Early and Pre-Clinical Detection of Prion Seeding Activity in Cerebrospinal Fluid of Goats using Real-Time Quaking-Induced Conversion Assay (original) (raw)
Introduction
Transmissible Spongiform Encephalopathies (TSEs) or prion diseases are fatal neurodegenerative diseases that include Creutzfeldt-Jakob disease (CJD) in humans, bovine spongiform encephalopathy (BSE) in cattle, scrapie in sheep and chronic wasting disease (CWD) in cervids. The infectious agent responsible for these diseases, the prion, appears to be composed primarily of an abnormal, misfolded and oligomeric (PrPSc) form of the cellular prion protein (PrPC)1,2. PrPSc induces the polymerization and conformational conversion of PrPC to its infectious form3,4,5 or, in a variety of in vitro conversion assays6, into alternative forms of the prion protein which are, similarly to PrPSc, partially resistant to digestion with proteases (PrPRes). The pathogenic isoform of the prion protein is therefore a marker associated to TSEs which acts as a seed allowing the ultrasensitive detection of PrPSc using in vitro prion amplification reactions such as Real-Time Quaking Induced Conversion (RT-QuIC)7.
The spread of BSE agent to small ruminants is a major issue in the surveillance of TSEs because BSE passage into a new host may change strain properties, make it difficult to recognize the original strain, and increasing the risk of epidemic spread8. Since 2005, two natural BSE cases have been reported in goats9,10. Furthermore, experimental transmissions of classical (C-BSE) and atypical (L-BSE) forms of BSE in goats were also reported11. An important goal to minimize further spreading of small ruminant TSEs is the development of an assay for highly sensitive and intra-vitam detection of prions in infected, but not clinically sick animals. Currently, small ruminant TSEs are definitively diagnosed by post-mortem examinations of brain stem to detect PrPSc [12](/articles/s41598-019-42449-7#ref-CR12 "Andreoletti, O. et al. Protocol for the evaluation of rapid post mortem tests to detect TSE in small ruminants. Scientific Opinion of the Panel on Biological Hazards (Question No EFSA-Q-2007-055). The EFSA Journal 509, https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2007.509
(2007)."). RT-QuIC assays can rapidly detect sub-infectious levels of prion seeding activity and have been used successfully to detect multiple human, cervid, ovine, hamster, and mouse prion strains in a variety of biological tissues, such as skin[13](/articles/s41598-019-42449-7#ref-CR13 "Wang, Z. et al. Early preclinical detection of prions in the skin of prion-infected animals. Nat Commun. 10, 247 (2019)."), cerebrospinal fluid[14](#ref-CR14 "Wilham, J. M. et al. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 6, e100121 (2010)."),[15](#ref-CR15 "Atarashi, R. et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med. 17, 175–178 (2011)."),[16](#ref-CR16 "McGuire, L. I. et al. Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann Neurol. 72, 278–285 (2012)."),[17](#ref-CR17 "Cramm, M. et al. Characteristic CSF prion seeding efficiency in humans with prion diseases. Mol Neurobiol. 51, 396–405 (2015)."),[18](/articles/s41598-019-42449-7#ref-CR18 "Cramm, M. et al. Stability and Reproducibility Underscore Utility of RT-QuIC for Diagnosis of Creutzfeldt-Jakob Disease. Mol Neurobiol. 53, 1896–1904 (2016)."), saliva[19](/articles/s41598-019-42449-7#ref-CR19 "Henderson, D. M. et al. Rapid Antemortem Detection of CWD Prions in Deer Saliva. PLoS One 8, e74377 (2013)."), blood[20](#ref-CR20 "Orrú, C. D. et al. Prion disease blood test using immunoprecipitation and improved quaking-induced conversion. MBio. 2, e00078–11 (2011)."),[21](#ref-CR21 "Vascellari, S. et al. Prion seeding activities of mouse scrapie strains with divergent PrPSc protease sensitivities and amyloid plaque content using RT-QuIC and eQuIC. PLoS One 7, e48969 (2012)."),[22](#ref-CR22 "Elder, A. M. et al. In vitro detection of prionemia in TSE-infected cervids and hamsters. PLoS One 8, e80203 (2013)."),[23](/articles/s41598-019-42449-7#ref-CR23 "Kramm, C. et al. Detection of Prions in Blood of Cervids at the Asymptomatic Stage of Chronic Wasting Disease. Sci Rep. 7, 17241 (2017)."), and nasal brushings[24](/articles/s41598-019-42449-7#ref-CR24 "Orrú, C. D. et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med. 371, 519–529 (2014)."), showing that this test has the potential of being used for _ante-mortem_ TSE diagnosis. Important studies on human cerebrospinal fluid from patients with sporadic Creutzfeldt-Jakob disease (sCJD) have shown that RT-QuIC has the potential to improve _ante-mortem_ diagnosis for this disease, with a high degree of specificity[15](#ref-CR15 "Atarashi, R. et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med. 17, 175–178 (2011)."),[16](#ref-CR16 "McGuire, L. I. et al. Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann Neurol. 72, 278–285 (2012)."),[17](/articles/s41598-019-42449-7#ref-CR17 "Cramm, M. et al. Characteristic CSF prion seeding efficiency in humans with prion diseases. Mol Neurobiol. 51, 396–405 (2015)."),[25](#ref-CR25 "Orrú, C. D. et al. Rapid and Sensitive RT-QuIC Detection of Human Creutzfeldt-Jakob disease Using Cerebrospinal Fluid. mBio 6, e02451–14 (2015)."),[26](#ref-CR26 "Foutz, A. et al. Diagnostic and prognostic value of human prion detection in cerebrospinal fluid. Ann Neurol. 81, 79–92 (2017)."),[27](#ref-CR27 "Groveman, B. R. et al. Extended and direct evaluation of RT-QuIC assays for Creutzfeldt-Jakob disease diagnosis. Ann Clin Transl Neurol. 4, 139–144 (2016)."),[28](#ref-CR28 "Franceschini, A. et al. High diagnostic value of second generation CSF RT-QuIC across the wide spectrum of CJD prions. Sci. Rep. 7, 10655 (2017)."),[29](/articles/s41598-019-42449-7#ref-CR29 "Bongianni, M. et al. Diagnosis of Human Prion Disease Using Real-Time Quaking-Induced Conversion Testing of Olfactory Mucosa and Cerebrospinal Fluid Samples. JAMA Neurol 74, 155–162 (2017)."). Results obtained by several laboratories with the first generation of this assay (referred to here as previous QuIC-CSF \[PQ-CSF\]), mainly using full-length (23–231) hamster recombinant PrP (rPrPSen) as the substrate, demonstrated a very high specificity but a suboptimal sensitivity, especially in sCJD subtypes associated with PrPSc type 2[30](/articles/s41598-019-42449-7#ref-CR30 "McGuire, L. I. et al. Cerebrospinal fluid real‐time quaking‐induced conversion is a robust and reliable test for sporadic creutzfeldt–jakob disease: An international study. Ann Neurol. 80, 160–165 (2016)."),[31](/articles/s41598-019-42449-7#ref-CR31 "Lattanzio, F. et al. Prion-specific and surrogate CSF biomarkers in Creutzfeldt-Jakob disease: diagnostic accuracy in relation to molecular subtypes and analysis of neuropathological correlates of p-tau and Aβ42 levels. Acta Neuropathol. 133, 559–578 (2017)."). However, Orrù _et al_.[25](/articles/s41598-019-42449-7#ref-CR25 "Orrú, C. D. et al. Rapid and Sensitive RT-QuIC Detection of Human Creutzfeldt-Jakob disease Using Cerebrospinal Fluid. mBio 6, e02451–14 (2015).") recently introduced an improved, second generation RT-QuIC for CSF (referred to here as improved QuIC-CSF \[IQ-CSF\]) assay which uses a truncated form of hamster rPrPSen (amino acids 90–231) as the substrate, higher incubation temperatures, and the addition of SDS. Initial evaluation of the IQ-CSF assay indicated greater analytical and diagnostic sensitivity, and markedly shorter testing times[25](#ref-CR25 "Orrú, C. D. et al. Rapid and Sensitive RT-QuIC Detection of Human Creutzfeldt-Jakob disease Using Cerebrospinal Fluid. mBio 6, e02451–14 (2015)."),[26](#ref-CR26 "Foutz, A. et al. Diagnostic and prognostic value of human prion detection in cerebrospinal fluid. Ann Neurol. 81, 79–92 (2017)."),[27](#ref-CR27 "Groveman, B. R. et al. Extended and direct evaluation of RT-QuIC assays for Creutzfeldt-Jakob disease diagnosis. Ann Clin Transl Neurol. 4, 139–144 (2016)."),[28](#ref-CR28 "Franceschini, A. et al. High diagnostic value of second generation CSF RT-QuIC across the wide spectrum of CJD prions. Sci. Rep. 7, 10655 (2017)."),[29](/articles/s41598-019-42449-7#ref-CR29 "Bongianni, M. et al. Diagnosis of Human Prion Disease Using Real-Time Quaking-Induced Conversion Testing of Olfactory Mucosa and Cerebrospinal Fluid Samples. JAMA Neurol 74, 155–162 (2017).").Studies have demonstrated that CSF tests that measure alterations in total PrP levels and prion seeding activity by RT-QuIC, may be useful in the identification of pre-clinical prion cases20,32. Still, it remains important to understand the time course of prion seeding activity accumulation in cerebrospinal fluid of prion-infected small ruminants in order to develop an early, sensitive, and specific test for ante-mortem and pre-clinical detection of PrPSc. Furthermore, to date there is no information on experimental conditions to amplify goat BSE prion strains by RT-QuIC. Moreover, IQ-CSF conditions have not been previously applied to animal CSF samples. Here, we report for the first time sensitive detection of different small ruminant prion strains in brain and CSF of TSE-infected goats by RT-QuIC assays. Furthermore, intra-vitam detection of preclinical L-BSE-infected goats at early time points is also described. Taken together these data are indicative of the great potential of this in vitro prion amplification assay as ante-mortem TSE test for live and asymptomatic small ruminants.
Results
RT-QuIC seeding activity in brains from natural scrapie and experimental C-BSE- and L-BSE-infected goats
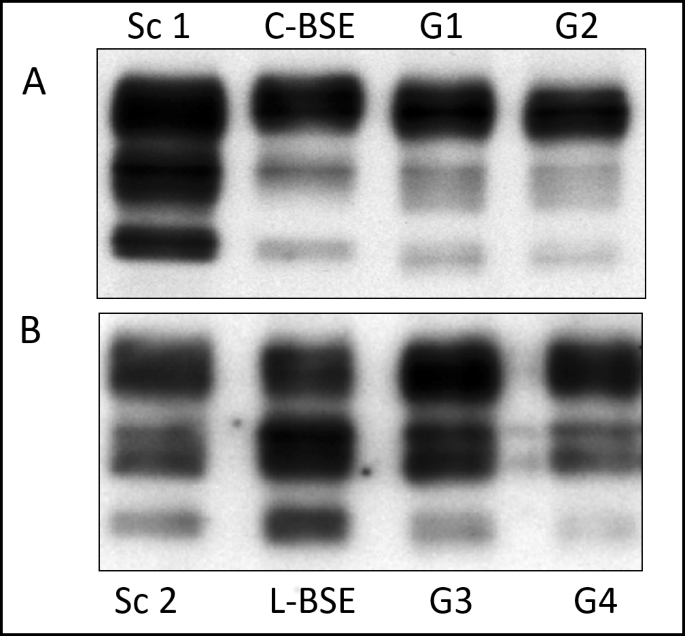
To test the presence of PrPSc before proceeding to subsequent RT-QuIC analyses, Western Blot (WB) analyses were carried out on brainstem collected from experimental C-BSE and L-BSE infected goats (Table 1), recently characterized in our work by Vallino Costassa et al.11. Brain tissue from cattle C-BSE and L-BSE samples, used for the inoculum, and previously confirmed classical scrapie goats served as controls. Western Blot analyses revealed strong immunosignals related to the presence of pathological prion protein in all analysed samples (Fig. 1).
Table 1 Study Populations.
Figure 1
Western blot analysis of brainstem of goats inoculated with C- or L-Type BSE. (A) PrPSc was extracted from the brainstem of two representative goats inoculated with C-type BSE (G1, G2). Brain tissue from cattle C-BSE, used for the inoculum, and previously confirmed classical scrapie goat (Sc 1) served as positive controls. (B) PrPSc was extracted from the brainstem of two representative goats inoculated with L-type BSE (G3, G4). Brain tissue from cattle L-BSE, used for the inoculum, and previously confirmed classical scrapie goats (Sc 2) served as controls. All samples were treated with Proteinase K. Applied tissue equivalents were 3 mg (C-BSE goats) or 8 mg (L-Type BSE goats). Membranes were probed with mAb SAF 84. Full length blots including negative controls and all goats inoculated with C- or L-type BSE are presented in Supplementary Figs S1 and S2, respectively.
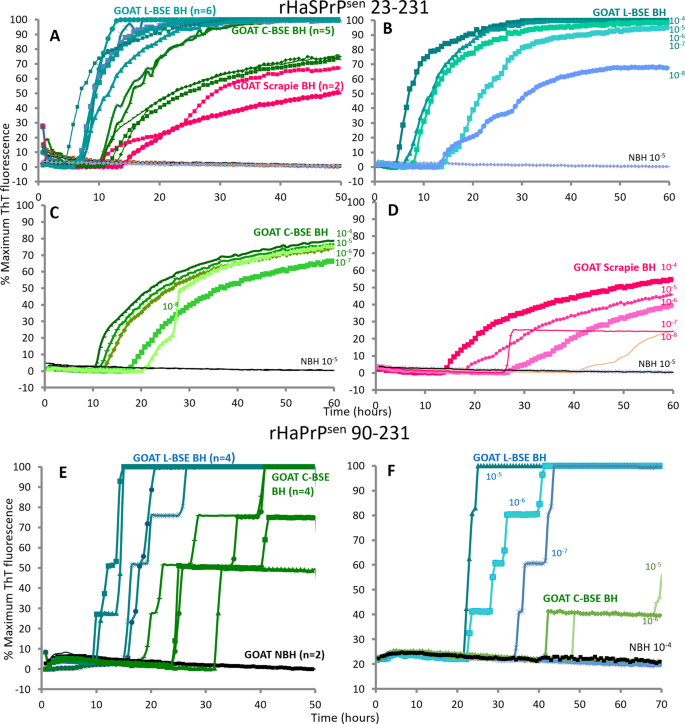
Chimeric hamster-sheep (Ha-S) rPrPSen was first used as substrate to evaluate RT-QuIC for the detection of different goat prion strains seeding activity in brain samples. A rapid increase in ThT fluorescence, indicative of newly formed seeded rPrP amyloid fibrils, was observed in each quadruplicate reaction seeded with 10−4 TSE-infected brain tissue dilutions within 24 h (Fig. 2A). As previously observed for brain homogenate samples from cattle33, the average fluorescence increase was stronger and faster for the L-BSE-infected samples, showing positive signals over the control samples as early as 5 h, compared to the 10–15 h it took for the BSE and scrapie-infected samples. Although the three goat prion strains exhibited distinct RT-QuIC seeded polymerization kinetics, our results show that Ha-S rPrPSen supports detection of goat C-BSE-, L-BSE- and scrapie-associated seeding activity.
Figure 2
RT-QuIC sensitive detection of PrPL-BSE, PrPC-BSE and PrPscrapie from goat brain homogenates. (A) rHaSPrPSen 23–231 substrate was used to detect both PrPL-BSE (azure), PrPC-BSE (green) and PrPscrapie (magenta) from brain homogenates. 10−4 brain tissue dilutions were used to seed quadruplicate RT-QuIC reactions. Normal control brain homogenates (black) showed no response. The number of samples is in parentheses. Representative sensitivity of detection for PrPL-BSE (shades of azure; B) PrPC-BSE (shades of green; C) and PrPscrapie (shades of magenta; D) in brain homogenates (BH) using rHaSPrPSen 23–231 as a substrate. Dilutions are indicated next to the curve. (E) rHaPrPSen 90–231 substrate was used to detect both PrPL-BSE (azure) and PrPC-BSE (green) from brain homogenates. 10−4 brain tissue dilutions were used to seed quadruplicate RT-QuIC reactions. Normal control brain homogenates (black) showed no response. The number of samples is in parentheses. (F) Representative sensitivity of detection for PrPC-BSE (shades of green) and PrPL-BSE (shades of azure) in brain homogenates (BH) using rHaPrPSen 90–231 as a substrate. Dilutions are indicated next to the curve. Similar results were obtained from two additional C-BSE-infected and two additional L-BSE-infected brain specimens (presented in Supplementary Fig. S3). Each ThT reading is indicated as the percentage of the maximum value achievable by the plate readers as a function of reaction time.
Next, RT-QuIC end-point dilution analysis of three representative clinical TSE-infected goat brain homogenates was performed to evaluate the detection sensitivity for each goat prion strain previously tested. We saw positive reactions with all prion strains using infected brain tissue dilutions down to 10−8 (Fig. 2B,D), although reactions seeded with more diluted scrapie samples (10−8) were positive only by 40 h incubation (Fig. 2D). This sensitivity was similar to that reported previously for rodent and human prion samples14. Spontaneous (unseeded) amyloid formation (rPrPspon) was observed in only one of the quadruplicate reactions seeded with normal brain tissue dilutions at 70 h (data not shown). Therefore, we chose 60 h as the reaction cut-off.
In order to explore the prion strain properties of goat C-BSE and L-BSE we tested their relative abilities to initiate polymerization of hamster 90–231 rPrPSen substrate. RT-QuIC reactions were seeded with 10−4 to 10−8 brain tissue dilutions and incubated for 60 h using the same experimental conditions described for Ha-S rPrPSen. Both goat BSE prion strains induced conformational conversion of Ha rPrPSen 90–231 giving the fastest response reactions with the L-BSE seed (Fig. 2E). Conversely to cattle C-BSE33, goat C-BSE was able to seed conversion of hamster 90–231 rPrPSen substrate under these conditions although the sensitivity was limited to less diluted brain tissue homogenates (Fig. 2F).
High diagnostic value of IQ-CSF across the broad spectrum of small ruminant prions
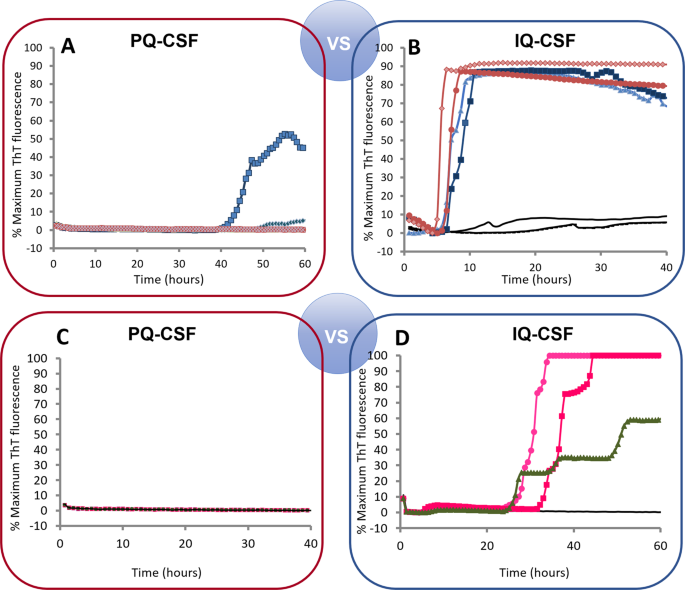
PQ-CSF and IQ-CSF performances were compared in order to develop experimental conditions for ante-mortem detection of prion-infected small ruminants. As previously reported for RT-QuIC-based sCJD diagnosis using CSF samples and N-terminally truncated hamster rPrPSen (90–231), we found initially that when pure goat CSF was analyzed, positive reactions occurred only after 40 h. However, pure CSF specimens lack SDS that was present in the previously studied brain homogenates, so we then applied RT-QuIC using the combination of the rHaPrPSen 90–231 substrate, 55 °C, and 0.002% SDS (referred to here as improved QuIC-CSF [IQ-CSF] conditions) to an initial panel of CSF samples collected from two symptomatic L-type BSE-infected goats and two patients affected with sCJD (compare Fig. 3A,B). Our findings indicated that we can sensitively detect PrPSc associated seeding activity using IQ-CSF in less than 10 h.
Figure 3
Comparison of first (PQ-CSF) and second generation (IQ-CSF) of RT-QuIC assays using rHaPrPSen 90–231. (A,B) Two L-BSE-infected (G3 and G4, blue) and uninfected (black) goat CSF were tested at 55 °C by using truncated rHaPrPSen 90–231 with (right) or without (left) the addition of 0.002% SDS. Two human CSF (red) from patients affected with sCJD were used as positive control. Distinct symbols represent separate samples. (C,D) Next, rHaSPrPSen 90–231 substrate was used to detect both PrPC-BSE (green) and PrPscrapie (magenta) from CSF of 3 symptomatic goats (G2, Sc1 and Sc2) using PQ-CSF (C) or IQ-CSF (D) of RT-QuIC assays.
Next, we also tested the effects of IQ-CSF conditions on CSF-seeded reaction mixtures using each goat prion strain and Ha 90–231 rPrPSen as substrate. Notably, 3 of the 3 samples that did not give positive responses under the PQ-CSF conditions (Fig. 3C), gave strong responses using the IQ-CSF conditions (Fig. 3D), while samples from non-TSE control cases remained negative. These findings provided initial evidence that these new reaction conditions improve the speed and diagnostic sensitivity of the RT-QuIC CSF assay for prion goat diseases while maintaining full specificity.
Early Detection of PrPSc in CSF of asymptomatic L-BSE infected goats by IQ-CSF
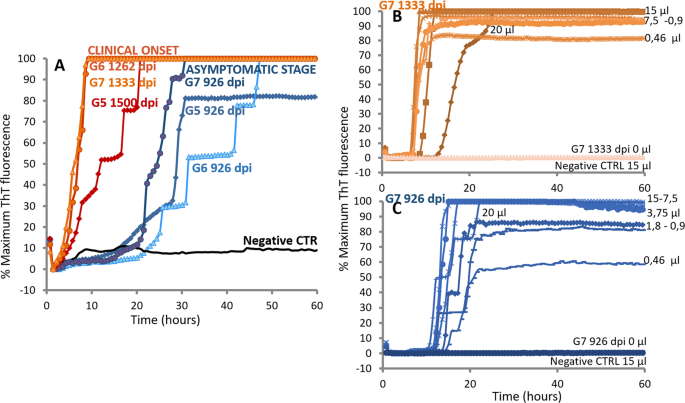
To investigate the potential of RT-QuIC to detect seeding activity at early time points after inoculation we applied IQ-CSF RT-QuIC to goat CSF collected from 3 L-BSE-infected goats during the pre-symptomatic stage (Table 2). Highly efficient detection of PrPL-BSE associated seeding activity was revealed with an average time of 439 days before clinical symptoms appeared (Fig. 4A).
Table 2 Preclinical timepoints at which CSF samples were collected, Survival time (SV), incubation period (IP) and IQ-CSF analysis in L-BSE infected goats.
Figure 4
Detection of pathological prion protein in CSF of L-BSE infected goats at the asymptomatic stage using IQ-CSF assay. (A) PrPSc detection in CSF of three L-BSE infected, but asymptomatic goats (shades of blue). CSF collected at 926 days post inoculum (dpi), and at clinical onset (shades of orange) from the same L-BSE-infected goat were tested at 55 °C by using truncated rHaPrPSen 90–231 with the addition of 0.002% SDS. Distinct symbols represent separate L-BSE samples. (B) analytical sensitivity of one goat L-BSE CSF collected at the clinical onset using IQ-CSF conditions. Reaction mixtures seeded with serial decreasing volume of L-BSE-infected CSF samples (20- to 0.46 µl equivalents of pure CSF) were tested. Distinct symbols represent separate volume of pure CSF seeded. (C) analytical sensitivity of goat L-BSE-infected CSF collected 407 days before clinical symptoms appeared using IQ-CSF conditions. Reaction mixtures seeded with serial decreasing volume of L-BSE-infected CSF samples (20- to 0.46 µl equivalents of pure CSF) were tested. Distinct symbols represent separate volume of pure CSF seeded.
Next, we compared the analytical sensitivities of RT-QuIC using IQ-CSF conditions on CSF samples collected at clinical onset and during pre-symptomatic stage that gave positive reactions in the above-described analyses. Four replicate reactions seeded with serial decreasing volume of 2 L-BSE CSF samples (20- to 0.46 µl equivalents of pure CSF) were tested. These measurements revealed equivalent analytical sensitivity between CSF samples collected at clinical onset and more than one year before clinical symptoms appeared. In each case positive reactions occurred until to 0.46 µl of pure CSF seeded (Fig. 4B,C).
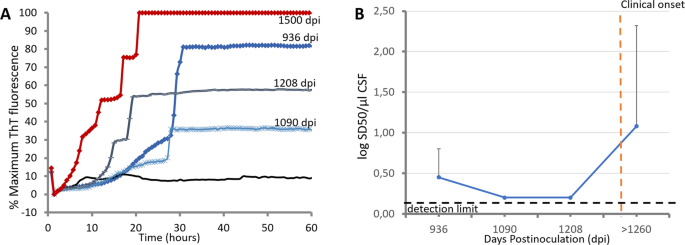
Furthermore, IQ-CSF conditions were used to evaluate time course of prion accumulation in the CSF of L-BSE-infected goats. Prion seeding activities detected in CSF samples collected 926 days post inoculum (dpi), 1080 dpi, 1200 dpi, and at clinical onset are showed in Fig. 5A. To directly compare the amount of PrPSc in each time point before clinical onset, we tested endpoint serial dilutions of these CSF samples collected from 2 L-BSE-infected goats. Finally, using Spearman-Karber analysis of the data, we estimated the dilution of pure CSF required to give 50% positive replicate wells under IQ-CSF conditions (the 50% seeding dose [SD50]). Initial Log SD50 per µl of CSF was measured at 926 dpi with an average value of 0.45. Soon thereafter, seeding activity began a nearly log-linear decrease down to a minimum of 0.2 LogSD50/µl CSF (Fig. 5B). This level of seeding activity was maintained during the remainder of the asymptomatic stage and appeared clearly increased at clinical onset.
Figure 5
Time course of prion seeding activity detected in CSF of L-BSE-infected goats after intracerebral inoculations. (A) Prion seeding activities detected in CSF samples collected at 926 days post inoculum (dpi), 1080 dpi, 1200 dpi, and at clinical onset from L-BSE-infected goat G5. (B) Log SD50 analysis of CSF collected from-L-BSE infected goats (G5, G6 and G7) at different time points of asymptomatic stage as indicated in Table 2. Spearman-Karber analysis of the data was used to calculate the dilution of pure CSF required to give 50% positive replicate wells under IQ-CSF conditions (the 50% seeding dose [SD50]). Data are displayed as the mean + the standard deviation.
Discussion
Identification of scrapie or BSE status in goats can be complicated due to the existence of several polymorphisms in the PRNP coding sequence which can delay the incubation period and PrPSc accumulation34,35,36. A recent study by Madsen-Bouterse et al.37 revealed that the polymorphisms in caprine PRNP can also affect the sensitivity of PrPSc detection in brain samples by anti-prion mAb-based immunoassays such as IHC and Western blot analysis. It therefore becomes important to have a rapid, sensitive, PRNP polymorphism-independent as well as antibody-independent detection test to diagnose goat TSE. A previous study already demonstrated sensitive and specific detection of classical scrapie prions in goat brains with different genotypes by RT-QuIC38 but to date there is no information on experimental conditions to detect BSE prion seeding activity in small ruminants. We showed that RT-QuIC can be applied successfully to a variety of brain samples from experimental and natural TSE-infected goats including scrapie and BSE. Our findings indicate that by using the Ha-S rPrPSen substrate, RT-QuIC assays can sensitively detect both C-BSE- and L-BSE-associated seeding activity in less than 24 h. Furthermore, conversely to cattle C-BSE33, goat C-BSE prion strain was able to seed conversion of hamster 90–231 rPrPSen substrate. These data confirm that RT-QuIC can also be used in prion strain typing, as different strains show slight differences in RT-QuIC response26,33,39,40,41,42.
In fact, one of the main problems related to the management of animal TSEs is the lack of rapid and sensitive tests for ante-mortem diagnosis. In small ruminants TSE is suspected diagnostically on the basis of clinical examination of symptomatic individuals and is confirmed post-mortem by neuropathological analysis and immunochemical detection of PrPSc in the central nervous system[12](/articles/s41598-019-42449-7#ref-CR12 "Andreoletti, O. et al. Protocol for the evaluation of rapid post mortem tests to detect TSE in small ruminants. Scientific Opinion of the Panel on Biological Hazards (Question No EFSA-Q-2007-055). The EFSA Journal 509, https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2007.509
(2007)."). One important limitation to this approach is the sensitivity of the detection system, because amounts of PrPSc high enough to be revealed by conventional methods are only present in the brain at late stages of the disease. However, animal transmission studies show that infectivity is present at a relatively early stage of the incubation period and gradually increases as the disease progresses[43](/articles/s41598-019-42449-7#ref-CR43 "Kimberlin, R. H. & Walker, C. A. Pathogenesis of experimental scrapie; in Bock G, Marsh J (eds): Novel Infectious Agent and the Central Nervous System. Ciba Found. Symp. 135, 37–62 (1988)."),[44](/articles/s41598-019-42449-7#ref-CR44 "Arnold, M. E. et al. Pathogenesis of experimental bovine spongiform encephalopathy (BSE): estimation of tissue infectivity according to incubation period. Vet Res. 40, 8 (2009)."). Our data indicate that RT-QuIC can amplify otherwise undetectable quantities of PrPSc from CSF of symptomatic and asymptomatic goats. Here, we demonstrate, for the first time, that using recombinant hamster 90–231 PrPSen as substrate in combination with IQ-CSF, the diagnostic sensitivity of RT-QuIC was clearly improved in CSF samples of both natural scrapie and experimental BSE infected goats, allowing rapid and specific prion seeded polymerization in less than 40 hours. One great advantage of this improved assay is that using hamster 90–231 rPrPSen substrate which can be universally applied to various animals with different PrP sequences[45](/articles/s41598-019-42449-7#ref-CR45 "Orrù, C. D. et al. Factors That Improve RT-QuIC Detection of Prion Seeding Activity. Viruses. 8, 140 (2016).") could avoid the potential problem of polymorphism in sheep or goat. Furthermore, the longitudinal study on experimental L-BSE goats showed that as early as two years and half after infection, i.e., at two fifths of the incubation time, it is possible to distinguish infected from non-infected animals by IQ-CSF analysis. In particular, highly efficient detection of PrPL-BSE associated seeding activity was revealed respectively 574, 336 and 407 days prior to onset of clinical signs. Also, the titration of seeding activity in Fig. [4C](/articles/s41598-019-42449-7#Fig4) showed very high seeding activity in CSF collected at 926 dpi, which suggests that this assay could be able to detect positive samples much earlier than 926 dpi. Moreover, Spearman-Karber analysis indicated that detectable but lower amount of PrPSc was maintained during the remainder of the asymptomatic stage. Similar time course in brain PrP deposition was observed from pre-clinical and clinical BSE cases reported by Bannach _et al_.[46](/articles/s41598-019-42449-7#ref-CR46 "Bannach, O. et al. Analysis of prion protein aggregates in blood and brain from pre-clinical and clinical BSE cases. Vet Microbiol. 166, 102–8 (2013)."). Collectively, these data provide the first indications of the kinetics of prion accumulation in the CSF of prion-infected goats. The observation that PrP seeding activity can be detected in the CSF during early preclinical stages of the disease is consistent with a previous report showing positive RT-QuIC signal in the CSF of intracerebrally scrapie-infected hamsters before the clinical onset[47](/articles/s41598-019-42449-7#ref-CR47 "Orrù, C. D. et al. Time Course of Prion Seeding Activity in Cerebrospinal Fluid of Scrapie-Infected Hamsters after Intratongue and Intracerebral Inoculations. J Clin Microbiol. 50, 1464–1466 (2012)."). Clearly, the accumulation time course was highly dependent upon the route of prion inoculation. Indeed, the same previous study already demonstrated that after intralingual scrapie inoculation, seeding activity appeared in hamsters CSF only with the onset of clinical signs, well after higher-level accumulation of seeding activity in CSF of intracerebral challenged animals[47](/articles/s41598-019-42449-7#ref-CR47 "Orrù, C. D. et al. Time Course of Prion Seeding Activity in Cerebrospinal Fluid of Scrapie-Infected Hamsters after Intratongue and Intracerebral Inoculations. J Clin Microbiol. 50, 1464–1466 (2012)."). However, data from recent study on sheep CSF of preclinical and clinical naturally occurring scrapie confirmed that alterations in PrP levels and conformation are primary events in the pathology of prion diseases preceding neuronal damage.Scrapie is still widespread, and the only available eradication measure is the genotype-based eradication program. However, this plan only shows effects in years and requires a high level of compliance by farmers, which is not always the case. Although, furthers studies are necessary to assess the ability of RT-QuIC to detect prion seeding activity in biological samples of more diagnostic interest as blood and excreta, the ante-mortem CSF test here described is one of few ante-mortem screening methods now available to detect asymptomatic TSE-infected goats48. It could represent a more rapid and sensitive approach to identify TSE-infected flocks, assaying symptomatics and all genetically susceptible animals. Infected animals could be eliminated at an early stage, with a rapid decrease of the potential environmental infection load. Such a surveillance system will also allow decreases the genetic selection pressure, maintaining some genetic variability in the populations, as desired by farmers.
Overall, our data suggest that CSF prion seeding activity may be related to early prion pathological events and may allow the design of a diagnostic test for preclinical and clinical animals, minimizing the risk of potential exposure to others in the herd.
Materials and Methods
Study Populations and Sample Collection
The analyses with RT-QuIC were carried out on brainstem and CSF samples collected from goats derived from two natural outbreaks of classical scrapie and from caprines intracranially inoculated with 1 ml of a 10% (wt./vol.) brain homogenate from a cow diagnosed with classical (C-BSE) or atypical BSE (L-BSE) as previously described11. The physical and neurological status of the animals was monitored monthly by a board certified veterinary neurologist. For this purpose, a clinical examination protocol, previously used for sheep49 was applied. CSF was collected from the lumbosacral site of each animal, while it was standing, as described by Mayhew et al.50. The animal was sedated with 0.05 mg/Kg xylazine (Rompun ®, Bayer Health care) IV. The collection site (7 × 7 cm) was clipped, surgically prepared and locally anaesthetized with 2.0 ml of procaine hydrochloride (Aticain®, Ati S.r.l.). Disposable 21 G 0.80 mm × 50.00 mm hypodermal needles (Terumo ®) were used to collect 5 mL of CSF by gentle syringe aspiration into a sterile tube (Uridraw, Terumo ®). In the presence of symptomatology related to the TSE, the animals were euthanized with i.v. enbutramide/mebezonium iodide/tetracaine hydrochloride (Tanax®, Intervet Inc. Merck). After euthanasia, CSF, the whole brain, the entire spinal cord and peripheral nervous tissues were sampled. Each sample was frozen at −80 °C.
PrPSc extraction and confirmatory Western blot (WB) analysis
PrPSc extraction and the following WB analyses were carried out on the brainstem samples collected at necropsy from the experimentally challenged goats. Brain tissue from healthy cattle, C-BSE and L-BSE samples, used for the inoculum, and goats previously confirmed positive or negative for classical scrapie were also analyzed as controls. The analyses were performed as previously reported11. PrPSc was detected using SAF84 (mouse; Cayman Chemical Co.; Cat. No. 189775) monoclonal antibody and an anti-mouse antiserum conjugated with alkaline phosphatase. Reaction was revealed by a chemiluminescent substrate and visualized on Hyperfilm ECL (Amersham GE-Healthcare, Life Sciences) or by a gel documentation (UVI Prochemi, Uvitec). Image LabTM analysis software (Bio-Rad) was used to analyse blot images.
Recombinant prion protein purification
Syrian golden hamster residues 90 to 231 (hamster 90–231 or Ha 90–231) and chimeric hamster-sheep (Ha-S; Syrian hamster residues 23 to 137 followed by sheep residues 141 to 234 of the R154Q171 polymorph [accession no. AY907689]) prion protein genes were ligated into the pET41 vector (EMD Biosciences). Escherichia coli carrying this vector was grown in Luria broth (LB) medium in the presence of kanamycin and chloramphenicol. rPrPSen expression was induced using Overnight Express Autoinduction system 1 (Novagen) and Bug Buster master mix (Novagen) to isolate inclusion bodies. Following solubilisation of the inclusion bodies in 8M guanidinium-HCl, the denatured protein was purified under 6M guanidinium-HCl denaturing conditions using nickel nitrilotriacetic acid (Ni-NTA) superflow resin (Qiagen) with an AKTA 25 L protein liquid chromatography instrument (GE-Healthcare, Life Sciences). The rPrPSen was subjected to on-column refolding using a linear gradient into phosphate buffer and then eluted using an imidazole gradient as previously described14. The purified protein was extensively dialyzed into 10 mM sodium phosphate buffer (pH 5.8). Then, following filtration (0.22-μm syringe filter; Sartorius), a concentration measurement by absorbance at 280 nm was performed and the rPrPSen was stored at −80 °C.
Brain homogenates preparation and RT-QuIC protocol
Normal (n = 2), scrapie-infected (n = 2), C-BSE-infected (n = 2), and L-BSE-infected (n = 2) goat brain homogenates (BHs) (10%, wt/vol) were prepared as previously described33 and stored at −80 °C. For RT-QuIC analysis, BHs were serially diluted in 0.1% SDS (Sigma)–N2 (Gibco)–PBS as previously reported14. The RT-QuIC reaction mix was composed of 10 mM phosphate buffer (pH 7.4), 300 mM NaCl, 10 μM ThT, 1 mM EDTA, and 0.1 mg/ml of rPrPSen. Aliquots of this mix (98 μl) were loaded into each well of a black 96-well plate with a clear bottom (Nunc) and seeded with 2 μl of 10−4 to 10−9 brain homogenate dilutions. Normal goat BH dilutions were used as negative controls (as shown in Fig. 2), and 10−4 brain homogenate dilutions from bovine or sheep with clinical BSE or scrapie, respectively, were initially included as positive controls when goat brain samples were tested for the first time. The plate was then sealed with a plate-sealer film (Nalgene Nunc International) and incubated for 70 h at 42 °C in a BMG Labtech FLUOstar plate reader with cycles of 1 min of shaking (700 rpm double orbital) and 1 min of rest throughout the incubation. ThT fluorescence measurements (excitation, 450 ± 10 nm; emission, 480 ± 10 nm; bottom read) were recorded every 45 min. RT-QuIC reactions were deemed acceptable when the negative controls remained negative for at least 50 h.
CSF preparation, PQ-CSF and IQ-CSF protocols
Native CSF samples were centrifuged at 1000 × g for 10 min to remove cell debris, using 5417 R Eppendorf centrifuge (or an equivalent). Supernatant was transferred to new 1.5-ml tubes and stored at −20 °C. IQ-CSF assays were performed as reported previously for sCJD patients25. Briefly, both PQ-CSF and IQ-CSF reactions were run with rPrPSen Ha 90–231 with or without the addition of 0.002% SDS to the reaction mix.
Data analysis
RT-QuIC fluorescence readings were analyzed as previously described33. Briefly, to compensate for differences between the fluorescence plate readers, data sets were normalized to a percentage of the maximal fluorescence response of the instrument, and the obtained values were plotted against the reaction times. Samples were judged to be RT-QuIC positive using criteria similar to those previously described for RT-QuIC analyses of brain specimens14,33. A 60 h time point was chosen based on multiple (n = 20) repeat runs in which no spontaneous conversions of the substrate in negative-control seeded reactions were observed up to the experimentally designated time point. Data are displayed as the average of four technical replicates except where indicated. Where multiple biological replicates are displayed, they are represented as the mean ± the standard deviation.
Ethics Statement
All procedures involving animals and their care were conducted in conformity with national and international laws and policies (EEC Council Directive 86/609, 63/2010; Italian Legislative Decree 116/92 and 26/2014). The study was approved by the Italian Ministry of Health with authorization number 694/2015-PR of 17th of July 2015.
References
- Borchelt, D. R., Scott, M., Taraboulos, A., Stahl, N. & Prusiner, S. B. Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J Cell Biol. 110, 743–752 (1990).
Article CAS Google Scholar - Caughey, B., Raymond, G. J. & Bessen, R. A. Strain-dependent differences in beta-sheet conformations of abnormal prion protein. The Journal of Biological Chemistry 273, 32230–32235 (1998).
Article CAS Google Scholar - Deleault, N. R., Harris, B. T., Rees, J. R. & Supattapone, S. Formation of native prions from minimal components in vitro. PNA 104, 9741–974 (2007).
Article ADS CAS Google Scholar - Kocisko, D. A. et al. Cell-free formation of protease-resistant prion protein. Nature 370, 471–474 (1994).
Article ADS CAS Google Scholar - Saborio, G. P., Permanne, B. & Soto, C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411, 810–813 (2001).
Article ADS CAS Google Scholar - Orrú, C. D. & Caughey, B. Prion Seeded Conversion and Amplification Assays. Topics of Current Chemistry 305, 121–133 (2011).
Article Google Scholar - Atarashi, R. et al. Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nature Methods 5, 211–212 (2008).
Article CAS Google Scholar - Seuberlich, T., Heim, D. & Zurbriggen, A. Atypical transmissible spongiform encephalopathies in ruminants: a challenge for disease surveillance and control. J Vet Diagn Invest 22, 823–842 (2010).
Article Google Scholar - Spiropoulos, J. et al. Isolation of Prion with BSE Properties from Farmed Goat. Emerg Infect Dis. 17, 2253–61 (2011).
Article CAS Google Scholar - Eloit, M. et al. BSE agent signatures in a goat. Vet Rec. 156, 523–524 (2005).
Article Google Scholar - Vallino Costassa, E. et al. Clinical, pathological, and molecular features of classical and L-type atypical-BSE in goats. PLoS One 13, e0198037 (2018).
Article Google Scholar - Andreoletti, O. et al. Protocol for the evaluation of rapid post mortem tests to detect TSE in small ruminants. Scientific Opinion of the Panel on Biological Hazards (Question No EFSA-Q-2007-055). The EFSA Journal 509, https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2007.509 (2007).
- Wang, Z. et al. Early preclinical detection of prions in the skin of prion-infected animals. Nat Commun. 10, 247 (2019).
Article ADS Google Scholar - Wilham, J. M. et al. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 6, e100121 (2010).
Article Google Scholar - Atarashi, R. et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med. 17, 175–178 (2011).
Article CAS Google Scholar - McGuire, L. I. et al. Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann Neurol. 72, 278–285 (2012).
Article Google Scholar - Cramm, M. et al. Characteristic CSF prion seeding efficiency in humans with prion diseases. Mol Neurobiol. 51, 396–405 (2015).
Article CAS Google Scholar - Cramm, M. et al. Stability and Reproducibility Underscore Utility of RT-QuIC for Diagnosis of Creutzfeldt-Jakob Disease. Mol Neurobiol. 53, 1896–1904 (2016).
Article CAS Google Scholar - Henderson, D. M. et al. Rapid Antemortem Detection of CWD Prions in Deer Saliva. PLoS One 8, e74377 (2013).
Article ADS CAS Google Scholar - Orrú, C. D. et al. Prion disease blood test using immunoprecipitation and improved quaking-induced conversion. MBio. 2, e00078–11 (2011).
Article Google Scholar - Vascellari, S. et al. Prion seeding activities of mouse scrapie strains with divergent PrPSc protease sensitivities and amyloid plaque content using RT-QuIC and eQuIC. PLoS One 7, e48969 (2012).
Article ADS CAS Google Scholar - Elder, A. M. et al. In vitro detection of prionemia in TSE-infected cervids and hamsters. PLoS One 8, e80203 (2013).
Article ADS CAS Google Scholar - Kramm, C. et al. Detection of Prions in Blood of Cervids at the Asymptomatic Stage of Chronic Wasting Disease. Sci Rep. 7, 17241 (2017).
Article ADS Google Scholar - Orrú, C. D. et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med. 371, 519–529 (2014).
Article Google Scholar - Orrú, C. D. et al. Rapid and Sensitive RT-QuIC Detection of Human Creutzfeldt-Jakob disease Using Cerebrospinal Fluid. mBio 6, e02451–14 (2015).
Article Google Scholar - Foutz, A. et al. Diagnostic and prognostic value of human prion detection in cerebrospinal fluid. Ann Neurol. 81, 79–92 (2017).
Article CAS Google Scholar - Groveman, B. R. et al. Extended and direct evaluation of RT-QuIC assays for Creutzfeldt-Jakob disease diagnosis. Ann Clin Transl Neurol. 4, 139–144 (2016).
Article Google Scholar - Franceschini, A. et al. High diagnostic value of second generation CSF RT-QuIC across the wide spectrum of CJD prions. Sci. Rep. 7, 10655 (2017).
Article ADS Google Scholar - Bongianni, M. et al. Diagnosis of Human Prion Disease Using Real-Time Quaking-Induced Conversion Testing of Olfactory Mucosa and Cerebrospinal Fluid Samples. JAMA Neurol 74, 155–162 (2017).
Article Google Scholar - McGuire, L. I. et al. Cerebrospinal fluid real‐time quaking‐induced conversion is a robust and reliable test for sporadic creutzfeldt–jakob disease: An international study. Ann Neurol. 80, 160–165 (2016).
Article CAS Google Scholar - Lattanzio, F. et al. Prion-specific and surrogate CSF biomarkers in Creutzfeldt-Jakob disease: diagnostic accuracy in relation to molecular subtypes and analysis of neuropathological correlates of p-tau and Aβ42 levels. Acta Neuropathol. 133, 559–578 (2017).
Article CAS Google Scholar - Llorens, F. et al. Cerebrospinal Fluid Prion Disease Biomarkers in Pre-clinical and Clinical Naturally Occu-rring Scrapie. Mol. Neurobiol. 55, 8586–8591 (2018).
Article CAS Google Scholar - Orrù, C. D. et al. Detection and discrimination of classical and atypical L-type bovine spongiform encephalopathy by real-time quaking-induced conversion. J. Clin. Microbiol. 53, 1115–1120 (2015).
Article Google Scholar - Dassanayake, R. P., White, S. N., Madsen-Bouterse, S. A., Schneider, D. A. & O’Rourke, K. I. Role of the PRNP S127 allele in experimental infection of goats with classical caprine scrapie. Anim Genet. 46, 341 (2015).
Article CAS Google Scholar - Goldmann, W. et al. Caprine prion gene polymorphisms are associated with decreased incidence of classical scrapie in goat herds in the United Kingdom. J Vet Res. 42, 110 (2011).
Article CAS Google Scholar - Lacroux, C. et al. Genetic resistance to scrapie infection in experimentally challenged goats. J Virol 88, 2406–2413 (2014).
Article Google Scholar - Madsen-Bouterse, S. A. et al. PRNP variants in goats reduce sensitivity of detection of PrP(Sc) by immunoassay. J Vet Diagn Invest. 27, 332–343 (2015).
Article CAS Google Scholar - Dassanayake, R. P. et al. Sensitive and specific detection of classical scrapie prions in the brains of goats by real-time quaking-induced conversion. J Gen Virol. 97, 803–812 (2016).
Article CAS Google Scholar - Kang, H. E. et al. Prion Diagnosis: Application of Real-Time Quaking-Induced Conversion. Biomed Res Int. 2017, 5413936 (2017).
ADS PubMed PubMed Central Google Scholar - Orrú, C. D. et al. Bank Vole Prion Protein as an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains. PLoS Pathog. 11, e1004983 (2015).
Article Google Scholar - Masujin, K. et al. Detection of Atypical H-Type Bovine Spongiform Encephalopathy and Discrimination of Bovine Prion Strains by Real-Time Quaking-Induced Conversion. J Clin Microbiol. 54, 676–86 (2016).
Article CAS Google Scholar - Hwang, S., Greenlee, J. J. & Nicholson, E. M. Use of bovine recombinant prion protein and real-time quaking-induced conversion to detect cattle transmissible mink encephalopathy prions and discriminate classical and atypical L- and H-Type bovine spongiform encephalopathy. PLoS One 12, e0172391 (2017).
Article Google Scholar - Kimberlin, R. H. & Walker, C. A. Pathogenesis of experimental scrapie; in Bock G, Marsh J (eds): Novel Infectious Agent and the Central Nervous System. Ciba Found. Symp. 135, 37–62 (1988).
CAS PubMed Google Scholar - Arnold, M. E. et al. Pathogenesis of experimental bovine spongiform encephalopathy (BSE): estimation of tissue infectivity according to incubation period. Vet Res. 40, 8 (2009).
Article Google Scholar - Orrù, C. D. et al. Factors That Improve RT-QuIC Detection of Prion Seeding Activity. Viruses. 8, 140 (2016).
Article Google Scholar - Bannach, O. et al. Analysis of prion protein aggregates in blood and brain from pre-clinical and clinical BSE cases. Vet Microbiol. 166, 102–8 (2013).
Article CAS Google Scholar - Orrù, C. D. et al. Time Course of Prion Seeding Activity in Cerebrospinal Fluid of Scrapie-Infected Hamsters after Intratongue and Intracerebral Inoculations. J Clin Microbiol. 50, 1464–1466 (2012).
Article Google Scholar - Monleón, E. et al. An assessment of the efficiency of PrPsc detection in rectal mucosa and third-eyelid biopsies from animals infected with scrapie. Vet Micro. 147, 237–43 (2011).
Article Google Scholar - D’Angelo, A. et al. Assessment of clinical criteria to diagnose scrapie in Italy. Vet J. 174, 106–12 (2007).
Article Google Scholar - Mayhew, I. G. Cervical vertebral fractures. Equine Veterinary Education 21, 536–539 (2009).
Article Google Scholar
Acknowledgements
The authors wish to thank the Istituto Zooprofilattico of Brescia for housing the animals and the members of the technical staff: Dr. Dante Pedretti, Giacomo Savoldi, and Claudio Barbeno for their assistance in medical examination and sampling. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply any recommendation. This study was funded by Italian Ministry of Health grants IZSPLV04/13RC to Dr. C. Corona, IZSPLV04/17RC to Dr. C. Corona and RF-2013-02354884 to Dr. C. Casalone. This work was supported in part by the Intramural Research Program of the NIAID, NIH (Caughey). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
- National Reference Laboratory of TSEs (CEA), Istituto Zooprofilattico Sperimentale del Piemonte, Liguria e Valle d’Aosta, Turin, Italy
Alessandra Favole, Maria Mazza, Elena Vallino Costassa, Paola Crociara, Elena Berrone, Marina Gallo, Claudia Palmitessa, Pier L. Acutis, Maria Caramelli, Cristina Casalone & Cristiano Corona - Dipartimento di Scienze Veterinarie, Sezione Clinica Medica, University of Turin, Grugliasco, Turin, Italy
Antonio D’Angelo - Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia Romagna, Brescia, Italy
Guerino Lombardi - Istituto Zooprofilattico Sperimentale Lazio e Toscana, Firenze, Italy
Paola Marconi - Rocky Mountain Laboratories, National Institute for Allergy and Infectious Diseases, National Institutes of Health, Hamilton, Montana, USA
Christina D. Orrù & Byron Caughey
Authors
- Alessandra Favole
You can also search for this author inPubMed Google Scholar - Maria Mazza
You can also search for this author inPubMed Google Scholar - Elena Vallino Costassa
You can also search for this author inPubMed Google Scholar - Antonio D’Angelo
You can also search for this author inPubMed Google Scholar - Guerino Lombardi
You can also search for this author inPubMed Google Scholar - Paola Marconi
You can also search for this author inPubMed Google Scholar - Paola Crociara
You can also search for this author inPubMed Google Scholar - Elena Berrone
You can also search for this author inPubMed Google Scholar - Marina Gallo
You can also search for this author inPubMed Google Scholar - Claudia Palmitessa
You can also search for this author inPubMed Google Scholar - Christina D. Orrù
You can also search for this author inPubMed Google Scholar - Byron Caughey
You can also search for this author inPubMed Google Scholar - Pier L. Acutis
You can also search for this author inPubMed Google Scholar - Maria Caramelli
You can also search for this author inPubMed Google Scholar - Cristina Casalone
You can also search for this author inPubMed Google Scholar - Cristiano Corona
You can also search for this author inPubMed Google Scholar
Contributions
A.F. carried out most of the RT-QuIC experiments, analyzed results, prepared the figures, and wrote the manuscript. M.M. and E.V.C. participated in designing the study, analyzed the data and supervised the execution of some experiments. Sample collection, acquisition, analysis and interpretation of data: A.D., G.L., P.M., P.C., E.B., M.G., C.P., P.L.A., C.C., C.D.O., B.C., M.C.; C. Corona was responsible for coordinating research activity, experimental design, data analysis, funding and writing the manuscript. All authors reviewed and corrected the manuscript.
Corresponding author
Correspondence toCristiano Corona.
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Favole, A., Mazza, M., Vallino Costassa, E. et al. Early and Pre-Clinical Detection of Prion Seeding Activity in Cerebrospinal Fluid of Goats using Real-Time Quaking-Induced Conversion Assay.Sci Rep 9, 6173 (2019). https://doi.org/10.1038/s41598-019-42449-7
- Received: 24 October 2018
- Accepted: 27 March 2019
- Published: 16 April 2019
- DOI: https://doi.org/10.1038/s41598-019-42449-7