Empagliflozin-based quadruple oral therapy for type 2 diabetes: a prospective cohort study (original) (raw)
Introduction
Type 2 diabetes mellitus (T2DM), a rapidly burgeoning chronic metabolic disease among the world population and one of the leading causes of mortality and reduced quality of life, is accounting for the most cases of all diagnosed diabetes (90–95%) in adults[1](/articles/s41598-024-84993-x#ref-CR1 "Cheng, D. Prevalence, predisposition and prevention of type II diabetes. Nutr. Metabolism. 2, 29. https://doi.org/10.1186/1743-7075-2-29
(2005)."),[2](/articles/s41598-024-84993-x#ref-CR2 "Buse, J. B. et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 63, 221–228.
https://doi.org/10.1007/s00125-019-05039-w
(2020)."). The pathophysiology of T2DM is characterized by a progressive deterioration in pancreatic beta cell function with simultaneous aggravation of insulin resistance[3](/articles/s41598-024-84993-x#ref-CR3 "American Diabetes, A. Diagnosis and classification of diabetes Mellitus. Diabetes Care. 37, S81–S90.
https://doi.org/10.2337/dc14-S081
(2013).").Proper management of blood sugar remains a main goal of treatment in the attempt to effectively prevent short-term and long-term complications associated with T2DM[4](/articles/s41598-024-84993-x#ref-CR4 "Reaven, P. D. et al. Intensive Glucose Control in patients with type 2 diabetes – 15-Year follow-up. N. Engl. J. Med. 380, 2215–2224. https://doi.org/10.1056/NEJMoa1806802
(2019)."). Obtaining balanced glucose concentrations is achieved mainly through drug therapies in patients with T2DM. However, choosing a patient-based effective treatment regimen remains challenging. Different antidiabetic drugs affect blood sugar with their distinct mechanisms of action. Increasing insulin levels, improving tissue sensitivity to insulin, reducing the intestinal absorption of glucose, and enhancing urine glucose excretion are the main mechanisms of action for antidiabetic drugs. Initiating an oral hypoglycemic agent (OHA) such as metformin is the first-line therapy for T2DM[5](/articles/s41598-024-84993-x#ref-CR5 "Early, K. B. & Stanley, K. Position of the Academy of Nutrition and Dietetics: the role of medical nutrition therapy and registered dietitian nutritionists in the prevention and treatment of prediabetes and type 2 diabetes. J. Acad. Nutr. Dietetics. 118, 343–353 (2018)."),[6](/articles/s41598-024-84993-x#ref-CR6 "Inzucchi, S. E. et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetologia 58, 429–442.
https://doi.org/10.1007/s00125-014-3460-0
(2015)."). However, when monotherapy fails to control blood sugar, combination therapy of two or three OHAs with complementary mechanisms of action is recommended[7](/articles/s41598-024-84993-x#ref-CR7 "Garber, A. J. et al. AACE/ACE Comprehensive Diabetes Management Algorithm 2015. Endocr. Pract. 21, 438–447.
https://doi.org/10.4158/EP15693.CS
(2015)."). Also, high-quality clinical studies demonstrated the superior efficiency and safety of combination therapy than monotherapy[2](/articles/s41598-024-84993-x#ref-CR2 "Buse, J. B. et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 63, 221–228.
https://doi.org/10.1007/s00125-019-05039-w
(2020)."),[8](#ref-CR8 "Bolen, S. et al. In Diabetes Medications for Adults with Type 2 Diabetes: An Update (Agency for Healthcare Research and Quality (US), 2016)."),[9](#ref-CR9 "Henry, R. R. et al. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int. J. Clin. Pract. 66, 446–456.
https://doi.org/10.1111/j.1742-1241.2012.02911.x
(2012)."),[10](#ref-CR10 "Matthews, D. R. et al. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet 394, 1519–1529.
https://doi.org/10.1016/S0140-6736(19)32131-2
(2019)."),[11](/articles/s41598-024-84993-x#ref-CR11 "Garber, A. J. et al. Endocr. Practice: Official J. Am. Coll. Endocrinol. Am. Association Clin. Endocrinologists 26, 107–139
https://doi.org/10.4158/cs-2019-0472
(2020)."). When the therapeutic goals of controlling diabetes with triple combination therapy are not met, guidelines recommend starting injectable insulin[11](#ref-CR11 "Garber, A. J. et al. Endocr. Practice: Official J. Am. Coll. Endocrinol. Am. Association Clin. Endocrinologists 26, 107–139
https://doi.org/10.4158/cs-2019-0472
(2020)."),[12](#ref-CR12 "8. Pharmacologic approaches to Glycemic Treatment. Diabetes Care. 40, S64–s74.
https://doi.org/10.2337/dc17-S011
(2017)."),[13](/articles/s41598-024-84993-x#ref-CR13 "Davies, M. J. et al. Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 41, 2669–2701.
https://doi.org/10.2337/dci18-0033
(2018)."). Despite the well-established efficacy of insulin therapy in improving glycemic control, suboptimal adherence remains a significant challenge due to various barriers[14](/articles/s41598-024-84993-x#ref-CR14 "American Diabetes, A. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care 44, S111-S124 (2020).
https://doi.org/10.2337/dc21-S009
"),[15](/articles/s41598-024-84993-x#ref-CR15 "Spann, S. J. et al. Management of type 2 diabetes in the primary care setting: a practice-based research network study. Ann. Fam. Med. 4, 23–31.
https://doi.org/10.1370/afm.420
(2006)."), including patients’ unwillingness to initiate insulin treatment, difficulties in using insulin devices, psychological concerns about injections, negative perceptions about disease progression[16](#ref-CR16 "Wang, H. F. & Yeh, M. C. Psychological resistance to insulin therapy in adults with type 2 diabetes: mixed-method systematic review. J. Adv. Nurs. 68, 743–757.
https://doi.org/10.1111/j.1365-2648.2011.05853.x
(2012)."),[17](#ref-CR17 "Bradley, C. & Speight, J. Patient perceptions of diabetes and diabetes therapy: assessing quality of life. Diab./Metab. Res. Rev. 18 (Suppl 3), S64–69.
https://doi.org/10.1002/dmrr.279
(2002)."),[18](/articles/s41598-024-84993-x#ref-CR18 "Brod, M., Kongsø, J. H., Lessard, S. & Christensen, T. L. Psychological insulin resistance: patient beliefs and implications for diabetes management. Qual. life Research: Int. J. Qual. life Aspects Treat. care Rehabilitation. 18, 23–32.
https://doi.org/10.1007/s11136-008-9419-1
(2009)."), and fears of hypoglycemia, weight gain, and reduced quality of life, all of which can have detrimental impacts on both patient outcomes and physician-patient relationships[19](#ref-CR19 "Korytkowski, M. When oral agents fail: practical barriers to starting insulin. Int. J. Obes. Relat. Metabolic Disorders: J. Int. Association Study Obes. 26 (Suppl 3), 18–24.
https://doi.org/10.1038/sj.ijo.0802173
(2002)."),[20](#ref-CR20 "Aronson, R. The role of comfort and discomfort in insulin therapy. Diabetes. Technol. Ther. 14, 741–747.
https://doi.org/10.1089/dia.2012.0038
(2012)."),[21](#ref-CR21 "Groop, L. C., Pelkonen, R., Koskimies, S., Bottazzo, G. F. & Doniach, D. Secondary failure to treatment with oral antidiabetic agents in non-insulin-dependent diabetes. Diabetes Care. 9, 129–133.
https://doi.org/10.2337/diacare.9.2.129
(1986)."),[22](#ref-CR22 "Meneghini, L. F., Lee, L. K., Gupta, S. & Preblick, R. Association of hypoglycaemia severity with clinical, patient-reported and economic outcomes in US patients with type 2 diabetes using basal insulin. Diabetes Obes. Metab. 20, 1156–1165.
https://doi.org/10.1111/dom.13208
(2018)."),[23](/articles/s41598-024-84993-x#ref-CR23 "Hur, K. Y. et al. Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association. Diabetes & metabolism journal 45, 461–481 (2021). (2021).
https://doi.org/10.4093/dmj.2021.0156
").Because of the mentioned reasons, adding an alternate OHA to the triple combination regimens seems to be a reasonable option. Empagliflozin, a relatively novel drug with a unique mechanism, is reported to be suitable as an add-on drug to combination therapy with all other classes of OHAs[6](/articles/s41598-024-84993-x#ref-CR6 "Inzucchi, S. E. et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetologia 58, 429–442. https://doi.org/10.1007/s00125-014-3460-0
(2015)."),[24](/articles/s41598-024-84993-x#ref-CR24 "Holmes-Truscott, E., Skinner, T. C., Pouwer, F. & Speight, J. Negative appraisals of insulin therapy are common among adults with type 2 diabetes using insulin: results from diabetes MILES – Australia cross-sectional survey. Diabet. Med. 32, 1297–1303.
https://doi.org/10.1111/dme.12729
(2015)."). Notably, the cardiovascular and renal benefits of empagliflozin in patients with T2DM encourage physicians to prescribe this OHA as a part of the treatment regimen for managing hyperglycemia[2](/articles/s41598-024-84993-x#ref-CR2 "Buse, J. B. et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 63, 221–228.
https://doi.org/10.1007/s00125-019-05039-w
(2020)."),[25](/articles/s41598-024-84993-x#ref-CR25 "Zinman, B. et al. Cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128.
https://doi.org/10.1056/NEJMoa1504720
(2015). Empagliflozin.").Despite the established benefits of Empagliflozin, ongoing debate exists regarding its efficacy and safety as an additional therapy to triple OHA regimens for long-term management of T2DM. While previous studies have examined the efficacy of Empagliflozin as part of a quadruple OHA regimen in patients with uncontrolled T2DM, the relatively short follow-up durations of these investigations may have led to an underestimation of the true long-term benefits of this therapeutic approach on glycemic control and cardiometabolic outcomes26,27,28. The present 7-year prospective study aimed to comprehensively assess the effectiveness and safety of a quadruple OHA regimen, including the addition of Empagliflozin, in patients with T2DM who exhibited poor glycemic control despite treatment with a triple OHA combination and were reluctant to transition to injectable insulin therapy. We investigated the efficacy of this quadruple OHA approach on glycemic control and cardiometabolic parameters, as well as factors that may influence treatment response and patient adherence to the regimen.
Materials and methods
Study design
We conducted a prospective cohort survey to study the effectiveness of a quadruple regimen of hypoglycemic agents on glycemic factors in patients with inadequately controlled type 2 diabetes who were on a triplet hypoglycemic agents’ regimen. A prospective observational clinical study was conducted on over a period of 1 to 8 years to assess the efficacy and safety of quadruple oral therapy. All participants maintained their routine daily activities, and adding excess exercise to their routine was not justified. Additionally, a licensed dietitian provided nutritional counseling to patients at every visit. The cohort included all patients with type 2 diabetes, aged 18 years or older, under-treated with three hypoglycemic agents, with a mean 2.5 years of follow-up, and who exhibited uncontrolled diabetes according to the ADA 2024 guidelines[29](/articles/s41598-024-84993-x#ref-CR29 "6. Glycemic goals and hypoglycemia: standards of Care in Diabetes-2024. Diabetes Care. 47, S111–s125. https://doi.org/10.2337/dc24-S006
(2024)."), due to various factors including concerns about insulin side effects and treatment complexity, fear of hypoglycemia, financial constraints, aversion to painful injections, uncertainty regarding treatment effectiveness, and apprehension about developing dependence on insulin. The diagnosis of type 2 diabetes was based on the American Diabetes Association criteria (ADA-2017). For any of the following conditions, patients were excluded from the study; pregnancy, type 1 diabetes, active infection, history of malignancy, GFR < 30 cc/min, significant medical comorbidities and dialysis. The majority of the study population comprised homogeneous middle-class individuals with middle to high-school education levels. Additionally, these individuals had access to healthcare facilities and insurance. The study protocol was approved by the Tehran University of Medical Sciences. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration. Informed consent was obtained from all individual participants included in the study.Data collection and laboratory evaluation
After reviewing the hospital’s diabetes clinic dataset, 651 patients who met the defined criteria were selected to be included in the cohort. Data on medications, the duration of diabetes, and demographic and socioeconomic characteristics were extracted. Anthropometric measures, such as waist circumference, weight, and height, were taken by skilled examiners. A rigid measuring tape was used to measure height without shoes, with an accuracy of 0.1 cm. Weight was measured upright with an accuracy of 0.1 kg with light clothes. Waist circumference (WC) was measured in a standing position over the uncovered abdomen between the iliac crest and the lowest rim of the rib cage using a non-stretchable standard tape (rounded to the nearest 0.1 cm). After a ten-minute rest period, the blood pressure was measured in a seated position on the right arm using a calibrated mercury sphygmomanometer. After fifteen minutes, the measurement was repeated, and the mean was documented. Laboratory test results, including hemoglobin A1C, 2-Hour Postprandial Blood Glucose (2hpp), Fasting Plasma Glucose (FPG), creatinine (Cr), and blood lipid profile (TG, TC, LDL-C, and HDL-C). HbA1c was evaluated using high-performance liquid chromatography (HPLC), and direct enzymatic colorimetry was used to determine the blood lipid profile using a Technicon RA-analyzer (Pars Azmoon, Karaj, Iran). The estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Definition of variables and outcome
The primary outcome of the current study was to evaluate the efficacy of quadruple therapy compared to insulin therapy in achieving target HbA1c levels among patients, also examining additional glycemic factors such as FPG and 2hPP levels. Furthermore, we investigated various clinical parameters, including BMI, duration of diabetes, and baseline HbA1c, to discern differences between patients who responded positively to treatment and those who did not. In the current study, treatment response was evaluated for each patient individually, in accordance with ADA guidelines from 2024 29. For adult patients, the target for HbA1C was set below 7.0%. However, for older adults (aged 65 years and above), the target range was adjusted to 7.0–7.5% for those with few coexisting chronic illnesses and intact cognitive and functional status. Additionally, for patients with at least three coexisting chronic illnesses, two or more instrumental activities of daily living impairments, or mild-to-moderate cognitive impairment, the HbA1C target was set below 8%. Based ADA 2024 guidelines, coexisting chronic illnesses were defined as conditions serious enough to require medications or lifestyle management and may include arthritis, cancer, heart failure, depression, emphysema, falls, hypertension, incontinence, stage 3 or worse chronic kidney disease, myocardial infarction, and stroke.
Results
Baseline characteristics of study participants
A total of 575 patients with type 2 diabetes receiving quadruple OHA therapy were enrolled in the study. The cohort had a mean age of 58.5 ± 10.3 years, with 53.6% being female. Table 1 summarizes the baseline characteristics of the study population. The average BMI was 29.8 ± 5.3 kg/m², and the mean waist circumference was 100.6 ± 10.5 cm. The mean HbA1c level was 7.77 ± 1.48%, with an average FPG of 159.16 ± 49.54 mg/dL and a 2hPP of 222.79 ± 77.05 mg/dL. Additionally, 33.7% of the participants presented with microvascular complications, and 28.2% had cardiovascular disease.
Table 1 Baseline characteristics of patients. Quantitative variables are presented as mean (SD) and qualitative variables are presented as frequency (%).
Table 2 Trend of glycemic profile of patients with diabetes on quadruple OHA during 7-year follow-up. Data is presented as mean ± SD.
Table 3 Comparison of baseline characteristics between patients with and without response to quadruple OHA after follow-up. Continuous variables are presents as mean (SD) and categorical variables are presented as N (%).
Glycemic profile trends
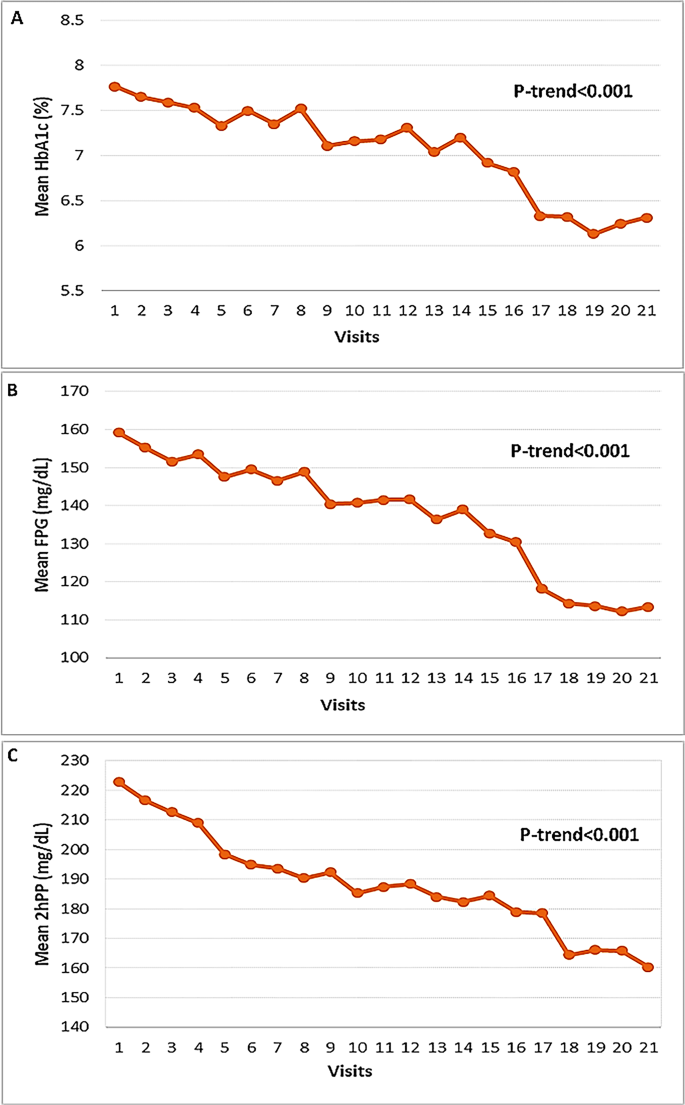
We assessed HbA1c (Fig. 1.A), FPG (Fig. 1.B), and 2hPP (Fig. 1.C) in patients over a 7-year period, across 22 visits conducted every 4 months. After the 68th month, all glycemic parameters showed consistent and significant (p < 0.001) reductions compared to baseline. Specifically, HbA1c decreased from 7.77 ± 1.48% to 6.31 ± 0.91%, FPG decreased from 159.16 ± 49.54 mg/dL to 114.3 ± 22.8 mg/dL, and 2hPP decreased from 222.79 ± 77.05 mg/dL to 164.4 ± 37.0 mg/dL. The first statistically significant reduction in HbA1c was observed at the 20-month visit (P = 0.019). Significant reductions in FPG were first noted at the 44-month visit (P = 0.045). For 2hPP, the initial significant reduction was seen at the 40-month visit (P = 0.033), and this significance persisted until month 52 (Table 2).
Fig. 1
Trend of glycemic profile in patients with diabetes on quadruple OHA, from visit 1(baseline) to visit 21(7-year follow-up) at 4-months interval. A) HbA1c, B) FPG and C) 2hPP. OHA; oral hypoglycemic agent, FPG; fasting plasma glucose, 2hPP; two- hour postprandial plasma glucose.
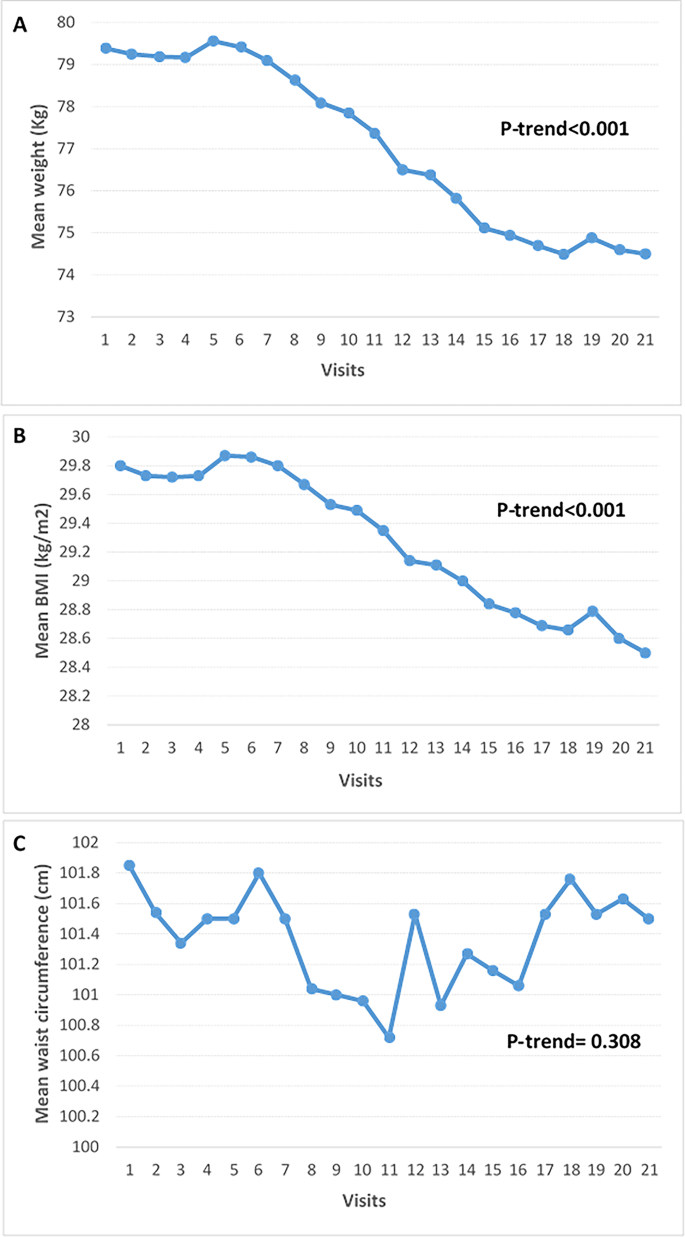
Fig. 2
Trend of anthropometric measures in patients with diabetes on quadruple OHA, from visit 1(baseline) to visit 21(7-year follow-up) at 4-months interval. (A) weight, (B) BMI and (C) waist circumference. OHA; oral hypoglycemic agent, BMI; body mass index.
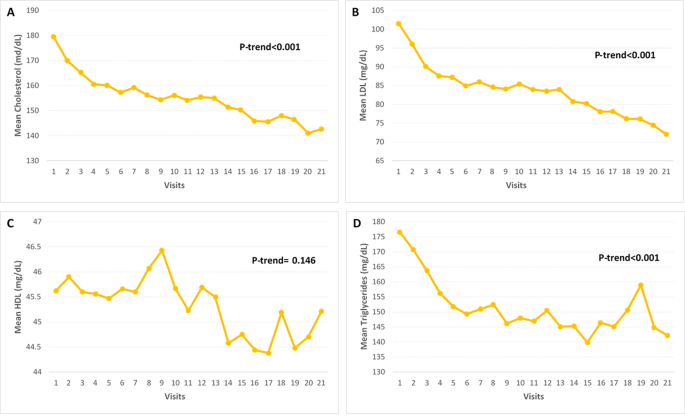
Fig. 3
Trend of lipid profile in patients with diabetes on quadruple OHA, from visit 1(baseline) to visit 21(7-year follow-up) at 4-months interval. A) Cholesterol, B) LDL, C) HDL and D) Triglycerides. OHA; oral hypoglycemic agent, LDL; low-density lipoprotein, HDL; high-density lipoprotein.
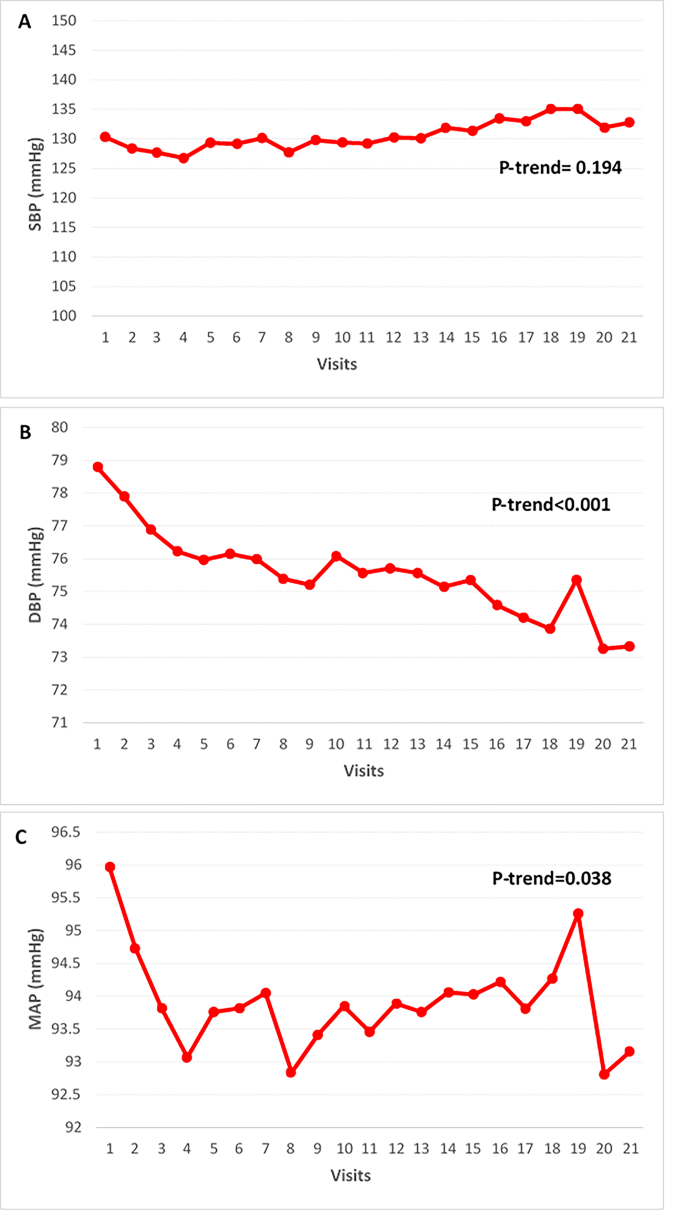
Fig. 4
Trend of blood pressure in patients with diabetes on quadruple OHA, from visit 1(baseline) to visit 21(7-year follow-up) at 4-months interval. A) SBP, B) DBP and C) MAP. OHA; oral hypoglycemic agent, SBP; systolic blood pressure, DBP; diastolic blood pressure, MAP; mean arterial blood pressure.
Assessment of therapeutic outcomes
In our continued investigations, we divided patients into two groups based on their response to quadruple OHA therapy. Over a period of 7 years, 532 patients (92.5%) achieved HbA1c levels below 7%, while 43 out of 575 patients (7.5%) did not achieve (Table 3). Our data indicated no significant differences between the responder and non-responder groups regarding age (p = 0.217), sex (p = 0.634), BMI (p = 0.080), waist circumference (p = 0.100), and initial glycemic parameters. Similarly, baseline lipid profiles, including cholesterol (p = 0.409), LDL (p = 0.141), HDL (p = 0.501), and triglycerides (p = 0.977), were not significantly different. Although the mean HbA1c levels at baseline were similar between the two groups (p = 0.494), the proportion of individuals with HbA1c levels greater than 9% was twice as high in the non-responder group compared to the responder group (p = 0.010). Additionally, significant differences were observed in ALT levels (p = 0.024) between the groups. Notably, the mean durability of response to quadruple OHA therapy was 57.9 (19.5) months, or approximately 4.8 (1.6) years, based on HbA1c levels.
Trend of anthropometric measures
We also analyzed the trend of anthropometric measures, including weight, BMI, and waist circumference, in diabetic patients undergoing quadruple OHA therapy over these 7 years (Fig. 2). Significant changes were observed in weight and BMI over the course of the study (P-trend < 0.001), while changes in waist circumference were not statistically significant (P-trend = 0.308). The mean weight decreased from 79.4 kg at baseline to 74.5 kg at the final visit (P-trend < 0.001). Similarly, the mean BMI declined from 29.8 ± 5.3 kg/m² at baseline to 28.5 kg/m² by the end of the study (P-trend < 0.001). In contrast, the mean waist circumference exhibited variability without a significant trend, starting at 100.6 ± 10.5 cm and fluctuating around this value throughout the study duration (P-trend = 0.308).
Trend of lipid profiles
Figure 4 illustrates the trends in blood pressure parameters, including systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP), over a 7-year period. Throughout the study, significant reductions were noted in DBP and MAP (P-trend < 0.001 and P-trend = 0.038, respectively), whereas SBP did not exhibit statistically significant changes (P-trend = 0.194). The mean SBP remained relatively stable, starting at 130.3 ± 47.5 mmHg at baseline and slightly decreasing to 132.8 ± 45.3 mmHg at the final visit (P-trend = 0.194). In contrast, the mean DBP significantly dropped from 78.8 ± 8.4 mmHg at baseline to 73.3 ± 8.0 mmHg by the end of the study (P-trend < 0.001). Similarly, the mean MAP showed a notable decrease from 96.0 ± 21.1 mmHg at baseline to 93.16 ± 19.3 mmHg at the final visit (P-trend = 0.038). These results indicate that while DBP and MAP demonstrated significant reductions over the 7-year period, SBP remained relatively constant.
Discussion
This prospective study found that quadruple therapy involving empagliflozin provided a substantial target achievement of 92.5% within the follow-up period of seven years in patients with T2DM who were inadequately controlled on a triple OHA regimen. Although the first significant decrease in each of the glycemic indices occurred earlier, from month 68 onwards, we observed a significantly profound decrease in all three indices simultaneously. The long-term Empagliflozin-based quadruple therapy demonstrated a significant and favorable decreasing trend in key glycemic parameters, including HbA1c, FPG, and 2hPP, as well as reductions in several additional cardiometabolic parameters, such as BMI, body weight, LDL-C, TC, TG, DBP, and MAP, after the 7-year follow-up period. The durability of the efficacy of Empagliflozin as a fourth agent was maintained for approximately 4.8 years. Additionally, evaluation of the clinical factors affecting empagliflozin response revealed that baseline HbA1c level<9% and higher ALT levels were significantly associated with higher response rates.
The utilization of Empagliflozin, a sodium-glucose cotransporter-2 (SGLT2) inhibitor, as either monotherapy or as an add-on to existing regimens has emerged as a promising therapeutic approach for patients with uncontrolled T2DM, particularly given the accompanied cardiorenal protective benefits. Recent meta-analyses have reported Empagliflozin’s ability to efficiently reduce HbA1c levels, with a range of 1% observed in monotherapy to 1.5% in add-on regimens[30](/articles/s41598-024-84993-x#ref-CR30 "Devi, R., Mali, G., Chakraborty, I., Unnikrishnan, M. K. & Abdulsalim, S. Efficacy and safety of empagliflozin in type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Postgrad. Med. 129, 382–392. https://doi.org/10.1080/00325481.2017.1259544
(2017).").Although substantial reductions in all glycemic indices, including HbA1c, FPG, and 2hPP, were observed from around year 6 onwards, the first significant improvements in each of these parameters were noted at the 20-month, 44-month, and 40-month visits, respectively, which is in contrast to other studies reporting an earlier treatment response onset within 3 months of initiating Empagliflozin therapy28,31,32. Given that the previously reported results indicate the higher efficacy of Empagliflozin in patients with higher baseline HbA1c levels, the difference in response onset between studies may be attributed to the differences in baseline HbA1c levels among the study populations, with the slower response onset observed in the current study potentially related to the lower baseline HbA1c levels compared to other investigations33,34. However, the overall mean reduction in HbA1c during the study period is in accordance with the 1-1.5% range reported in previous meta-analysis 30,35.
Although our results seemingly show that patients with baseline HbA1c <9% have a higher response rate (<7% HbA1c), which is inconsistent with previous findings underscoring the greater reductions in HbA1c at higher baseline HbA1c, it is important to note several key differences in our study design and methodology. Firstly, we followed patients for a substantially longer duration of 7 years, in contrast to the shorter 6 month follow-up periods reported in previous studies. This extended observation period likely allowed for the capture of longer-term adaptive responses and cumulative effects of the quadruple therapy, yielding an overall impressive total response rate of 92.5% - significantly higher than the 21% response rates reported with shorter follow-ups33,[34](/articles/s41598-024-84993-x#ref-CR34 "Cho, Y. K. et al. Clinical efficacy of quadruple oral therapy for type 2 diabetes in real-world practice: a retrospective observational study. Diabetes Ther. 11, 2029–2039. https://doi.org/10.1007/s13300-020-00881-3
(2020)."). Nonetheless, the relationship between the mean HbA1c reduction and baseline HbA1c remains acceptable, with our data showing 93.7% and 86.1% response rates in the <9% and >9% baseline HbA1c groups, respectively. While the response rate was slightly higher in the lower baseline HbA1c group, we did not analyze the mean HbA1c reduction within each group. It is possible that a small proportion of patients in the >9% baseline HbA1c group, who did not reach the <7% target after 7 years, still experienced considerable HbA1c reductions, but did not meet the strict response criteria.The durability of the empagliflozin-based quadruple therapy, as measured by HbA1c levels, in our study was approximately 4.8 years. This duration was higher than the previously reported 3-year durability observed in other studies31. This difference can be attributed to two potential factors: The baseline HbA1c levels in our study population were substantially lower compared to the participants in other reported investigations. This lower starting point may have enabled patients to sustain the quadruple therapy regimen for a longer duration. Additionally, the longer follow-up duration in our study, relative to prior research, may have allowed for the observation of a more protracted durability period for the empagliflozin-based quadruple therapy approach. Moreover, since we did not report any adverse effects in our study, the explanation for the longer durability may be relatively complex.
It is of note that the incorporation of Empagliflozin as the fourth arm of the treatment regimen exhibits promising effects on cardiometabolic parameters among individuals with type 2 diabetes as well. The significant decreasing trend in body weight following empagliflozin quadruple therapy has also been observed in previous studies31. Despite no significant differences in glycemic control efficacy and the proportion of target achievement among the three add-on hypoglycemic agent classes (thiazolidinediones, SGLT2 inhibitors, and DPP4 inhibitors), the weight-lowering effect is the defining characteristic of SGLT2 inhibitor therapy, in contrast to the other two drug classes[34](/articles/s41598-024-84993-x#ref-CR34 "Cho, Y. K. et al. Clinical efficacy of quadruple oral therapy for type 2 diabetes in real-world practice: a retrospective observational study. Diabetes Ther. 11, 2029–2039. https://doi.org/10.1007/s13300-020-00881-3
(2020).").. Given the higher glycemic control and weight-lowering potential of empagliflozin in patients with higher BMI or body weight, there is a preference for selecting SGLT2 inhibitors, such as empagliflozin, as the fourth OHA in obese patients with diabetes[33](/articles/s41598-024-84993-x#ref-CR33 "Inzucchi, S. E. et al. Empagliflozin treatment effects across categories of baseline HbA1c, body weight and blood pressure as an add-on to metformin in patients with type 2 diabetes. Diabetes Obes. Metabolism. 23, 425–433 (2021)."). While previous studies have reported a significant reduction in waist circumference with empagliflozin treatment[36](/articles/s41598-024-84993-x#ref-CR36 "Bilgin, S. et al. Sodium glucose co-transporter-2 inhibitor, Empagliflozin, is associated with significant reduction in weight, body mass index, fasting glucose, and A1c levels in type 2 diabetic patients with established coronary heart disease: the SUPER GATE study. Ir. J. Med. Sci. (1971-). 191, 1647–1652 (2022)."),[37](/articles/s41598-024-84993-x#ref-CR37 "Neeland, I. J. et al. The impact of empagliflozin on obstructive sleep apnea and cardiovascular and renal outcomes: an exploratory analysis of the EMPA-REG OUTCOME trial. Diabetes care. 43, 3007–3015 (2020)."), the results from our analysis presented a more variable trend, rather than a consistent downward trajectory. The dual effects of empagliflozin on triglyceride synthesis were observed across patient groups with differing baseline visceral fat levels. While empagliflozin suppressed triglyceride synthesis in the lower visceral fat group, higher triglyceride levels were detected in the greater visceral fat group despite overall weight loss. These contrasting findings indicate the metabolic effects of empagliflozin on central adiposity and visceral fat distribution may be more complex 38.While empagliflozin monotherapy has been shown to increase HDL and LDL leading to an overall increase in TC and decrease in Tg[39](/articles/s41598-024-84993-x#ref-CR39 "Mukai, J., Yoshiyama, A. & Kubota, R. Clinical relevance between sodium-glucose co-transporter 2 inhibitors and lipid profiles in Asian patients with type 2 diabetes mellitus: a systematic review with a meta-analysis of randomized controlled trials. J. Pharm. Health Care Sci. 6, 4. https://doi.org/10.1186/s40780-020-00160-0
(2020)."), the lipid changes observed with empagliflozin in our quadruple therapy regimen did not align with this monotherapy profile. The expected trend in lipid profile alterations with empagliflozin quadruple therapy is a decrease in TC, Tg, and LDL-cholesterol, coupled with an increase in HDL-cholesterol[27](/articles/s41598-024-84993-x#ref-CR27 "Ku, E. J., Lee, D. H., Jeon, H. J. & Oh, T. K. Effectiveness and safety of empagliflozin-based quadruple therapy compared with insulin glargine‐based therapy in patients with inadequately controlled type 2 diabetes: an observational study in clinical practice. Diabetes Obes. Metabolism. 21, 173–177 (2019)."),[40](/articles/s41598-024-84993-x#ref-CR40 "Doğanay, B., Celebi, O. O., EFFECT OF SODIUM GLUCOSE & CO-TRANSPORTER 2 INHIBITORS ON LIPID LEVELS IN NEWLY DIAGNOSED HYPERTENSIVE PATIENTS WITH TYPE 2 DIABETES MELLITUS. Eskisehir Med. J. 4, 73–79 (2023)."). Our findings corroborated the reductions in TC, Tg, and LDL-cholesterol, but did not demonstrate the anticipated increase in HDL-cholesterol.Clinical trials have demonstrated that empagliflozin can potentially reduce SBP, DBP, and MAP in patients with type 2 diabetes mellitus, suggesting beneficial effects on vascular resistance and arterial stiffness[41](/articles/s41598-024-84993-x#ref-CR41 "Chilton, R. et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes. Metab. 17, 1180–1193. https://doi.org/10.1111/dom.12572
(2015)."). Previous studies examining the effects of empagliflozin as part of a quadruple therapy regimen have highlighted the beneficial impact of empagliflozin on both SBP and DBP in patients with T2DM[26](/articles/s41598-024-84993-x#ref-CR26 "Ku, E. J., Lee, D. H., Jeon, H. J. & Oh, T. K. Empagliflozin versus dapagliflozin in patients with type 2 diabetes inadequately controlled with metformin, glimepiride and dipeptidyl peptide 4 inhibitors: a 52-week prospective observational study. Diabetes Res. Clin. Pract. 151, 65–73 (2019)."),[27](/articles/s41598-024-84993-x#ref-CR27 "Ku, E. J., Lee, D. H., Jeon, H. J. & Oh, T. K. Effectiveness and safety of empagliflozin-based quadruple therapy compared with insulin glargine‐based therapy in patients with inadequately controlled type 2 diabetes: an observational study in clinical practice. Diabetes Obes. Metabolism. 21, 173–177 (2019)."). Moreover, the results of a recent meta-analysis have also demonstrated a greater beneficial reduction in SBP compared to DBP with empagliflozin treatment[42](/articles/s41598-024-84993-x#ref-CR42 "You, Y., Zhao, Y., Chen, M., Pan, Y. & Luo, Z. Effects of empagliflozin on serum uric acid level of patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetol. Metab. Syndr. 15, 202.
https://doi.org/10.1186/s13098-023-01182-y
(2023).")\-[44](/articles/s41598-024-84993-x#ref-CR44 "Zhang, Q., Zhou, S. & Liu, L. Efficacy and safety evaluation of SGLT2i on blood pressure control in patients with type 2 diabetes and hypertension: a new meta-analysis. Diabetol. Metab. Syndr. 15, 118.
https://doi.org/10.1186/s13098-023-01092-z
(2023)."). While our findings indicate a significant decreasing trend in MAP and DBP over 7 years of empagliflozin quadruple therapy, the results did not reach statistical significance for SBP. In the context of long-term follow-up, it appears that the reduction in arterial stiffness associated with Empagliflozin may no longer be sufficient to significantly control systolic blood pressure or overcome the progression of underlying atherosclerotic changes. This suggests that the cardiovascular benefits of Empagliflozin may have limitations in managing blood pressure and vascular function over extended treatment durations.Although the present study lacked a control group, previous randomized controlled trials have compared the efficacy of empagliflozin-based quadruple therapy to other add-on treatments, including GLP-1 receptor agonists. These studies have demonstrated the superior efficacy of the empagliflozin-containing quadruple regimen, with significantly greater reductions in HbA1c, improvements in insulin resistance, weight loss, and enhancements in DBP and HDL cholesterol, compared to GLP-1 receptor agonist therapy28. In the clinical dilemma of selecting fourth-line agent or insulin therapy for patients with poorly controlled T2DM, comparative investigations have revealed that a quadruple therapy regimen incorporating empagliflozin demonstrated more pronounced target achievement, greater reductions in HbA1c, and more efficient body weight reduction compared to an insulin-based treatment approach31. Compared to add-on therapies incorporating dapagliflozin, the empagliflozin-containing quadruple regimen has demonstrated superior efficacy in improving glycemic control, body weight reduction, and lowering LDL cholesterol26. Moreover, The Empagliflozin add-on has been shown to be superior to dose escalation of existing medication in poor glycemic control conditions[45](/articles/s41598-024-84993-x#ref-CR45 "Shin, Y., Moon, J. H., Chin, H. J., Ferrannini, E. & Lim, S. Glycemic efficacy and metabolic consequences of an Empagliflozin add-on versus conventional dose-increasing strategy in patients with type 2 diabetes inadequately controlled by Metformin and Sulfonylurea. Endocrinol. Metab. (Seoul). 35, 329–338. https://doi.org/10.3803/EnM.2020.35.2.329
(2020).").While previous research has firmly established that empagliflozin-based quadruple therapy does not cause severe macrovascular or microvascular events and has proven to be well-tolerated among patients[46](/articles/s41598-024-84993-x#ref-CR46 "Moon, J. S. et al. Efficacy and safety of treatment with quadruple oral hypoglycemic agents in uncontrolled type 2 diabetes Mellitus: a Multi-center, Retrospective, Observational Study. Diabetes Metab. J. 45, 675–683. https://doi.org/10.4093/dmj.2020.0107
(2021)."), even in comparison to other SGLT2 inhibitors (dapagliflozin)[26](/articles/s41598-024-84993-x#ref-CR26 "Ku, E. J., Lee, D. H., Jeon, H. J. & Oh, T. K. Empagliflozin versus dapagliflozin in patients with type 2 diabetes inadequately controlled with metformin, glimepiride and dipeptidyl peptide 4 inhibitors: a 52-week prospective observational study. Diabetes Res. Clin. Pract. 151, 65–73 (2019)."), other types of OHAs[34](/articles/s41598-024-84993-x#ref-CR34 "Cho, Y. K. et al. Clinical efficacy of quadruple oral therapy for type 2 diabetes in real-world practice: a retrospective observational study. Diabetes Ther. 11, 2029–2039.
https://doi.org/10.1007/s13300-020-00881-3
(2020)."),[47](/articles/s41598-024-84993-x#ref-CR47 "Kim, M. et al. The efficacy of treatment intensification by quadruple oral therapy compared to GLP-1RA therapy in poorly controlled type 2 diabetes Mellitus patients: a real-world Data Study. Diabetes Metab. J. 47, 135–139.
https://doi.org/10.4093/dmj.2021.0373
(2023)."), or even insulin therapy[27](/articles/s41598-024-84993-x#ref-CR27 "Ku, E. J., Lee, D. H., Jeon, H. J. & Oh, T. K. Effectiveness and safety of empagliflozin-based quadruple therapy compared with insulin glargine‐based therapy in patients with inadequately controlled type 2 diabetes: an observational study in clinical practice. Diabetes Obes. Metabolism. 21, 173–177 (2019)."), the present study lacks data regarding the incidence of such complications during the 7-year follow-up period.The long-term, 7-year prospective design of this study allows for a robust assessment of the durability and sustainability of the beneficial impacts observed with the empagliflozin-based quadruple therapy approach. This extended evaluation period provides valuable insights into the ability of this treatment strategy to maintain improvements in glycemic control and cardiometabolic risk factors over a clinically meaningful timeframe.
While the present study offers valuable insights, it is important to acknowledge several limitations that should be considered. Firstly, this investigation was conducted at a single center, and further multi-center studies will be necessary to verify and corroborate the multifaceted benefits of this empagliflozin-based quadruple therapy approach across broader patient populations. Additionally, the current study did not include a control group, which would have allowed for a direct comparison of the efficacy of the empagliflozin-containing quadruple regimen to other potential add-on drug selections for a fourth-line therapy or even insulin-based treatment strategies. The lack of a control group limits the ability to definitively attribute the observed improvements in glycemic control and cardiometabolic parameters solely to the empagliflozin-centered quadruple therapy. Moreover, a notable limitation of the present study is the lack of data regarding the impact of the empagliflozin-based quadruple therapy on microvascular and macrovascular complications in this patient population with poorly controlled type 2 diabetes mellitus, as the prevention and mitigation of these complications are crucial considerations in the holistic care of patients with type 2 diabetes.
Conclusion
In conclusion, the findings of this study underscore empagliflozin as a versatile and efficacious add-on choice to triple therapy regimens for patients with unmet glycemic control needs. The observed improvements in glycemic indices, body weight, and cardiometabolic parameters suggest the potential of Empagliflozin-containing quadruple therapy as a promising long-term management approach without eliciting concerns about hypoglycemic events, particularly among patients hesitant about initiating injection therapy.
Data availability
All data generated or analyzed during this study are available upon request from the first author, F. Moosaie (email: moosaie.fateme@gmail.com).
References
- Cheng, D. Prevalence, predisposition and prevention of type II diabetes. Nutr. Metabolism. 2, 29. https://doi.org/10.1186/1743-7075-2-29 (2005).
Article CAS MATH Google Scholar - Buse, J. B. et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 63, 221–228. https://doi.org/10.1007/s00125-019-05039-w (2020).
Article PubMed MATH Google Scholar - American Diabetes, A. Diagnosis and classification of diabetes Mellitus. Diabetes Care. 37, S81–S90. https://doi.org/10.2337/dc14-S081 (2013).
Article MATH Google Scholar - Reaven, P. D. et al. Intensive Glucose Control in patients with type 2 diabetes – 15-Year follow-up. N. Engl. J. Med. 380, 2215–2224. https://doi.org/10.1056/NEJMoa1806802 (2019).
Article CAS PubMed PubMed Central MATH Google Scholar - Early, K. B. & Stanley, K. Position of the Academy of Nutrition and Dietetics: the role of medical nutrition therapy and registered dietitian nutritionists in the prevention and treatment of prediabetes and type 2 diabetes. J. Acad. Nutr. Dietetics. 118, 343–353 (2018).
Article Google Scholar - Inzucchi, S. E. et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetologia 58, 429–442. https://doi.org/10.1007/s00125-014-3460-0 (2015).
Article PubMed Google Scholar - Garber, A. J. et al. AACE/ACE Comprehensive Diabetes Management Algorithm 2015. Endocr. Pract. 21, 438–447. https://doi.org/10.4158/EP15693.CS (2015).
Article PubMed MATH Google Scholar - Bolen, S. et al. In Diabetes Medications for Adults with Type 2 Diabetes: An Update (Agency for Healthcare Research and Quality (US), 2016).
MATH Google Scholar - Henry, R. R. et al. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int. J. Clin. Pract. 66, 446–456. https://doi.org/10.1111/j.1742-1241.2012.02911.x (2012).
Article CAS PubMed MATH Google Scholar - Matthews, D. R. et al. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet 394, 1519–1529. https://doi.org/10.1016/S0140-6736(19)32131-2 (2019).
Article CAS PubMed MATH Google Scholar - Garber, A. J. et al. Endocr. Practice: Official J. Am. Coll. Endocrinol. Am. Association Clin. Endocrinologists 26, 107–139 https://doi.org/10.4158/cs-2019-0472 (2020).
Article Google Scholar - 8. Pharmacologic approaches to Glycemic Treatment. Diabetes Care. 40, S64–s74. https://doi.org/10.2337/dc17-S011 (2017).
Article MATH Google Scholar - Davies, M. J. et al. Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 41, 2669–2701. https://doi.org/10.2337/dci18-0033 (2018).
Article PubMed PubMed Central MATH Google Scholar - American Diabetes, A. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care 44, S111-S124 (2020). https://doi.org/10.2337/dc21-S009
- Spann, S. J. et al. Management of type 2 diabetes in the primary care setting: a practice-based research network study. Ann. Fam. Med. 4, 23–31. https://doi.org/10.1370/afm.420 (2006).
Article PubMed PubMed Central MATH Google Scholar - Wang, H. F. & Yeh, M. C. Psychological resistance to insulin therapy in adults with type 2 diabetes: mixed-method systematic review. J. Adv. Nurs. 68, 743–757. https://doi.org/10.1111/j.1365-2648.2011.05853.x (2012).
Article PubMed MATH Google Scholar - Bradley, C. & Speight, J. Patient perceptions of diabetes and diabetes therapy: assessing quality of life. Diab./Metab. Res. Rev. 18 (Suppl 3), S64–69. https://doi.org/10.1002/dmrr.279 (2002).
Article MATH Google Scholar - Brod, M., Kongsø, J. H., Lessard, S. & Christensen, T. L. Psychological insulin resistance: patient beliefs and implications for diabetes management. Qual. life Research: Int. J. Qual. life Aspects Treat. care Rehabilitation. 18, 23–32. https://doi.org/10.1007/s11136-008-9419-1 (2009).
Article Google Scholar - Korytkowski, M. When oral agents fail: practical barriers to starting insulin. Int. J. Obes. Relat. Metabolic Disorders: J. Int. Association Study Obes. 26 (Suppl 3), 18–24. https://doi.org/10.1038/sj.ijo.0802173 (2002).
Article MathSciNet CAS Google Scholar - Aronson, R. The role of comfort and discomfort in insulin therapy. Diabetes. Technol. Ther. 14, 741–747. https://doi.org/10.1089/dia.2012.0038 (2012).
Article PubMed PubMed Central MATH Google Scholar - Groop, L. C., Pelkonen, R., Koskimies, S., Bottazzo, G. F. & Doniach, D. Secondary failure to treatment with oral antidiabetic agents in non-insulin-dependent diabetes. Diabetes Care. 9, 129–133. https://doi.org/10.2337/diacare.9.2.129 (1986).
Article CAS PubMed MATH Google Scholar - Meneghini, L. F., Lee, L. K., Gupta, S. & Preblick, R. Association of hypoglycaemia severity with clinical, patient-reported and economic outcomes in US patients with type 2 diabetes using basal insulin. Diabetes Obes. Metab. 20, 1156–1165. https://doi.org/10.1111/dom.13208 (2018).
Article CAS PubMed PubMed Central Google Scholar - Hur, K. Y. et al. Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association. Diabetes & metabolism journal 45, 461–481 (2021). (2021). https://doi.org/10.4093/dmj.2021.0156
- Holmes-Truscott, E., Skinner, T. C., Pouwer, F. & Speight, J. Negative appraisals of insulin therapy are common among adults with type 2 diabetes using insulin: results from diabetes MILES – Australia cross-sectional survey. Diabet. Med. 32, 1297–1303. https://doi.org/10.1111/dme.12729 (2015).
Article CAS PubMed Google Scholar - Zinman, B. et al. Cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128. https://doi.org/10.1056/NEJMoa1504720 (2015). Empagliflozin.
Article CAS PubMed MATH Google Scholar - Ku, E. J., Lee, D. H., Jeon, H. J. & Oh, T. K. Empagliflozin versus dapagliflozin in patients with type 2 diabetes inadequately controlled with metformin, glimepiride and dipeptidyl peptide 4 inhibitors: a 52-week prospective observational study. Diabetes Res. Clin. Pract. 151, 65–73 (2019).
Article CAS PubMed Google Scholar - Ku, E. J., Lee, D. H., Jeon, H. J. & Oh, T. K. Effectiveness and safety of empagliflozin-based quadruple therapy compared with insulin glargine‐based therapy in patients with inadequately controlled type 2 diabetes: an observational study in clinical practice. Diabetes Obes. Metabolism. 21, 173–177 (2019).
Article CAS Google Scholar - Lee, E. Y. et al. Glucometabolic control of once-weekly dulaglutide switched from DPP4 inhibitor versus daily empagliflozin add-on in patients with type 2 diabetes inadequately controlled with metformin, sulfonylurea, and DPP4 inhibitor: a randomised trial. Diabetes Res. Clin. Pract. 203, 110884 (2023).
Article CAS PubMed MATH Google Scholar - 6. Glycemic goals and hypoglycemia: standards of Care in Diabetes-2024. Diabetes Care. 47, S111–s125. https://doi.org/10.2337/dc24-S006 (2024).
Article MATH Google Scholar - Devi, R., Mali, G., Chakraborty, I., Unnikrishnan, M. K. & Abdulsalim, S. Efficacy and safety of empagliflozin in type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Postgrad. Med. 129, 382–392. https://doi.org/10.1080/00325481.2017.1259544 (2017).
Article CAS PubMed Google Scholar - Ku, E. J. & Oh, T. K. Long-term effectiveness of Quadruple Combination Therapy with Empagliflozin Versus basal long-acting insulin therapy in patients with type 2 diabetes: 3-Year retrospective observational study. Diabetes Therapy. 14, 1471–1479 (2023).
Article CAS PubMed PubMed Central Google Scholar - Bae, J., Huh, J. H., Lee, M., Lee, Y. H. & Lee, B. W. Glycaemic control with add-on thiazolidinedione or a sodium‐glucose co‐transporter‐2 inhibitor in patients with type 2 diabetes after the failure of an oral triple antidiabetic regimen: a 24‐week, randomized controlled trial. Diabetes Obes. Metabolism. 23, 609–618 (2021).
Article CAS MATH Google Scholar - Inzucchi, S. E. et al. Empagliflozin treatment effects across categories of baseline HbA1c, body weight and blood pressure as an add-on to metformin in patients with type 2 diabetes. Diabetes Obes. Metabolism. 23, 425–433 (2021).
Article CAS Google Scholar - Cho, Y. K. et al. Clinical efficacy of quadruple oral therapy for type 2 diabetes in real-world practice: a retrospective observational study. Diabetes Ther. 11, 2029–2039. https://doi.org/10.1007/s13300-020-00881-3 (2020).
Article CAS PubMed PubMed Central MATH Google Scholar - Zhang, Y. J. et al. Efficacy and safety of empagliflozin for type 2 diabetes mellitus: Meta-analysis of randomized controlled trials. Med. (Baltim). 97, e12843. https://doi.org/10.1097/md.0000000000012843 (2018).
Article CAS MATH Google Scholar - Bilgin, S. et al. Sodium glucose co-transporter-2 inhibitor, Empagliflozin, is associated with significant reduction in weight, body mass index, fasting glucose, and A1c levels in type 2 diabetic patients with established coronary heart disease: the SUPER GATE study. Ir. J. Med. Sci. (1971-). 191, 1647–1652 (2022).
Article CAS Google Scholar - Neeland, I. J. et al. The impact of empagliflozin on obstructive sleep apnea and cardiovascular and renal outcomes: an exploratory analysis of the EMPA-REG OUTCOME trial. Diabetes care. 43, 3007–3015 (2020).
Article CAS PubMed PubMed Central MATH Google Scholar - Lee, M. H. et al. A randomized clinical trial evaluating the effect of empagliflozin on triglycerides in obese adults: role of visceral fat. Metabolism Open. 13, 100161. https://doi.org/10.1016/j.metop.2021.100161 (2022).
Article CAS PubMed Google Scholar - Mukai, J., Yoshiyama, A. & Kubota, R. Clinical relevance between sodium-glucose co-transporter 2 inhibitors and lipid profiles in Asian patients with type 2 diabetes mellitus: a systematic review with a meta-analysis of randomized controlled trials. J. Pharm. Health Care Sci. 6, 4. https://doi.org/10.1186/s40780-020-00160-0 (2020).
Article PubMed PubMed Central Google Scholar - Doğanay, B., Celebi, O. O., EFFECT OF SODIUM GLUCOSE & CO-TRANSPORTER 2 INHIBITORS ON LIPID LEVELS IN NEWLY DIAGNOSED HYPERTENSIVE PATIENTS WITH TYPE 2 DIABETES MELLITUS. Eskisehir Med. J. 4, 73–79 (2023).
Google Scholar - Chilton, R. et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes. Metab. 17, 1180–1193. https://doi.org/10.1111/dom.12572 (2015).
Article CAS PubMed PubMed Central MATH Google Scholar - You, Y., Zhao, Y., Chen, M., Pan, Y. & Luo, Z. Effects of empagliflozin on serum uric acid level of patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetol. Metab. Syndr. 15, 202. https://doi.org/10.1186/s13098-023-01182-y (2023).
Article CAS PubMed PubMed Central Google Scholar - Tanaka, A. et al. Blood pressure reduction with empagliflozin in Japanese patients with type 2 diabetes and cardiovascular diseases: a post-hoc sub-analysis of the placebo-controlled randomized EMBLEM trial. Hypertens. Res. https://doi.org/10.1038/s41440-024-01725-4 (2024).
Article PubMed PubMed Central MATH Google Scholar - Zhang, Q., Zhou, S. & Liu, L. Efficacy and safety evaluation of SGLT2i on blood pressure control in patients with type 2 diabetes and hypertension: a new meta-analysis. Diabetol. Metab. Syndr. 15, 118. https://doi.org/10.1186/s13098-023-01092-z (2023).
Article CAS PubMed PubMed Central MATH Google Scholar - Shin, Y., Moon, J. H., Chin, H. J., Ferrannini, E. & Lim, S. Glycemic efficacy and metabolic consequences of an Empagliflozin add-on versus conventional dose-increasing strategy in patients with type 2 diabetes inadequately controlled by Metformin and Sulfonylurea. Endocrinol. Metab. (Seoul). 35, 329–338. https://doi.org/10.3803/EnM.2020.35.2.329 (2020).
Article CAS PubMed Google Scholar - Moon, J. S. et al. Efficacy and safety of treatment with quadruple oral hypoglycemic agents in uncontrolled type 2 diabetes Mellitus: a Multi-center, Retrospective, Observational Study. Diabetes Metab. J. 45, 675–683. https://doi.org/10.4093/dmj.2020.0107 (2021).
Article PubMed MATH Google Scholar - Kim, M. et al. The efficacy of treatment intensification by quadruple oral therapy compared to GLP-1RA therapy in poorly controlled type 2 diabetes Mellitus patients: a real-world Data Study. Diabetes Metab. J. 47, 135–139. https://doi.org/10.4093/dmj.2021.0373 (2023).
Article CAS PubMed Google Scholar
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
- Endocrinology and Metabolism Research Center (EMRC), School of Medicine, Vali-Asr Hospital, Tehran University of Medical Sciences, P.O. Box: 13145-784, Tehran, Iran
Fatemeh Moosaie, Shiva Abedinzadeh, Soghra Rabizadeh, Kimia Daneshvar, Mohammadamin Noorafrooz, Fatemeh Alsadat Mojtahedi, Niloofar Deravi, Akam Ramezani, Sahar Karimpour Reyhan, Mahsa Abbaszadeh, Manouchehr Nakhjavani & Alireza Esteghamati - International Surgical Research Association (ISRA), Universal Scientific Education and Research Network (USERN), Tehran University of Medical Sciences, Tehran, Iran
Fatemeh Moosaie - Psychosomatic Medicine Research Center, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran
Fatemeh Moosaie & Marzieh Hajibabaei - Ophthalmic Research Center, Research Institute for Ophthalmology and Vision Science, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Kimia Daneshvar - Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Niloofar Deravi - Department of Pediatrics, Imam Ali Hospital, Alborz University of Medical Sciences, Karaj, Iran
Seyede Marzie Fatemi Abhari - Department of Community Medicine, Tehran University of Medical Sciences, Tehran, Iran
Alipasha Meysamie
Authors
- Fatemeh Moosaie
- Shiva Abedinzadeh
- Soghra Rabizadeh
- Kimia Daneshvar
- Mohammadamin Noorafrooz
- Fatemeh Alsadat Mojtahedi
- Niloofar Deravi
- Seyede Marzie Fatemi Abhari
- Akam Ramezani
- Alipasha Meysamie
- Marzieh Hajibabaei
- Sahar Karimpour Reyhan
- Mahsa Abbaszadeh
- Manouchehr Nakhjavani
- Alireza Esteghamati
Contributions
Conception and design of work was conducted by A.E, F.M and S.R. Drafting the manuscript was done by S.A, K.D, M.A.N, S.M.F.A, N.D, A.R and M.H. Data analysis and interpretation was conducted by F.M and A.M. Critical revision of the article was done by A.E, F.M, M.N S.K.R and M.A. All the authors approved the final version of the manuscript.
Corresponding author
Correspondence toAlireza Esteghamati.
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent: Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Moosaie, F., Abedinzadeh, S., Rabizadeh, S. et al. Empagliflozin-based quadruple oral therapy for type 2 diabetes: a prospective cohort study.Sci Rep 15, 1427 (2025). https://doi.org/10.1038/s41598-024-84993-x
- Received: 04 August 2024
- Accepted: 30 December 2024
- Published: 09 January 2025
- Version of record: 09 January 2025
- DOI: https://doi.org/10.1038/s41598-024-84993-x