Incident Parkinson’s disease in kidney transplantation recipients: a nationwide population-based cohort study in Korea (original) (raw)
Introduction
Parkinson’s disease is the second most prevalent neurodegenerative disorder after Alzheimer’s disease and is characterized by bradykinesia, resting tremor, rigidity, and postural instability[1](/articles/s41598-021-90130-9#ref-CR1 "de Lau, L. M. & Breteler, M. M. Epidemiology of Parkinson’s disease. Lancet Neurol. 5, 525–535. https://doi.org/10.1016/S1474-4422(06)70471-9
(2006)."). It has an estimated prevalence of 0.5% in the entire population and 1%-2% in individuals aged > 60 years[2](/articles/s41598-021-90130-9#ref-CR2 "Nussbaum, R. L. & Ellis, C. E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 348, 1356–1364.
https://doi.org/10.1056/NEJM2003ra020003
(2003)."). Patients with Parkinson’s disease present with poor outcomes and it is associated with an increased risk of mortality compared with that in the healthy population (hazard ratio \[HR\] 1.5–2.7 times higher)[1](/articles/s41598-021-90130-9#ref-CR1 "de Lau, L. M. & Breteler, M. M. Epidemiology of Parkinson’s disease. Lancet Neurol. 5, 525–535.
https://doi.org/10.1016/S1474-4422(06)70471-9
(2006)."). The pathogenesis of Parkinson’s disease is thought to involve progressive neuron loss in brain structures, including the substantia nigra[3](/articles/s41598-021-90130-9#ref-CR3 "Tolosa, E., Wenning, G. & Poewe, W. The diagnosis of Parkinson’s disease. Lancet Neurol. 5, 75–86.
https://doi.org/10.1016/S1474-4422(05)70285-4
(2006)."). Previous epidemiological studies have reported several genetic and environmental risk factors for Parkinson’s disease[1](/articles/s41598-021-90130-9#ref-CR1 "de Lau, L. M. & Breteler, M. M. Epidemiology of Parkinson’s disease. Lancet Neurol. 5, 525–535.
https://doi.org/10.1016/S1474-4422(06)70471-9
(2006)."),[4](/articles/s41598-021-90130-9#ref-CR4 "Schapira, A. H. & Jenner, P. Etiology and pathogenesis of Parkinson’s disease. Mov. Disord. 26, 1049–1055.
https://doi.org/10.1002/mds.23732
(2011)."),[5](/articles/s41598-021-90130-9#ref-CR5 "Lee, A. & Gilbert, R. M. Epidemiology of Parkinson disease. Neurol. Clin. 34, 955–965.
https://doi.org/10.1016/j.ncl.2016.06.012
(2016).").Several recent cohort studies have reported an association of chronic kidney disease (CKD)[6](/articles/s41598-021-90130-9#ref-CR6 "Lin, H. L., Lin, H. C. & Chen, Y. H. Increased risks of parkinsonism in the 3 years after chronic renal failure. Int. J. Clin. Pract. 66, 499–503. https://doi.org/10.1111/j.1742-1241.2012.02896.x
(2012)."),[7](/articles/s41598-021-90130-9#ref-CR7 "Nam, G. E. et al. Chronic renal dysfunction, proteinuria, and risk of Parkinson’s disease in the elderly. Mov. Disord.
https://doi.org/10.1002/mds.27704
(2019).") and end-stage renal disease (ESRD)[8](/articles/s41598-021-90130-9#ref-CR8 "Wang, I. K. et al. Increased risk of Parkinson’s disease in patients with end-stage renal disease: a retrospective cohort study. Neuroepidemiology 42, 204–210.
https://doi.org/10.1159/000358921
(2014).") with incident Parkinson’s disease. This is speculated to involve metabolic derangements, including hypoxia, uremic toxins, and metabolic acidosis with resulting persistent vasogenic edema in the basal ganglia and eventual permanent Parkinsonism characterized by cytotoxic derangements in the globus pallidus lesion[9](#ref-CR9 "Kim, T. K., Seo, S. I., Kim, J. H., Lee, N. J. & Seol, H. Y. Diffusion-weighted magnetic resonance imaging in the syndrome of acute bilateral basal ganglia lesions in diabetic uremia. Mov. Disord. 21, 1267–1270.
https://doi.org/10.1002/mds.20932
(2006)."),[10](#ref-CR10 "Wang, H. C., Brown, P. & Lees, A. J. Acute movement disorders with bilateral basal ganglia lesions in uremia. Mov. Disord. 13, 952–957.
https://doi.org/10.1002/mds.870130615
(1998)."),[11](/articles/s41598-021-90130-9#ref-CR11 "Raskin, N. H. & Fishman, R. A. Neurologic disorders in renal failure (first of two parts). N. Engl. J. Med. 294, 143–148.
https://doi.org/10.1056/NEJM197601152940306
(1976)."). However, a recent epidemiology study reported that patients undergoing tissue transplants, including kidney, heart, lung, and bone marrow transplantation, were at a lower risk of Parkinson’s disease than the general population[12](/articles/s41598-021-90130-9#ref-CR12 "Fan, J., Searles Nielsen, S., Faust, I. M. & Racette, B. A. Transplant and risk of Parkinson disease. Parkinsonism Relat. Disord. 63, 149–155.
https://doi.org/10.1016/j.parkreldis.2019.02.013
(2019)."). They speculated that immunosuppressants may reduce the risk of Parkinson’s disease given the possible involvement of inflammation in the pathophysiology of Parkinson’s disease.Kidney transplantation (KT) is considered the best treatment option for patients with ESRD mainly due to the resulting survival benefits and quality of life even with increase in age and comorbidities of contemporary transplant recipients[13](#ref-CR13 "Wolfe, R. A. et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 341, 1725–1730. https://doi.org/10.1056/NEJM199912023412303
(1999)."),[14](#ref-CR14 "Tonelli, M. et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am. J. Transplant. 11, 2093–2109.
https://doi.org/10.1111/j.1600-6143.2011.03686.x
(2011)."),[15](/articles/s41598-021-90130-9#ref-CR15 "Jeon, H. J. et al. Outcomes of end-stage renal disease patients on the waiting list for deceased donor kidney transplantation: a single-center study. Kidney Res. Clin. Pract. 38, 116–123.
https://doi.org/10.23876/j.krcp.18.0068
(2019)."). Moreover, KT is becoming increasingly popular among older adults with ESRD with improvements in survival and graft loss in this population[16](/articles/s41598-021-90130-9#ref-CR16 "McAdams-DeMarco, M. A., James, N., Salter, M. L., Walston, J. & Segev, D. L. Trends in kidney transplant outcomes in older adults. J. Am. Geriatr. Soc. 62, 2235–2242.
https://doi.org/10.1111/jgs.13130
(2014)."). Given that KT recipients live longer with a functioning graft compared to dialysis-dependent patients with ESRD, they are at risk of incident age-related conditions, including neurodegenerative diseases. Therefore, there is a need to assess the effect of KT on the risk of incident Parkinson’s disease. In this study, we aimed to estimate the risk of incident Parkinson’s disease among KT recipients compared with patients with ESRD and the general population.Results
Study population
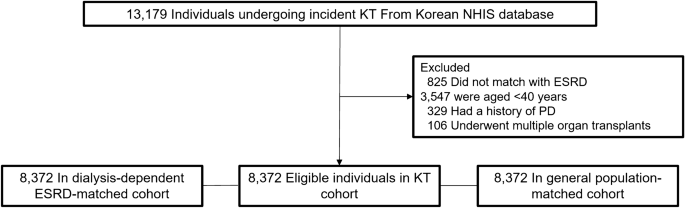
We followed 8372 KT recipients, dialysis-dependent patients with ESRD, and individuals in the general population for 45,723, 38,357, and 47,476 patient-years, respectively until the outcome date, death, or December 31, 2017 (Fig. 1). We performed among-group comparisons of the patients’ baseline characteristics (Table 1). Their mean age was 51.2 ± 7.0 years and men were 60.1%. There were lower and higher proportions of the KT recipients with Medical Aid coverage compared with dialysis-dependent patients with ESRD and the general population, respectively. The KT recipients had a similar and higher Charlson’s comorbidity index compared with dialysis-dependent patients with ESRD and the general population, respectively. KT recipients received preemptive KT in 29.8% and underwent hemodialysis mainly before KT. Further, 16.2%, 86.6%, and 82% of the KT recipients underwent pre-KT desensitization therapy, induction treatment using basiliximab, and immunosuppression maintenance using tacrolimus, respectively. KT recipients had a lower frequency of use of nonsteroidal anti-inflammatory drugs compared to that of dialysis-dependent patients with ESRD and the general population.
Figure 1
Study population. KT, kidney transplantation; NHIS, National Health Insurance Service; ESRD, end-stage renal disease; PD, Parkinson’s disease.
Table 1 Baseline characteristics of patients among the kidney transplants, dialysis-dependent ESRD, and general population groups.
Between-group comparison of the incidence probability of Parkinson’s disease
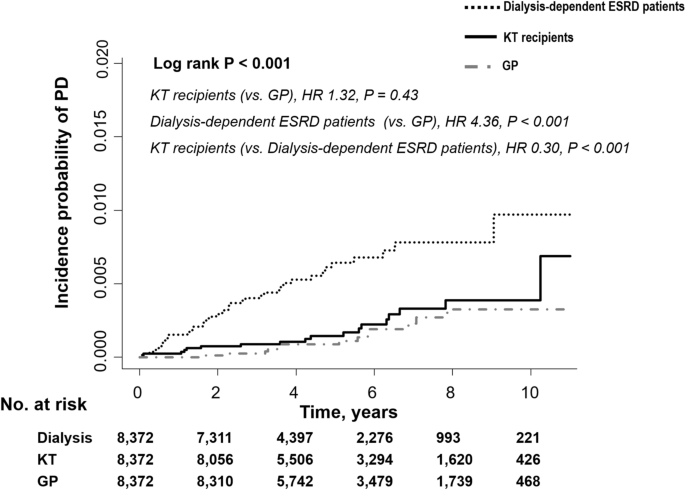
Figure 2 shows the Kaplan–Meier curves of the incidence probability of Parkinson’s disease for up to 10 years in the three groups. Within the observation periods, 19, 53, and 15 KT recipients, dialysis-dependent patients with ESRD, and individuals in the general population developed Parkinson’s disease, respectively. The annual incidence rate of Parkinson’s disease in KT recipients, dialysis-dependent patients with ESRD, and the general population was 0.42, 1.38, and 0.32 per 1000-person-years, respectively. The KT recipients showed a similar and significantly lower risk of incident Parkinson’s disease compared to the general population group (adjusted HR 0.86, 95% confidence interval [CI] 0.35 to 2.13, P = 0.75) and dialysis-dependent patients (adjusted HR 0.31, 95% CI, 0.18 to 0.53, P < 0.001), respectively. The results remained significant even after adjusting for age, sex, diabetes mellitus, hypertension, dyslipidemia, income, Charlson’s comorbidity index, dialysis vintage period, and use of immunosuppressive treatment before inclusion and nonsteroidal anti-inflammatory drugs (Table 2).
Figure 2
The risk of Parkinson’s disease among the transplant, dialysis-dependent ESRD, and general population groups. ESRD, end-stage renal disease; KT, kidney transplantation; GP, general population; PD, Parkinson’s disease; HR, hazard ratio.
Table 2 Risk of Parkinson’s disease.
Predictors of Parkinson’s disease in KT recipients
The KT recipients who developed Parkinson’s disease were older at the time of KT (51.2 vs. 56.0 years old: P = 0.003) (see Table 3 and Supplementary Table S1 online). However, incident Parkinson’s disease was not associated with any comorbidities including, diabetes mellitus, hypertension, and dyslipidemia. Moreover, there was no association of incident Parkinson’s disease with income, sex, previous dialysis type and vintage period, and use of immunosuppressive treatment before inclusion (Table 3).
Table 3 Risk prediction models for incident Parkinson’s disease in post KT recipients.
Discussion
In this nationwide population-based, large-scale, cohort study, we examined the risk of incident Parkinson’s disease in KT recipients compared with that in dialysis patients and the general population. We found that the risk of incident Parkinson’s disease in KT recipients was significantly lower and similar compared with that in dialysis-dependent patients with ESRD and the general population, respectively. Older age at the KT time was an independent risk factor for incident Parkinson’s disease in KT recipients.
We found that the annual incidence rate of Parkinson’s disease in dialysis-dependent patients with ESRD averaged 51-year was 1.38 per 1000-person-year; moreover, they had a significantly higher risk of incident Parkinson’s disease than the KT recipients and the general population. Previous studies have reported an association between chronic uremia and Parkinson’s disease. A 3-year follow-up study in Taiwan based on the Longitudinal Health Insurance Database reported that uremic patients had a 1.81-fold higher risk of incident Parkinson’s disease than nonuremic patients[6](/articles/s41598-021-90130-9#ref-CR6 "Lin, H. L., Lin, H. C. & Chen, Y. H. Increased risks of parkinsonism in the 3 years after chronic renal failure. Int. J. Clin. Pract. 66, 499–503. https://doi.org/10.1111/j.1742-1241.2012.02896.x
(2012)."). A 2.56-year follow-up cohort study based on the same database reported an association of dialysis-dependent ESRD with an increased risk of incident Parkinson’s disease (adjusted HR of 1.73, 95% CI 1.39–2.15)[8](/articles/s41598-021-90130-9#ref-CR8 "Wang, I. K. et al. Increased risk of Parkinson’s disease in patients with end-stage renal disease: a retrospective cohort study. Neuroepidemiology 42, 204–210.
https://doi.org/10.1159/000358921
(2014)."). A 5.2-year follow-up cohort study based on the National Health Insurance Service of South Korea reported that an association of a lower estimated glomerular filtration rate and an increased proteinuria severity on dipstick test with a higher occurrence of Parkinson’s disease[7](/articles/s41598-021-90130-9#ref-CR7 "Nam, G. E. et al. Chronic renal dysfunction, proteinuria, and risk of Parkinson’s disease in the elderly. Mov. Disord.
https://doi.org/10.1002/mds.27704
(2019)."). Consistent with these previous findings, we found an association of dialysis-dependent ESRD with an increased risk of incident Parkinson’s disease.In our study, the annual incidence rate of Parkinson’s disease was 0.42 and 0.32 per 1000-person-years in KT recipients and the general population, respectively. This is consistent with the findings of previous studies on the general population aged 50 years[1](/articles/s41598-021-90130-9#ref-CR1 "de Lau, L. M. & Breteler, M. M. Epidemiology of Parkinson’s disease. Lancet Neurol. 5, 525–535. https://doi.org/10.1016/S1474-4422(06)70471-9
(2006)."),[17](/articles/s41598-021-90130-9#ref-CR17 "de Lau, L. M. et al. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology 63, 1240–1244.
https://doi.org/10.1212/01.wnl.0000140706.52798.be
(2004)."). Moreover, in our study, the risk of incident Parkinson’s disease in KT recipients was similar to that in the general population. Only one study has assessed the association of incident Parkinson’s disease with organ transplants. In contrast to our results, this previous study, which was based on information obtained from Medicare claims in the United States, reported a lower risk of developing Parkinson’s disease in KT recipients (odds ratio 0.63, 95% CI 0.47–0.84) than that in the general population[12](/articles/s41598-021-90130-9#ref-CR12 "Fan, J., Searles Nielsen, S., Faust, I. M. & Racette, B. A. Transplant and risk of Parkinson disease. Parkinsonism Relat. Disord. 63, 149–155.
https://doi.org/10.1016/j.parkreldis.2019.02.013
(2019)."). They attributed their findings to immunosuppressive agents potentially lowering the risk of Parkinson’s disease given the possible involvement of inflammation in the pathophysiology of Parkinson’s disease[12](/articles/s41598-021-90130-9#ref-CR12 "Fan, J., Searles Nielsen, S., Faust, I. M. & Racette, B. A. Transplant and risk of Parkinson disease. Parkinsonism Relat. Disord. 63, 149–155.
https://doi.org/10.1016/j.parkreldis.2019.02.013
(2019)."),[18](/articles/s41598-021-90130-9#ref-CR18 "Racette, B. A. et al. Immunosuppressants and risk of Parkinson disease. Ann. Clin. Transl. Neurol. 5, 870–875.
https://doi.org/10.1002/acn3.580
(2018)."). However, the analysis of the same database did not yield similar results. In our study, there was no correlation of immunosuppressant usage with the risk of Parkinson’s disease even with thorough analysis and adjustment of confounding factors, including desensitization, induction, and maintenance (data not shown).KT has been shown to reverse the underlying mechanisms that cause cognitive impairment in CKD, including uremic toxins, hyperparathyroidism, and Klotho deficiency[19](/articles/s41598-021-90130-9#ref-CR19 "Van Sandwijk, M. S. et al. Cognitive changes in chronic kidney disease and after transplantation. Transplantation 100, 734–742. https://doi.org/10.1097/TP.0000000000000968
(2016)."). Initially, as the new transplant begins functioning, ischemia–reperfusion injury causes the up-regulation of pro-inflammatory neurotoxic molecules, uremic toxin removal, restoration of normal calcium-phosphate homeostasis, acute fluid disappearance, and osmotic shift[19](/articles/s41598-021-90130-9#ref-CR19 "Van Sandwijk, M. S. et al. Cognitive changes in chronic kidney disease and after transplantation. Transplantation 100, 734–742.
https://doi.org/10.1097/TP.0000000000000968
(2016)."). These positive effects counteract the possible neurotoxic effects of infection[20](/articles/s41598-021-90130-9#ref-CR20 "Fishman, J. A. Infection in solid-organ transplant recipients. N. Engl. J. Med. 357, 2601–2614.
https://doi.org/10.1056/NEJMra064928
(2007).") and immunosuppressive drugs, especially corticosteroids and calcineurin inhibitors[21](/articles/s41598-021-90130-9#ref-CR21 "Anghel, D. et al. Neurotoxicity of immunosuppressive therapies in organ transplantation. Maedica (Buchar) 8, 170–175 (2013)."),[22](/articles/s41598-021-90130-9#ref-CR22 "Bechstein, W. O. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl. Int. 13, 313–326.
https://doi.org/10.1007/s001470050708
(2000)."). From this perspective, our findings suggest that dialysis (uremic) patients are at a higher risk of Parkinson’s disease than KT recipients (non-uremic individuals or those with controlled chronic uremia). Consistent with previous reports that KT improves cognitive function[23](#ref-CR23 "Griva, K. et al. Neuropsychological performance after kidney transplantation: a comparison between transplant types and in relation to dialysis and normative data. Nephrol. Dial. Transplant. 19, 1866–1874.
https://doi.org/10.1093/ndt/gfh141
(2004)."),[24](#ref-CR24 "Harciarek, M., Biedunkiewicz, B., Lichodziejewska-Niemierko, M., Debska-Slizien, A. & Rutkowski, B. Continuous cognitive improvement 1 year following successful kidney transplant. Kidney Int. 79, 1353–1360.
https://doi.org/10.1038/ki.2011.40
(2011)."),[25](#ref-CR25 "Radic, J. et al. Kidney transplantation improves cognitive and psychomotor functions in adult hemodialysis patients. Am. J. Nephrol. 34, 399–406.
https://doi.org/10.1159/000330849
(2011)."),[26](#ref-CR26 "Griva, K. et al. Cognitive functioning pre- to post-kidney transplantation–a prospective study. Nephrol. Dial. Transplant. 21, 3275–3282.
https://doi.org/10.1093/ndt/gfl385
(2006)."),[27](/articles/s41598-021-90130-9#ref-CR27 "Harciarek, M., Biedunkiewicz, B., Lichodziejewska-Niemierko, M., Debska-Slizien, A. & Rutkowski, B. Cognitive performance before and after kidney transplantation: a prospective controlled study of adequately dialyzed patients with end-stage renal disease. J. Int. Neuropsychol. Soc. 15, 684–694.
https://doi.org/10.1017/S1355617709990221
(2009)."), our findings suggest that KT may lower the risk of Parkinson’s disease even with long standing kidney disease and/or neurotoxic immunosuppressant usage. Moreover, previous case reports have shown that uremia-associated acute parkinsonism is reversible, as well as the selective vulnerability of the basal ganglia to metabolic derangements, including hypoxia, uremic toxins, and metabolic acidosis[10](/articles/s41598-021-90130-9#ref-CR10 "Wang, H. C., Brown, P. & Lees, A. J. Acute movement disorders with bilateral basal ganglia lesions in uremia. Mov. Disord. 13, 952–957.
https://doi.org/10.1002/mds.870130615
(1998)."). Although the mechanisms underlying the development of Parkinson’s disease in patients with CKD remain unclear, uremia could lead to reversible[10](/articles/s41598-021-90130-9#ref-CR10 "Wang, H. C., Brown, P. & Lees, A. J. Acute movement disorders with bilateral basal ganglia lesions in uremia. Mov. Disord. 13, 952–957.
https://doi.org/10.1002/mds.870130615
(1998).") and/or permanent destruction of brain structures, including the basal ganglia[9](/articles/s41598-021-90130-9#ref-CR9 "Kim, T. K., Seo, S. I., Kim, J. H., Lee, N. J. & Seol, H. Y. Diffusion-weighted magnetic resonance imaging in the syndrome of acute bilateral basal ganglia lesions in diabetic uremia. Mov. Disord. 21, 1267–1270.
https://doi.org/10.1002/mds.20932
(2006)."), and sequelae of movement disorders. Therefore reducing the risk of Parkinson’s disease may also be a reason for recommending KT in older dialysis-dependent patients with ESRD at risk of incident age-related conditions, including neurodegenerative diseases.This study has several limitations. First, this study involved a short-term follow up of KT recipients, who tended to have a higher risk of incident Parkinson’s disease than that in the general population after 10 years (Fig. 2). Therefore, there is a need for long-term follow-up studies. Second, previous studies have reported that the presymptomatic phase from the onset of neuronal loss to the onset of symptoms was 4.7–5.6 years in Parkinson’s disease[28](/articles/s41598-021-90130-9#ref-CR28 "Fearnley, J. M. & Lees, A. J. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(Pt 5), 2283–2301. https://doi.org/10.1093/brain/114.5.2283
(1991)."),[29](/articles/s41598-021-90130-9#ref-CR29 "Hilker, R. et al. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch. Neurol. 62, 378–382.
https://doi.org/10.1001/archneur.62.3.378
(2005)."). The incidence possibility of Parkinson’s disease in dialysis-dependent patients with ESRD seemed to be higher than that in KT recipients at 2 years from the beginning of the study. Neuronal loss may have been ongoing at the start of the present study. Not all dialysis-dependent ESRD patients were on the transplant waitlist, and therefore, potential selection bias remains. To overcome this, history of major diseases including Charlson’s comorbidity index were also analyzed. Third, since the diagnosis of Parkinson’s disease was completely based on information in the claims, there could have been under- or over-estimation of Parkinson’s disease and ambiguity in clinical diagnostic criteria of Parkinson’s disease. Fourth, we analyzed observational data; therefore, it remains unclear why KT recipients are less likely to develop Parkinson’s disease. In our study, the small incidence of Parkinson’s disease impeded evaluation of the association between post-KT renal function and incident Parkinson’s disease. There is a need for future long-term studies on this association to help explain the low incidence of Parkinson’s disease in KT recipients compared with that in dialysis patients. Fifth, since we analyzed only a single ethnic group, our findings cannot be generalized across races. Sixth, due to the limited information obtainable from claim data, we could not adjust for several variables that could affect the occurrence of Parkinson’s disease, including family history of Parkinson’s disease; smoking habits; body mass index; laboratory findings such as serum glucose, creatinine, uric acid, and parathyroid hormone; and dialysis adequacy. Nonetheless, this is the first, large-scale Asian study to compare the incidence of Parkinson’s disease among KT recipients, dialysis-dependent patients with ESRD, and the general population using a nationwide data set in which the insured are covered by a single compulsory insurance with a 100% claim rate. In addition, using a national registry that reports rare intractable disease including Parkinson’s disease leads to yielding more robust entries and the number of patients who are missing is small. Our findings demonstrated that KT was associated with the lower risk of Parkinson’s disease compared with that in dialysis-dependent patients with ESRD. Therefore, KT may be suggested in older dialysis-dependent patients with ESRD at risk of incident Parkinson’s disease[8](/articles/s41598-021-90130-9#ref-CR8 "Wang, I. K. et al. Increased risk of Parkinson’s disease in patients with end-stage renal disease: a retrospective cohort study. Neuroepidemiology 42, 204–210.
https://doi.org/10.1159/000358921
(2014).").In conclusion, this study provides evidence that the risk of incident Parkinson’s disease in KT recipients is significantly lower than that in dialysis patients and similar to that in the general population even after adjustment for potential confounding factors. Moreover, we found that older age at the time of KT is an independent risk factor for Parkinson’s disease occurrence in KT recipients.
Methods
Data source
The National Health Security System is a mandatory social insurance program in Korea that comprises of the National Health Insurance and Medical Aid[30](/articles/s41598-021-90130-9#ref-CR30 "Song, S. O. et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab. J. 38, 395–403. https://doi.org/10.4093/dmj.2014.38.5.395
(2014)."). National Health Insurance System, which was launched in 2000 under the supervision of the Ministry of Health and Welfare, is subscribed to by approximately 97% of the Korean population and provides universal health coverage. The remaining 3% of the population is covered by the Medical Aid program. Information on patient demographics, medical service use, disease diagnosis, and life style from both healthcare programs is incorporated into a single National Health Insurance Service database that is accessible for researchers.In 2006, the South Korean government established a registration program for copayment reduction of up to 10% for patients with rare intractable diseases, including Parkinson’s disease, after a confirmed physician’s diagnosis according to the National Health Insurance diagnostic criteria[7](/articles/s41598-021-90130-9#ref-CR7 "Nam, G. E. et al. Chronic renal dysfunction, proteinuria, and risk of Parkinson’s disease in the elderly. Mov. Disord. https://doi.org/10.1002/mds.27704
(2019).").Study Participants
We analyzed incident KT recipients registered in the Korean National Health Insurance Service database between 2007 and 2015. Moreover, we established two control groups comprised of dialysis-dependent patients with ESRD and individuals in the general population through 1:1 direct matching with the study population. Individuals in the general population without a previous ESRD history were directly matched according to age, sex, and inclusion year. Moreover, dialysis-dependent patients with ESRD without a previous KT history were directly matched according to age, sex, era, hypertension, and diabetes mellitus. During matching, those who failed to find a matched pair were excluded from the study. Subsequently, we excluded matched pairs aged < 40 years, with a history of Parkinson’s disease, or with multi-organ transplantation.
Study outcomes and follow-up
The primary outcome was incident Parkinson’s disease based on the International Classification of Disease, 10th Revision (ICD-10) codes for Parkinson’s disease (G20) and Parkinson’s disease registration code (V124) during the observation period. The follow-up period lasted from the baseline to the outcome date, death, or December 31, 2017. Additional censoring was conducted for each study group, when KT recipients returned to maintenance dialysis, dialysis patients received KT, or subjects in the general population group received any renal replacement therapy.
Data collection
We assessed demographic characteristics, including age, sex, and economic status. Economic status was divided into the aid or quartile groups based on the individuals’ insurance fees. We identified baseline comorbidities, including diabetes mellitus, hypertension, and dyslipidemia, based on clinical and pharmacy codes of ICD-10 within 3 years before the inclusion date. We additionally identified microscopic polyangiitis (M31.7), Goodpasture’s syndrome (M31.0), systemic lupus erythematosus (M32), and rapid eye movement sleep behavior disorder (G47.8) based on codes of ICD-10. Moreover, we calculated the Charlson’s comorbidity index[31](/articles/s41598-021-90130-9#ref-CR31 "Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40, 373–383. https://doi.org/10.1016/0021-9681(87)90171-8
(1987)."). Unique codes given for maintenance dialysis (V001 or O7011-O7020 for hemodialysis, V003 or O7071-7075 for peritoneal dialysis, and V005 or R3280 for transplantation) allow identification of information regarding kidney replacement therapy[32](/articles/s41598-021-90130-9#ref-CR32 "Choi, H. S. et al. The risk of end-stage renal disease in systemic lupus erythematosus: A nationwide population-based study in Korea. Medicine (Baltimore) 98, e16420.
https://doi.org/10.1097/MD.0000000000016420
(2019)."). We recorded the duration of dialysis before the inclusion date for KT recipients and dialysis-dependent patients with ESRD as categorical variables (< 5 years, ≥ 5 years). We recorded the main dialysis modality as that performed for the longest duration before the inclusion date. Further, if peritoneal dialysis and hemodialysis were performed during similar periods, we recorded the dialysis type as mixed. For the KT recipients, we defined preemptive transplantation as having a previous dialysis duration of < 3 months. To presume the etiologies of ESRD, use of immunosuppressive treatment from 5 year to 6 months before inclusion was obtained (tacrolimus, cyclosporine, mycophenolate mofetil, rituximab, steroids, and cyclophosphamide). Immunosuppressive agent after inclusion (for KT recipients) was recorded as follows: desensitization therapy, including rituximab or plasmapheresis; induction treatment (IL-1 receptor antagonist, anti-thymocyte globulin, or none); and maintenance immunosuppressants except for steroids (tacrolimus, cyclosporine, mycophenolate mofetil, or others). The use of nonsteroidal anti-inflammatory drugs was obtained from the baseline.Statistical analysis
We presented baseline characteristics as means and standard deviations for continuous variables and as frequencies (percentages) for categorical variables. Among-group differences in continuous and categorical variables were analyzed using the Mann–Whitney U or Kruskal–Wallis tests and the chi-square or Fisher’s exact test, respectively. We plotted Kaplan–Meier curves to present the cumulative incidence probability of Parkinson’s disease and performed the log-rank test to examine among-group differences. We calculated the incidence of Parkinson’s disease as the number of events per 1000 person-years. We performed Cox proportional hazards analyses to evaluate the risk of incident Parkinson’s disease in KT recipients compared with that in dialysis-dependent patients with ESRD and the general population; moreover, we estimated the HR and 95% CI. A two-sided P value of < 0.05 was considered statistically significant. All analyses and calculations were performed using SAS 9.4 program (SAS Institute, United States).
Ethical statement
This study was conducted according to the 2008 Declaration of Helsinki and approved by the independent Institutional Review Board of Seoul National University Hospital (IRB number: H1903-098–1018) with no written informed consent because patients records/information was anonymized and de-identified prior to analysis. The need of the Informed Consent was waived by the Institutional Review Board of Seoul National University Hospital.
Data availability
All data generated or analyzed during this study are included in this published article.
References
- de Lau, L. M. & Breteler, M. M. Epidemiology of Parkinson’s disease. Lancet Neurol. 5, 525–535. https://doi.org/10.1016/S1474-4422(06)70471-9 (2006).
Article PubMed Google Scholar - Nussbaum, R. L. & Ellis, C. E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 348, 1356–1364. https://doi.org/10.1056/NEJM2003ra020003 (2003).
Article CAS PubMed Google Scholar - Tolosa, E., Wenning, G. & Poewe, W. The diagnosis of Parkinson’s disease. Lancet Neurol. 5, 75–86. https://doi.org/10.1016/S1474-4422(05)70285-4 (2006).
Article PubMed Google Scholar - Schapira, A. H. & Jenner, P. Etiology and pathogenesis of Parkinson’s disease. Mov. Disord. 26, 1049–1055. https://doi.org/10.1002/mds.23732 (2011).
Article PubMed Google Scholar - Lee, A. & Gilbert, R. M. Epidemiology of Parkinson disease. Neurol. Clin. 34, 955–965. https://doi.org/10.1016/j.ncl.2016.06.012 (2016).
Article PubMed Google Scholar - Lin, H. L., Lin, H. C. & Chen, Y. H. Increased risks of parkinsonism in the 3 years after chronic renal failure. Int. J. Clin. Pract. 66, 499–503. https://doi.org/10.1111/j.1742-1241.2012.02896.x (2012).
Article PubMed Google Scholar - Nam, G. E. et al. Chronic renal dysfunction, proteinuria, and risk of Parkinson’s disease in the elderly. Mov. Disord. https://doi.org/10.1002/mds.27704 (2019).
Article PubMed Google Scholar - Wang, I. K. et al. Increased risk of Parkinson’s disease in patients with end-stage renal disease: a retrospective cohort study. Neuroepidemiology 42, 204–210. https://doi.org/10.1159/000358921 (2014).
Article PubMed Google Scholar - Kim, T. K., Seo, S. I., Kim, J. H., Lee, N. J. & Seol, H. Y. Diffusion-weighted magnetic resonance imaging in the syndrome of acute bilateral basal ganglia lesions in diabetic uremia. Mov. Disord. 21, 1267–1270. https://doi.org/10.1002/mds.20932 (2006).
Article PubMed Google Scholar - Wang, H. C., Brown, P. & Lees, A. J. Acute movement disorders with bilateral basal ganglia lesions in uremia. Mov. Disord. 13, 952–957. https://doi.org/10.1002/mds.870130615 (1998).
Article CAS PubMed Google Scholar - Raskin, N. H. & Fishman, R. A. Neurologic disorders in renal failure (first of two parts). N. Engl. J. Med. 294, 143–148. https://doi.org/10.1056/NEJM197601152940306 (1976).
Article CAS PubMed Google Scholar - Fan, J., Searles Nielsen, S., Faust, I. M. & Racette, B. A. Transplant and risk of Parkinson disease. Parkinsonism Relat. Disord. 63, 149–155. https://doi.org/10.1016/j.parkreldis.2019.02.013 (2019).
Article PubMed Google Scholar - Wolfe, R. A. et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 341, 1725–1730. https://doi.org/10.1056/NEJM199912023412303 (1999).
Article CAS PubMed Google Scholar - Tonelli, M. et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am. J. Transplant. 11, 2093–2109. https://doi.org/10.1111/j.1600-6143.2011.03686.x (2011).
Article CAS PubMed Google Scholar - Jeon, H. J. et al. Outcomes of end-stage renal disease patients on the waiting list for deceased donor kidney transplantation: a single-center study. Kidney Res. Clin. Pract. 38, 116–123. https://doi.org/10.23876/j.krcp.18.0068 (2019).
Article PubMed PubMed Central Google Scholar - McAdams-DeMarco, M. A., James, N., Salter, M. L., Walston, J. & Segev, D. L. Trends in kidney transplant outcomes in older adults. J. Am. Geriatr. Soc. 62, 2235–2242. https://doi.org/10.1111/jgs.13130 (2014).
Article PubMed PubMed Central Google Scholar - de Lau, L. M. et al. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology 63, 1240–1244. https://doi.org/10.1212/01.wnl.0000140706.52798.be (2004).
Article PubMed Google Scholar - Racette, B. A. et al. Immunosuppressants and risk of Parkinson disease. Ann. Clin. Transl. Neurol. 5, 870–875. https://doi.org/10.1002/acn3.580 (2018).
Article CAS PubMed PubMed Central Google Scholar - Van Sandwijk, M. S. et al. Cognitive changes in chronic kidney disease and after transplantation. Transplantation 100, 734–742. https://doi.org/10.1097/TP.0000000000000968 (2016).
Article CAS PubMed Google Scholar - Fishman, J. A. Infection in solid-organ transplant recipients. N. Engl. J. Med. 357, 2601–2614. https://doi.org/10.1056/NEJMra064928 (2007).
Article CAS PubMed Google Scholar - Anghel, D. et al. Neurotoxicity of immunosuppressive therapies in organ transplantation. Maedica (Buchar) 8, 170–175 (2013).
Google Scholar - Bechstein, W. O. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl. Int. 13, 313–326. https://doi.org/10.1007/s001470050708 (2000).
Article CAS PubMed Google Scholar - Griva, K. et al. Neuropsychological performance after kidney transplantation: a comparison between transplant types and in relation to dialysis and normative data. Nephrol. Dial. Transplant. 19, 1866–1874. https://doi.org/10.1093/ndt/gfh141 (2004).
Article PubMed Google Scholar - Harciarek, M., Biedunkiewicz, B., Lichodziejewska-Niemierko, M., Debska-Slizien, A. & Rutkowski, B. Continuous cognitive improvement 1 year following successful kidney transplant. Kidney Int. 79, 1353–1360. https://doi.org/10.1038/ki.2011.40 (2011).
Article PubMed Google Scholar - Radic, J. et al. Kidney transplantation improves cognitive and psychomotor functions in adult hemodialysis patients. Am. J. Nephrol. 34, 399–406. https://doi.org/10.1159/000330849 (2011).
Article PubMed Google Scholar - Griva, K. et al. Cognitive functioning pre- to post-kidney transplantation–a prospective study. Nephrol. Dial. Transplant. 21, 3275–3282. https://doi.org/10.1093/ndt/gfl385 (2006).
Article PubMed Google Scholar - Harciarek, M., Biedunkiewicz, B., Lichodziejewska-Niemierko, M., Debska-Slizien, A. & Rutkowski, B. Cognitive performance before and after kidney transplantation: a prospective controlled study of adequately dialyzed patients with end-stage renal disease. J. Int. Neuropsychol. Soc. 15, 684–694. https://doi.org/10.1017/S1355617709990221 (2009).
Article PubMed Google Scholar - Fearnley, J. M. & Lees, A. J. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(Pt 5), 2283–2301. https://doi.org/10.1093/brain/114.5.2283 (1991).
Article PubMed Google Scholar - Hilker, R. et al. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch. Neurol. 62, 378–382. https://doi.org/10.1001/archneur.62.3.378 (2005).
Article PubMed Google Scholar - Song, S. O. et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab. J. 38, 395–403. https://doi.org/10.4093/dmj.2014.38.5.395 (2014).
Article PubMed PubMed Central Google Scholar - Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40, 373–383. https://doi.org/10.1016/0021-9681(87)90171-8 (1987).
Article CAS PubMed Google Scholar - Choi, H. S. et al. The risk of end-stage renal disease in systemic lupus erythematosus: A nationwide population-based study in Korea. Medicine (Baltimore) 98, e16420. https://doi.org/10.1097/MD.0000000000016420 (2019).
Article Google Scholar
Acknowledgements
This research was supported in study design and data collection by the grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant HI18C1604).
Author information
Authors and Affiliations
- Department of Internal Medicine, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Gyeonggi-do, Republic of Korea
Seon Ha Baek - Department of Biomedical Medicine, Seoul National University Hospital, Seoul, Republic of Korea
Sehoon Park & Yon Su Kim - Department of Internal Medicine, Armed Forces Capital Hospital, Seongnam, Gyeonggio-do, Republic of Korea
Sehoon Park - Department of Internal Medicine, Hanyang University Guri Hospital, Guri, Gyeonggio-do, Republic of Korea
Mi-yeon Yu - Department of Internal Medicine, Korea University Guro Hospital, Seoul, Republic of Korea
Ji Eun Kim - Department of Medical Statistics, College of Medicine, Catholic University of Korea, Seoul, Republic of Korea
Sang Hyun Park - Department of Statistics and Actuarial Science, Soongsil University, Seoul, Republic of Korea
Kyungdo Han - Department of Internal Medicine, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Korea
Yong Chul Kim, Dong Ki Kim, Kwon Wook Joo, Yon Su Kim & Hajeong Lee - Kidney Research Institute, Seoul National University, Seoul, Republic of Korea
Dong Ki Kim, Kwon Wook Joo & Yon Su Kim
Authors
- Seon Ha Baek
You can also search for this author inPubMed Google Scholar - Sehoon Park
You can also search for this author inPubMed Google Scholar - Mi-yeon Yu
You can also search for this author inPubMed Google Scholar - Ji Eun Kim
You can also search for this author inPubMed Google Scholar - Sang Hyun Park
You can also search for this author inPubMed Google Scholar - Kyungdo Han
You can also search for this author inPubMed Google Scholar - Yong Chul Kim
You can also search for this author inPubMed Google Scholar - Dong Ki Kim
You can also search for this author inPubMed Google Scholar - Kwon Wook Joo
You can also search for this author inPubMed Google Scholar - Yon Su Kim
You can also search for this author inPubMed Google Scholar - Hajeong Lee
You can also search for this author inPubMed Google Scholar
Contributions
S.H.B., S.P., M.Y., J.E.K., K.H., Y.C.K., and H.L. participated in research design. S.H.B. and H.L. participated in the writing of the paper. S.H.B., S.P., M.Y., J.E.K., K.H., Y.C.K., and H.L. participated in the performance of the research. D.K.K., K.W.J., Y.S.K. contributed interpretation of data. S.H.P. and K.H. participated in data analysis. All authors reviewed the manuscript.
Corresponding author
Correspondence toHajeong Lee.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baek, S.H., Park, S., Yu, My. et al. Incident Parkinson’s disease in kidney transplantation recipients: a nationwide population-based cohort study in Korea.Sci Rep 11, 10541 (2021). https://doi.org/10.1038/s41598-021-90130-9
- Received: 11 September 2020
- Accepted: 19 March 2021
- Published: 18 May 2021
- DOI: https://doi.org/10.1038/s41598-021-90130-9