretion-imbued swab to 2 cm anterior to the lizard´s snout. Then, we noted the number of TF directed to the swab along 1 min since the first TF. We also measured the latency, which is the time (s) that females required from the initial exposure of the swab to the first TF. We tested for potential differences in TF rates and latency of females using repeated measures ANOVAs with the type of scent (i.e., water vs. E-males vs. C-males) as a within factor. Differences between pairs of treatments were tested with post hoc Tukey’s tests.
All of the variables were previously log-transformed to ensure normality (Shapiro-Wilk’s test) as well as the homogeneity of variances (Levene’s test) in statistical analyses, which were conducted with R, version 3.3.1, and SPSS 20.0.0.
References
- Zahavi, A. & Zahavi, A. The handicap principle: A missing piece of Darwins puzzle (Oxford University Press, 1999).
- Zahavi, A. Mate selection—a selection for a handicap. J. Theor. Biol. 53, 205–214 (1975).
Article CAS PubMed Google Scholar - Wyatt, T. D. Pheromones and animal behavior: chemical signals and signatures (Cambridge University Press, 2014).
- Weber, M. G., Mitko, L., Eltz, T. & Ramírez, S. R. Macroevolution of perfume signalling in orchid bees. Ecol. Lett. 19, 1314–1323 (2016).
Article PubMed Google Scholar - Maynard Smith, J. & Harper, D. Animal signals: Oxford series in ecology and evolution. Oxf. Univ. Press NY pp. 1–166 (2003).
Google Scholar - Bradbury, J. W. & Vehrencamp, S. L. Principles of animal communication. Second edn (2011).
- Mason, R. T. & Parker, M. R. Social behavior and pheromonal communication in reptiles. J. Comp. Physiol. A 196, 729–749 (2010).
Article CAS Google Scholar - Martín, J. & López, P. Condition-dependent chemosignals in reproductive behavior of lizards. Horm. Behav. 68, 14–24, doi:10.1016/j.yhbeh.2014.06.009 (2015).
Article PubMed Google Scholar - Weldon, P. J., Flachsbarth, B. & Schulz, S. Natural products from the integument of nonavian reptiles. Nat. Prod. Rep. 25, 738–756 (2008).
Article CAS PubMed Google Scholar - Martín, J. & López, P. In Reproductive Biology and Phylogeny of Lizards and Tuatara (eds Rheubert, J.L. Siegel, D.S. & S.E. Trauth) 43-75 (CRC Press, Boca Raton, Florida, 2014).
- Mayerl, C., Baeckens, S. & Van Damme, R. Evolution and role of the follicular epidermal gland system in non-ophidian squamates. Amphibia-Reptilia 36, 185–206 (2015).
Article Google Scholar - García-Roa, R., Cabido, C., López, P. & Martín, J. Interspecific differences in chemical composition of femoral gland secretions between two closely related wall lizard species, Podarcis bocagei and Podarcis carbonelli. Biochem. Syst. Ecol. 64, 105–110 (2016).
Article Google Scholar - García-Roa, R., Carreira, S., López, P. & Martín, J. Genders matters: Sexual differences in chemical signals of Liolaemus wiegmannii lizards (Iguania, Liolaemidae). Biochem. Syst. Ecol. 69, 108–114 (2016).
Article Google Scholar - Runemark, A., Gabirot, M. & Svensson, E. Population divergence in chemical signals and the potential for premating isolation between islet‐and mainland populations of the Skyros wall lizard (Podarcis gaigeae). J. Evol. Biol. 24, 795–809 (2011).
Article CAS PubMed Google Scholar - López, P. & Martín, J. Male Iberian rock lizards may reduce the costs of fighting by scent matching of the resource holders. Behav. Ecol. Soc. 65, 1891–1898 (2011).
Article Google Scholar - Martín, J., Moreira, P. & López, P. Status‐signalling chemical badges in male Iberian rock lizards. Funct. Ecol. 21, 568–576 (2007).
Article Google Scholar - Carazo, P., Font, E. & Desfilis, E. Beyond ‘nasty neighbours’ and ‘dear enemies’? Individual recognition by scent marks in a lizard (Podarcis hispanica). Anim. Behav. 76, 1953–1963 (2008).
Article Google Scholar - Henneken, J., Goodger, J. Q., Jones, T. M. & Elgar, M. A. Diet-Mediated Pheromones and Signature Mixtures can Enforce Signal Reliability. Front. Ecol. Evol. 4, 145 (2017).
Article Google Scholar - Heathcote, R. J., Bell, E., d’Ettorre, P., While, G. M. & Uller, T. The scent of sun worship: basking experience alters scent mark composition in male lizards. Behav. Ecol. Soc. 68, 861–870 (2014).
Article Google Scholar - Traber, M. G. & Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 43, 4–15 (2007).
Article CAS PubMed PubMed Central Google Scholar - Brigelius-Flohe, R. & Traber, M. G. Vitamin E: function and metabolism. FASEB J. 13, 1145–1155 (1999).
CAS PubMed Google Scholar - Dierenfeld, E. S. Vitamin E deficiency in zoo reptiles, birds, and ungulates. J. Zoo Wildl. Med. 3–11 (1989).
- Mardones, P. & Rigotti, A. Cellular mechanisms of vitamin E uptake: relevance in α-tocopherol metabolism and potential implications for disease. J. Nutr. Biochem. 15, 252–260 (2004).
Article CAS PubMed Google Scholar - Kopena, R., López, P. & Martín, J. What are carotenoids signaling? Immunostimulatory effects of dietary vitamin E, but not of carotenoids, in Iberian green lizards. Naturwissenschaften 101, 1107–1114 (2014).
Article ADS CAS PubMed Google Scholar - Kopena, R., Martín, J., López, P. & Herczeg, G. Vitamin E supplementation increases the attractiveness of males’ scent for female European green lizards. PLoS One 6, e19410 (2011).
Article ADS CAS PubMed PubMed Central Google Scholar - Martín, J. & López, P. Multimodal sexual signals in male ocellated lizards Lacerta lepida: vitamin E in scent and green coloration may signal male quality in different sensory channels. Naturwissenschaften 97, 545–553 (2010).
Article ADS PubMed Google Scholar - Ryan, M. J. & Keddy-Hector, A. Directional patterns of female mate choice and the role of sensory biases. Am. Nat. S4–S35 (1992).
- Pellitteri-Rosa, D. et al. Chemical polymorphism in male femoral gland secretions matches polymorphic coloration in common wall lizards (Podarcis muralis). Chemoecology 24, 67–78 (2014).
Article CAS Google Scholar - López, P., Moreira, P. L. & Martín, J. Chemical polymorphism and chemosensory recognition between Iberolacerta monticola lizard color morphs. Chem. Senses 34, 723–731 (2009).
Article PubMed Google Scholar - García‐Roa, R., Jara, M., López, P., Martín, J. & Pincheira‐Donoso, D. Heterogeneous tempo and mode of evolutionary diversification of compounds in lizard chemical signals. Ecology and Evolution 7, 1286–1296 (2017).
Article PubMed PubMed Central Google Scholar - Kopena, R., López, P. & Martín, J. Relative contribution of dietary carotenoids and vitamin E to visual and chemical sexual signals of male Iberian green lizards: an experimental test. Behav. Ecol. Soc. 68, 571–581 (2014).
Article Google Scholar - Gvoždík, L. To heat or to save time? Thermoregulation in the lizard Zootoca vivipara (Squamata: Lacertidae) in different thermal environments along an altitudinal gradient. Can. J. Zool. 80, 479–492 (2002).
Article Google Scholar - Wolf, R., Wolf, D. & Ruocco, V. Vitamin E: the radical protector. J. Eur. Acad. Dermatol. Venereol. 10, 103–117 (1998).
Article CAS PubMed Google Scholar - Martín, J. & López, P. Links between male quality, male chemical signals, and female mate choice in Iberian rock lizards. Funct. Ecol. 20, 1087–1096 (2006).
Article Google Scholar - Divers, S. J. & Mader, D. R. Reptile medicine and surgery (Elsevier Health Sciences, 2005).
- Bender, D. A. Nutritional biochemistry of the vitamins (Cambridge University Press, 2003).
- Lessells, C. & Boag, P. T. Unrepeatable repeatabilities: a common mistake. The Auk 116–121 (1987).
- Smits, J., Bortolotti, G. R. & Tella, J. L. Simplifying the phytohaemagglutinin skin‐testing technique in studies of avian immunocompetence. Funct. Ecol. 13, 567–572 (1999).
Article Google Scholar - Kennedy, M. W. & Nager, R. G. The perils and prospects of using phytohaemagglutinin in evolutionary ecology. Trends Ecol. Evol. 21, 653–655 (2006).
Article PubMed Google Scholar - Lochmiller, R. L., Vestey, M. R. & Boren, J. C. Relationship between protein nutritional status and immunocompetence in northern bobwhite chicks. The Auk, 503–510 (1993).
- Demas, G. E., Zysling, D. A., Beechler, B. R., Muehlenbein, M. P. & French, S. S. Beyond phytohaemagglutinin: assessing vertebrate immune function across ecological contexts. J. Anim. Ecol. 80, 710–730 (2011).
Article PubMed Google Scholar - Martin, L. et al. Phytohemagglutinin‐induced skin swelling in birds: histological support for a classic immunoecological technique. Funct. Ecol. 20, 290–299 (2006).
Article Google Scholar - Elgert, K. D. Immunology: understanding the immune system (John Wiley & Sons, 2009).
- Oppliger, A., Giorgi, M., Conelli, A., Nembrini, M. & John-Alder, H. Effect of testosterone on immunocompetence, parasite load, and metabolism in the common wall lizard (Podarcis muralis). Can. J. Zool. 82, 1713–1719 (2004).
Article CAS Google Scholar - Calsbeek, R., Bonneaud, C. & Smith, T. B. Differential fitness effects of immunocompetence and neighbourhood density in alternative female lizard morphs. J. Anim. Ecol. 77, 103–109 (2008).
Article PubMed Google Scholar - Huyghe, K. et al. Seasonal changes in parasite load and a cellular immune response in a colour polymorphic lizard. Oecologia 163, 867–874 (2010).
Article ADS PubMed Google Scholar - Cooper, W. E. Chemical discrimination by tongue-flicking in lizards: a review with hypotheses on its origin and its ecological and phylogenetic relationships. J. Chem. Ecol. 20, 439–487 (1994).
Article CAS PubMed Google Scholar - Baeckens, S., Van Damme, R. & Cooper, W. E. How phylogeny and foraging ecology drive the level of chemosensory exploration in lizards and snakes. J. Evol. Biol (2016).
- Cooper, W. E. Foraging mode, prey chemical discrimination, and phylogeny in lizards. Anim. Behav. 50, 973–985 (1995).
Article Google Scholar
Acknowledgements
We thank two anonymous reviewers for helpful comments and “El Ventorrillo” MNCN field station for the use of its facilities. We are also grateful to Frutos García García for his drawings of the Fig. 2. RGR benefited from FPI grant BES-2012–054387 from the Ministerio de Ciencia e Innovación (research project MICIIN-CGL2011-24150/BOS). Financial support for lab and field work was provided by the Ministerio de Economía y Competitividad (research projects CGL2011-24150/BOS, CGL2014-53523-P and AGL2012-37201).
Author information
Authors and Affiliations
- Department of Evolutionary Ecology, National Museum of Natural Sciences, Spanish Research Council (MNCN- CSIC), José Gutiérrez Abascal, 2, 28006, Madrid, Spain
Roberto García-Roa, Pilar López & José Martín - Department of Instrumental Analysis and Environmental Chemistry, Spanish Research Council (IQOG- CSIC), Juan de la Cierva, 3, 28006, Madrid, Spain
Jorge Sáiz & Belén Gómara
Authors
- Roberto García-Roa
You can also search for this author inPubMed Google Scholar - Jorge Sáiz
You can also search for this author inPubMed Google Scholar - Belén Gómara
You can also search for this author inPubMed Google Scholar - Pilar López
You can also search for this author inPubMed Google Scholar - José Martín
You can also search for this author inPubMed Google Scholar
Contributions
The study was designed by R.G.R. and J.M., conducted by R.G.R. and P.L. and analysed by R.G.R. and J.M. Chemical analyses were carried out by R.G.R., J.S. and B.G. The article was conceived and written by R.G.R. with input from J.S., B.G., P.L. and J.M.
Corresponding author
Correspondence toRoberto García-Roa.
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
García-Roa, R., Sáiz, J., Gómara, B. et al. Dietary constraints can preclude the expression of an honest chemical sexual signal.Sci Rep 7, 6073 (2017). https://doi.org/10.1038/s41598-017-06323-8
- Received: 13 March 2017
- Accepted: 12 June 2017
- Published: 20 July 2017
- DOI: https://doi.org/10.1038/s41598-017-06323-8