Randomised controlled trial comparing single agent paclitaxel vs epidoxorubicin plus paclitaxel in patients with advanced ovarian cancer in early progression after platinum-based chemotherapy: an Italian Collaborative Study from the ‘Mario Negri’ Institute, Milan, G.O.N.O. (Gruppo Oncologico Nord Ovest) group and I.O.R. (Istituto Oncologico Romagnolo) group (original) (raw)
Main
Ovarian cancer, fifth cause of cancer death in European women, is the most common cause of death among women with gynaecological malignancies. An estimated 25 200 new cases of ovarian cancer are diagnosed annually in the US and about 14 500 women die every year (Landis et al, 1999). Ovarian cancer is often diagnosed in advanced phase (stage III and IV) and the prognosis is generally poor despite activity shown by chemotherapy agents. Although up to 80% of cases achieve an objective response with platinum-containing regimens, about 22% of patients progress while receiving initial platinum-based treatment and are defined refractory to chemotherapy (Kavanagh et al, 1995), 40% of patients generally relapse or progress within 12 months and 20% after 12 months from the end of first-line chemotherapy (Ozols and Young, 1991; Thigpen, 1999). The probability of a second response increases with the relapse-free period (Seltzer et al, 1985; Gore et al, 1990; Markman et al, 1991; Weiss et al, 1991; Zanaboni et al, 1991; Eisenhauer et al, 1997; Cantù et al, 2002).
For these patients, second-line treatment include drugs that are noncrossresistant with platinum compounds including ifosfamide, anthracyclines at standard or high dose (Markman et al, 1992; Thigpen et al, 1993; Vermorken et al, 1994; Vermorken and Pecorelli, 1996) and, more recently, taxanes.
In a review analysis, Vermorken et al (1999) showed that 25 out of 75 (33%) chemotherapy patients from several trials achieved an objective response to single agent doxorubicin. The results of single agent epirubicin appear to be better in prior platinum-exposed patients. Paclitaxel, an antimitotic agent derived from bark of Pacific Yew tree Taxus brevifolia (Schiff et al, 1979), proved to be active in relapsed and platinum-resistant ovarian cancer (McGuire et al, 1989; Einzig et al, 1992; Trimble et al, 1993; Kohn et al, 1994; Gore et al, 1995) and was well tolerated by most patients: significant side effects other than alopecia were uncommon (Seidman et al, 1996). Moreover, preliminary data in advanced breast cancer patients seemed to suggest that the combination of paclitaxel plus doxorubicin might have a potential interaction in efficacy (Gianni et al, 1994). In absence of sound data to support the use of paclitaxel as single agent or in combination with anthracyclines in the second-line treatment of ovarian cancer, we set up a randomised multicenter phase III trial. This trial aimed at evaluating the efficacy and feasibility of paclitaxel as single agent compared to a combination including paclitaxel and epidoxorubicin, administered as second-line chemotherapy for epithelial ovarian cancer patients progressing within 1 year from the end of first-line platinum-based chemotherapy. Patients exposed to largely sub-maximally cumulative doses of anthracyclines in first-line platinum-based chemotherapies were deemed still eligible for this study.
Materials and methods
Design
The study was run as parallel trial, adopting a randomisation ratio between arms of 1 : 1. Randomisation was performed calling the coordinating centre located at the Istituto di Ricerche Farmacologiche ‘Mario Negri’, Milan. A stratification was adopted considering centre and response achieved after first-line chemotherapy, in terms of progression during treatment (refractory patients), partial or stable disease, relapse within 12 months.
Ethics committee
The study protocol was revised and accepted by local ethical committees; informed consent was obtained before randomisation from all patients in accordance with national legislation following the principles enunciated in the Declaration of Helsinki (Vastag, 2000).
Eligibility
Patients with histological proven ovarian carcinoma platinum resistant, relapsed or progressed within 12 months since the end of a first-line therapy containing cisplatin or carboplatin, were eligible for the study. Further eligibility criteria included: measurable disease, World Health Organization (WHO) performance status ⩽2; adequate bone marrow function (absolute granulocyte count ⩾2 000 mm−3, platelet count ⩾100 000 mm−3); adequate renal, hepatic and cardiac functions. Patients were considered not eligible if they had received more than one previous chemotherapy line or if the first-line chemotherapy contained taxanes. Prior anticancer treatment with anthracyclines was allowed provided patients had not received cumulative doses in excess of 300 or 360 mg m−2 for doxorubicin or epidoxorubicin, respectively.
Chemotherapy regimen
Patients were randomised to receive T or ET, intravenously, at 3-week intervals, as soon as possible after randomisation.
Paclitaxel was given 175 mg m−2 over 3 h infusion. Premedication given to reduce the risk of paclitaxel hypersensitivity, was as follows: Desamethasone 40 mg, given as 20 mg at 12 and 6 h before paclitaxel, 300 mg of Cimetidine and 10 mg of Chlorpheniramine intravenously, 30 min before the treatment. Epidoxorubicin 80 mg m−2 intravenously was administered as a bolus followed by paclitaxel 175 mg m−2 over 3 h infusion, as already described. Each patient had to receive a minimum of four cycles of chemotherapy but patients with progressing disease at the first 2 month evaluation were considered off study and the assigned treatment was interrupted. After completion of four cycles, two additional cycles of protocol treatment were proposed to all patients not showing disease progression. Patients were allowed to receive any third-line chemotherapy at investigators' discretion.
Treatment modifications
Toxiciy was graded on a scale of 1–4 according to the WHO criteria. If, at the time of scheduled retreatment, white blood cell count was ⩾1 500 mm−3 and platelets count ⩾100 000 mm−3, chemotherapy was given without reductions or delays, otherwise it was delayed by 1 week or reduced according to type and grade of toxicity. The following indications of toxicity led to dose modification: (a) nadir neutrophil count between 500 and 1 000 mm−3 or nadir platelet count between 50 000 and 100 000 mm−3: dose level – 1 (see below); (b) nadir neutrophil count less than 500 mm−3 or nadir platelet count less than 50 000 mm−3: dose level – 2 (see below); (c) any nonhaematologic grade 2 toxicity: dose level – 1; (d) any nonhaematologic toxicity more than grade 2: treatment to be interrupted until the adverse effects resolve. Dose levels were as follows for paclitaxel and epidoxorubicin, respectively. Initial dose, 175 and 80 mg m−2; level – 1, 135 and 65 mg m−2; level – 2 110 and 50 mg m−2.
Assessment of response
Response to study drug, assessed using ECOG criteria (Miller et al, 1981), was based on objective tumour evaluation every two cycles.
A complete response (CR) was defined as complete disappearance of all measurable and assessable lesions no new lesions and no related symptoms. A partial response (PR) was documented in patients with a ⩾50% decrease in the sum of the products of bidimensional perpendicular diameters of all measurable lesions. Progressive disease (PD) was said to occur in patients with a ⩾50% increase in the sum of the products of bidimensionally measured lesions over the smallest sum obtained at best response, or clear worsening of any assessable disease, or failure to return to evaluation because of death or deteriorating condition, or the appearance of any new lesion or site. Patients were classified as having stable disease if they did not qualify for CR, PR or PD.
Follow-up programme
Once patients were off the protocol treatments, they were monitored for assessment of disease status and long-term toxicities every 3 months for 2 years and every 6 months thereafter. Follow-up procedures comprised clinical examination, blood chemistry and CA 125 (optional) estimation. ECG and echocardiography for cardiac function assessment were scheduled at 6 and 12 months and yearly thereafter; routine computed tomography, abdomen echography or chest radiography were not required but were requested if biomarkers rose or symptoms developed.
Analysis
The primary outcome measure was overall survival; secondary outcomes were progression-free survival and response to treatment.
The trial was designed to have an 80% power to detect a 33% relative reduction in mortality (i.e. increasing median overall survival of about 6 months), corresponding to a hazard ratio of 0.67 with a two-sided α of 0.05. We anticipated that 230 patients would have to be recruited to the trial to meet these specifications. The final analysis was designated to occur when at least 85% of the eligible patients experienced either progression or death.
We compared Kaplan–Meier curves for overall survival and progression-free survival using the Mantel–Cox version of the log-rank test (Kaplan and Meier, 1958; Makuch and Simon, 1982). The Cox proportional hazards regression model (Parmar and Machin, 1995) was also used for progression-free and overall survival to estimate the treatment relative hazards (HRs), while adjusting for multiple prognostic factors.
Response to chemotherapy treatments was compared with Mantel–Haenszel _χ_2 test for trend.
Overall survival was defined as the time from randomisation to death from any cause; patients known to be still alive at the time of the analysis were censored at the time of their last follow-up. Progression-free survival was defined as the time from randomisation to first appearance of PD or death from any cause; patients known to be alive and without PD at the time of analysis were censored at the time of their last follow-up. Absolute benefits at specific time points were calculated using the Kaplan–Meier estimate of survival in the control group at the time point (control survival) and hazard ratio with the expression: absolute benefit=exp (hazard ratio × log (control survival))−control survival. This approach was also adopted for the end point of progression-free survival. Although this approach implicitly assumes proportional hazards, it is preferable to reading differences between Kaplan–Meier curves at individual time points. Differences in medians were calculated in a similar way but using the expression difference in medians=(control median/hazard ratio)−control median. This approach assumes approximately exponentially distributed survival times. The relative benefits of platinum-based chemotherapy on survival were assessed in an exploratory manner in subgroups defined by prior use of anthracyclines and refractory status. To test for differences in the relative size of effect in different subgroups, we used a _χ_2 test for interaction, or, when appropriate, a _χ_2 test for trend.
All analyses were performed on an intention-to-treat basis on eligible patients except for the analyses of toxicity. The latter analyses were restricted to all patients who received at least one cycle of allocated treatment. All _P_-values are two-sided. Analyses were carried out using SAS System Version 8.20. No interim analysis was planned or performed.
Results
Accrual
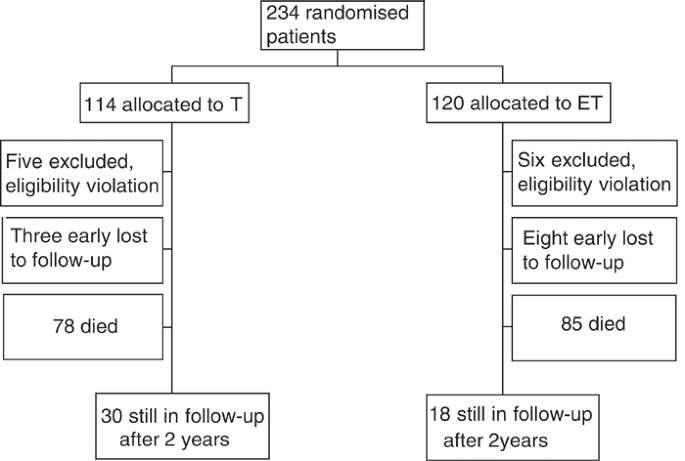
Between October 1994 and June 1999, 234 patients were enrolled into the trial from 34 Italian centres. Final survival analysis was performed on 212 patients, as there were 11 major violations of the protocol (six patients were given paclitaxel in first-line therapy, two had more then one first-line therapy, two patients had a disease-free interval greater than 12 months and one patient had cardiac impairment) and 11 further patients were early lost to follow-up. Figure 1 reports the flow chart of the progress of patients through the trial.
Figure 1
Flow chart of the progress of patients through the trial
Patient characteristics
Pretreatment characteristics were well balanced within randomised groups as listed in Table 1: median age was about 60 years, platinum-based monochemotherapy was the first-line treatment in 43% of patients. Polichemotherapy containing anthracyclines was the preferred first-line therapy in 22% of patients. Progression during first-line chemotherapy was observed in 32% of cases. Median time from the end of first-line chemotherapy was 3 months.
Table 1 Patient characteristics
Compliance and toxicity
In all, 87 and 85% of the patients completed the scheduled therapy in T and ET, respectively. However, modification of dosage or time accounted for 9 and 41% of cases (P<0.001), and interruption due to toxicity were 9 and 13% (_P_=NS), respectively. Four percent of patients in both arms refused therapy after randomisation. A median number of six courses, with an interquartile range from 4 to 6, was given to T group and a median number of five courses, with an interquartile range from 3 to 6, was given to ET group. Table 2 shows the proportion of patients with toxic effects observed during treatment. Levels of haematological toxicity were quite different across the arms. The ET was associated with higher rates of grade 3 or 4 haematological toxicity: leuckopenia and neutropenia. Other nonhaematological toxicities occurred as expected and the toxicity profiles of the two groups were similar.
Table 2 Toxicity evaluation
Response
Response evaluation results are shown in Table 3. Data were available in 181 patients. The overall response rate was 46.9% in T (CR: 20.2%; PR: 26.7%) and 37.4% in ET (CR: 15.4%; PR: 22.0%), while PD was observed in 27.8 and 36.3% of patients, respectively (Mantel–Haenszel _χ_2 test for trend: 1.89, _P_=0.17). The overall high rate of overall response was largely due to the patients progressing after 6 months since the end of first-line chemotherapy. In fact, a subset analysis in this population showed an overall response rate as high as 60% in T and 48% in ET.
Table 3 Response evaluation
Overall survival
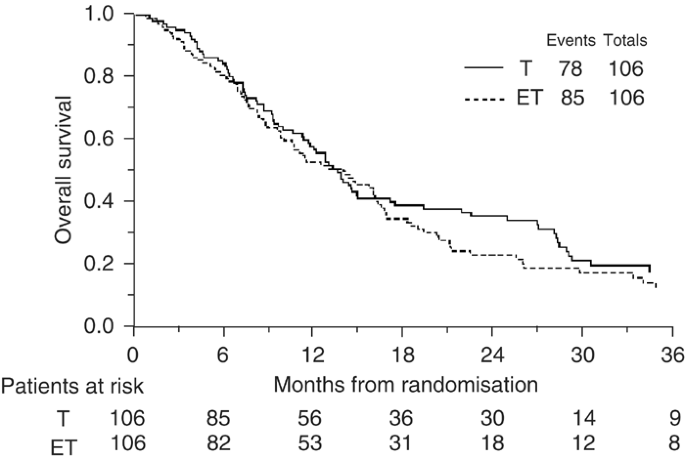
At a median follow-up of 48 months, 163 (76.9%) patients have died. Comparing the survival curves (Figure 2), the hazard ratio of 1.17 (95% CI: 0.86–1.59; _P_=0.33) translates into an absolute difference in 1-year survival of 6% (95% CI for difference: −5 to 16%) in favour of single agent paclitaxel, from 50 to 56%. In terms of median survival, the hazard ratio translates into a difference of 2 months (95% CI for difference: -2 to 5 months) with a median survival of 14 months on the paclitaxel alone arm and 12 months on the paclitaxel plus epidoxorubicin chemotherapy arm.
Figure 2
Overall Survival
Progression-free survival
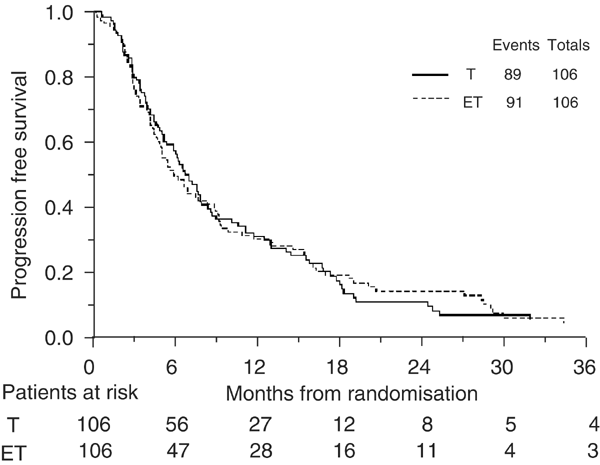
A total of 180 (84.9%) patients have progressed or died. Progression-free interval curves are shown in Figure 3. The hazard ratio of 1.00 (95% CI: 0.75–1.35; _P_=0.96) translates into an absolute difference in 6-months survival of 0% (95% CI for difference: −10 to 10%). In terms of median survival, the hazard ratio translates into a difference of 0 months (95% CI for difference: −2 to 2 months) with a median survival of 6 months on both chemotherapy arms.
Figure 3
Progression-free survival
Effects in different subgroups
We found no definite evidence that paclitaxel plus epidoxorubicin was more or less effective than paclitaxel alone in any subgroup for either overall survival or progression-free survival (Table 4). However, a statistically borderline test for interaction suggests that previous exposure to anthracyclines-based first-line chemotherapy might interact in the relative performance of the two regimens, favouring ET combination.
Table 4 Effect of chemotherapy in different subgroups
Discussion
Salvage therapies in relapsed epithelial ovarian cancer are rarely curative and usually result in low response rates with small impact on survival. The current management of patients with recurrent ovarian carcinoma is based on the results of the initial chemotherapy. In the early 1990s, paclitaxel became the major second-line treatment for ovarian cancer and soon after gained the role of standard first-line treatment in combination with platinum compounds (Thigpen et al, 1994; Kavanagh et al, 1995). More recently, the negative results of two large-scale clinical trials (Muggia et al, 2000; ICON3 group, 2002) have challenged the general consensus on paclitaxel as a first-line therapeutic approach. In accordance with these new evidences, for example, the UK National Institute for Clinical Excellence (NICE) has revised its guidance on the use of paclitaxel in the treatment of ovarian cancer in January 2003 ([NICE, 2003](/articles/6601787#ref-CR20 "NICE (2003) National Institute for Clinical Excellence Technology Appraisal Guidance No 55. London: NICE www.nice.org.uk
")) and recommended that paclitaxel in combination with platinum-based compound or platinum-based therapy alone are offered as alternatives for first-line chemotherapy. This means that the patients who were eligible for our study still possibly represent a significant subset of the ovarian cancer patients currently referring to oncologic outpatient's departments. The most important aspect emerging from this study, which, to our knowledge, is one of the largest phase III randomised trials ever performed in recurrent ovarian cancer, is that the combination of two noncrossresistant drugs failed to achieve better results and was associated to an higher toxicity than the single agent paclitaxel in the management of advanced ovarian cancer patients in early progression after platinum-based chemotherapy. Our data can be compared with the results emerged from the study of [Bolis et al (1999)](/articles/6601787#ref-CR1 "Bolis G, Parazzini F, Scarfone G, Villa A, Amoroso M, Rabaiotti E, Polatti A, Reina S, Piroetti E (1999) Paclitaxel vs epidoxorubicin plus paclitaxel as second-line therapy for platinum-refractory and resistant ovarian cancer. Gynecol Oncol 72: 60–64"). In this multicenter phase III randomized trial, combination of paclitaxel plus epidoxorubicin gave a higher response rate than single agent paclitaxel. In 81 evaluable patients, the overall response rates was 17% in paclitaxel arm and 34% in the combination arm but, in keeping with our findings, toxicity was higher in combination therapy, duration of response was limited to a few months, and the 2-year survival was similar between the two groups. Typically, response rates to second-line chemotherapy in platinum-resistant recurrent ovarian cancer is modest although overall response rates up to 40% have been reported in the literature ([Salom et al, 2002](/articles/6601787#ref-CR23 "Salom E, Almeida Z, Mirhashemi R (2002) Management of recurrent ovarian cancer: evidence-based decisions. Curr Opin Oncol 14: 519–527")). The high proportion of patients achieving an objective response in our study is mainly due to the fact than about a quarter of our study population had a disease-free interval greater than 6 months (but less than 12 months), while most of the series of platinum-resistant patients included only patients recurring within 6 months. However, comparing response rates across studies makes clear that a strong relationship between response and survival is not to be expected in this setting and therefore clinical outcome measures related to survival or quality of life should be preferred for efficacy assessment.The findings of this trial indicate that the combination of paclitaxel plus epidoxorubicin does not seem to be more effective than single agent paclitaxel in patients in early progression after first-line platinum-based treatment. In subset analyses, the appearance of a statistically borderline test for interaction suggesting a different performance on survival of ET when compared with T in patients who were given anthracyclines in first-line therapy came unexpected. We deemed this finding devoid of a real clinical value apart from confirming that anthracyclines resistance did not develop in these patients.
If the combination of common cytotoxic drugs does not seem to improve survival in patients with refractory ovarian cancer, further studies are necessary to address the open questions regarding the best second-line therapy in the recurrent disease setting. There is now evidence suggesting that drug resistance is partly a result of defects in the apoptotic pathway that, after previous chemotherapy, can determine selection of tumour cells, more resistant to subsequent agents (Cannistra, 2002). Also, the role of paclitaxel has to be further defined: recent data suggest that a possible role of paclitaxel may be in cisplatin-resistant clones of cells that overexpress mutated p53 protein (Smith-Sorensen et al, 1998; Judson et al, 1999). Large comparative trials of second-line treatment should be planned to test the available new active agents, which have different mechanisms of action and potentially limited serious toxicity, while offering the best framework to improve our knowledge of predictive factors.
Collaborators and affiliations
Avellino, Casa di Cura Malzoni: A Vernaglia Lombardi, M Malzoni; Centro di Riferimento Oncologico IRCCS, Aviano: S Tumolo; Ospedale Policlinico Universitario, Bari: G Cormio; Ospedale degli Infermi ASL 12, Biella: A Monaco; Ospedale Regionale, Bolzano: F Welponer; Ospedale Cannizzaro, Catania: R Ruggeri, P Scollo; Ospedale L Currò, Catania: D Priolo; Ospedale Generale Valduce, Como: L Redaelli; Ospedale di Circolo, Desio: G Orfanotti; Azienda Ospedaliera S Anna, Ferrara: R Martinello; Ospedale S Antonio Abate, Gallarate: M Borsani, S Garsia; Ospedale Santa Maria Goretti, Latina: M D'Aprile; Azienda Ospedaliera, Lecco: N Natale; Azienda Ospedaliera C Poma, Mantova: G Cavazzini; Istituto Europeo di Oncologia, Milano: G Parma, L Bocciolone; Ospedale San Carlo Borromeo, Milano: MC Locatelli; Ospedale San Gerardo dei Tintori, Università Milano-Bicocca, Monza: MG Cantù, S Chiari; Ospedale Civile, Padova: MO Nicoletto; Università di Padova, Pat. Ginecologica: UM Fiorentino; Ospedale V Cervello, Palermo: D Gueli Alletti, C Strazzeri; Ospedale Civile Agnelli, Savigliano, Cuneo: L Galletto; Unità Sanitaria Locale Roma no 26, Tivoli, Roma: G Corrado; Università Dipartimento Scienze Ginecologiche e Ostetriche, Torino: A Durando; Ospedale Civile Consortile, Treviglio: R Grassi; Azienda Ospedaliera, Verona: C Griso.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
- Bolis G, Parazzini F, Scarfone G, Villa A, Amoroso M, Rabaiotti E, Polatti A, Reina S, Piroetti E (1999) Paclitaxel vs epidoxorubicin plus paclitaxel as second-line therapy for platinum-refractory and resistant ovarian cancer. Gynecol Oncol 72: 60–64
Article CAS Google Scholar - Cannistra S (2002) Is there a ‘best’ choice of second-line agent in the treatment of recurrent, potentially platinum-sensitive ovarian cancer? J Clin Oncol 20: 1158–1160
Article Google Scholar - Cantù MG, Buda A, Parma G, Rossi R, Floriani I, Bonazzi C, Dell'Anna T, Torri V Colombo N (2002) Randomized controlled trial of single-agent paclitaxel versus cyclophosphamide, doxorubicin, and cisplatin in patients with recurrent ovarian cancer who responded to first-line platinum-based regimens. J Clin Oncol 20: 1232–1237
Article Google Scholar - Einzig AI, Wiernik PH, Sasloff J, Runowicz CD, Goldberg GL (1992) Phase II and long-term follow-up of patients treated with Paclitaxel for advanced ovarian adenocarcinoma. J Clin Oncol 10: 1748–1753
Article CAS Google Scholar - Eisenhauer EA, Vermorken JB, van Glabbeke M (1997) Predictors response to subsequent chemotherapy in platinum pre-treated ovarian cancer: a multivariate analysis of 704 patients. Ann Oncol 8: 963–968
Article CAS Google Scholar - Gianni L, Straneo M, Capri G, Villani F, Munzone E, Bonadonna G (1994) Optimal dose and sequence finding study of paclitaxel (p) by 3 h infusion combined with bolus doxorubicin (d) in untreated metastatic breast cancer patients (pts). Proc Am Soc Clin Oncol 74: 97
Google Scholar - Gore ME, Fryatt I, Wiltshaw E, Dawson T (1990) Treatment of relapsed carcinoma of the ovary with cisplatin or carboplatin following initial treatment with these compounds. Gynecol Oncol 36: 707–711
Article Google Scholar - Gore ME, Levy V, Rustin G, Perren T, Calvert AH, Earl H, Thompson JM (1995) Paclitaxel (Paclitaxel) in relapsed and refractory ovarian cancer: the UK and Eire experience. Br J Cancer 72: 1016–1019
Article CAS Google Scholar - Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53: 457–481
Article Google Scholar - Kavanagh J, Tresukosol D, Edwards C, Freedman R, Gonzales de Leon C, Fishman A, Mante R, Hord M, Kudelka A (1995) Carboplatin reinduction after taxane in patients with platinum-refractory epithelial ovarian cancer. J Clin Oncol 13: 1584–1588
Article CAS Google Scholar - Kohn EC, Sarosy G, Bicher A, Link C, Christian M, Steinberg SM, Rothenberg M, Adamo DO, Davis P, Ognibene FP (1994) Dose-intense Paclitaxel: response rate in patients with platinum-resistant recurrent ovarian cancer. J Natl Cancer Inst 86: 18–24
Article CAS Google Scholar - Judson PL, Watson JM, Gehrig PA, Fowler Jr WC, Haskill JS (1999) Cisplatin inhibits paclitaxel-induced apoptosis in cisplatin-resistant ovarian cancer cell lines: possible explanation for failure of combination therapy. Cancer Res. 59: 2425–2432
CAS PubMed Google Scholar - Landis SH, Murray T, Bolden S, Wingo PA (1999) Cancer statistics, 1999. CA Cancer J Clin 49: 8–31
Article CAS Google Scholar - Makuch RW, Simon RM (1982) Sample size requirements for comparing time-to-failure among k treatment groups. J Chron Dis 35: 861–867
Article CAS Google Scholar - Markman M, Hakes T, Reichman B, Lewis Jr JL, Rubin S, Jones W, Almadrones L, Pizzuto F, Hoskins W (1992) Ifosfamide and mesna in previously treated advanced epithelial ovarian cancer: activity in platinum-resistant disease. J Clin Oncol 10: 243–248
Article CAS Google Scholar - Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, Jones W, Almadrones L, Lewis Jr JL (1991) Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol 9: 389–393
Article CAS Google Scholar - McGuire WP, Rowinsky EK, Rosenshein NB, Grumbine FC, Ettinger DS, Armstrong DK, Donehower RC (1989) Taxol: a unique anti-neoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med 111: 273–279
Article CAS Google Scholar - Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47: 207–214
Article CAS Google Scholar - Muggia FM, Braly PS, Brady MF, Sutton G, Niemann TH, Lentz SL, Alvarez RD, Kucera PR, Small JM (2000) Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 18: 106–115
Article CAS Google Scholar - NICE (2003) National Institute for Clinical Excellence Technology Appraisal Guidance No 55. London: NICE www.nice.org.uk
- Ozols RF, Young RC (1991) Chemotherapy of ovarian cancer. Semin Oncol 3: 222–232
Google Scholar - Parmar MKB, Machin D (1995) Survival Analysis: a Practical Approach. Chichester: John Wiley
Google Scholar - Salom E, Almeida Z, Mirhashemi R (2002) Management of recurrent ovarian cancer: evidence-based decisions. Curr Opin Oncol 14: 519–527
Article Google Scholar - Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtubule assembly in vitro by Paclitaxel. Nature 11: 716
Google Scholar - Seidman AD, Hochhauser D, Gollub M, Edelman B, Yao TJ, Hudis CA, Francis P, Fennelly D, Gilewski TA, Moynahan ME, Currie V, Baselga J, Tong W, O'Donaghue M, Salvaggio R, Auguste L, Spriggs D, Norton L (1996) Ninety-six hour Paclitaxel infusion after progression during short taxane exposure: a phase II pharmacokinetic and pharmacodynamic study in metastatic breast cancer. J Clin Oncol 14: 1877–1884
Article CAS Google Scholar - Seltzer V, Vogl S, Kaplan B (1985) Recurrent ovarian carcinoma: treatment utilizing combination chemotherapy including cisdiamminedichloroplatinum in patients previously responding to this agent. Gynecol Oncol 21: 167–176
Article CAS Google Scholar - Smith-Sorensen B, Kaern J, Holm R, Dorum A, Trope C, Borresen-Dale AL (1998) Therapy effect of either Paclitaxel or cyclophosphamide combination treatment in patients with epithelial ovarian cancer and relation to TP53 gene status. Br J Cancer 78: 375–381
Article CAS Google Scholar - The International Collaborative Ovarian Cancer Neoplasm (ICON) Group (2002) Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet 360: 505–515
- Thigpen JT, Blessing JA, Ball H, Hummel SJ, Barrett RJ (1994) Phase II trial of Paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: a Gynecologic Oncology Group study. J Clin Oncol 12: 1748–1753
Article CAS Google Scholar - Thigpen JT, Vance RB, Khansur T (1993) Second-line chemotherapy for recurrent carcinoma of the ovary. Cancer 71: 1559–1564
Article CAS Google Scholar - Thigpen T (1999) Second-Line Therapy for Ovarian Carcinoma: General Concepts, ASCO Educational book: Atlanta, GA: American Society of Clinical Oncology 564–566
- Trimble EL, Adams JD, Vena D, Hawkins MJ, Friedman MA, Fisherman JS, Christian MC, Canetta R, Onetto N, Hayn R (1993) Paclitaxel for platinum-refractory ovarian cancer: results from the first 1,000 patients registered to National Cancer Institute Treatment Referral Center 9103. J Clin Oncol 11: 2405–2410
Article CAS Google Scholar - Vastag B (2000) Helsinki discord? A Controversial Declaration. JAMA 284: 2983–2985
Article CAS Google Scholar - Vermorken JB, Bolis G, van Rijswijk RE, Kobierska A, Chevallier B, van der Burg ME, Zanaboni F, Lentz MA, Burger CW, Kenemans P, Pinedo HM (1994) High-dose intensity regimens with epirubicin in ovarian cancer. Semin Oncol 21: 17–22
CAS PubMed Google Scholar - Vermorken JB, Harper PG, Buyse M (1999) The role of anthracyclines in epithelial ovarian cancer. Ann Oncol 10: 43–50
Article Google Scholar - Vermorken JB, Pecorelli S (1996) Clinical trials in patients with epithelial ovarian cancer: past, present and future. Eur J Surg Oncol 22: 455–466
Article CAS Google Scholar - Weiss G, Geen S, Albert DS, Thigpen JT, Hines HE, Hanson K, Pierce HI, Baker LH, Goodwin JW (1991) Second-line treatment of advanced measurable ovarian cancer with iproplatin: a Southwest Oncology Group Study. Eur J Cancer 27: 135–138
Article CAS Google Scholar - Zanaboni F, Scarfone G, Presti M, Maggi R, Borello C, Bolis G (1991) Salvage chemotherapy for ovarian cancer recurrence: weekly cisplatin in combination with epirubicin or etoposide. Gynecol Oncol 43: 24–28
Article CAS Google Scholar
Acknowledgements
We thank all the women who participated in this trial and Fondazione Mattioli, who provided support for Data Management. We also thank all the research staff at centres who helped to recruit patients and provide data.
Author information
Authors and Affiliations
- Istituto di Ricerche Farmacologiche ‘Mario Negri’, Milan, Italy
A Buda, I Floriani, V Torri & R Fossati - Ospedale San Gerardo, Monza, Italy
R Rossi & C Mangioni - European Institute of Oncology, Milan, Italy
N Colombo - Ospedale S Chiara, Policlinico Universitario, Pisa, Italy
P F Conte - Ospedale degli Infermi, Rimini, Italy
A Ravaioli
Authors
- A Buda
You can also search for this author inPubMed Google Scholar - I Floriani
You can also search for this author inPubMed Google Scholar - R Rossi
You can also search for this author inPubMed Google Scholar - N Colombo
You can also search for this author inPubMed Google Scholar - V Torri
You can also search for this author inPubMed Google Scholar - P F Conte
You can also search for this author inPubMed Google Scholar - R Fossati
You can also search for this author inPubMed Google Scholar - A Ravaioli
You can also search for this author inPubMed Google Scholar - C Mangioni
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toA Buda.
Additional information
Presented in part at the Thirty-Six Annual Meeting of the American Society of Clinical Oncology, New Orleans, LA, May 20–23, 2000.
Other collaborators and their affiliation listed at the end of paper.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Buda, A., Floriani, I., Rossi, R. et al. Randomised controlled trial comparing single agent paclitaxel vs epidoxorubicin plus paclitaxel in patients with advanced ovarian cancer in early progression after platinum-based chemotherapy: an Italian Collaborative Study from the ‘Mario Negri’ Institute, Milan, G.O.N.O. (Gruppo Oncologico Nord Ovest) group and I.O.R. (Istituto Oncologico Romagnolo) group.Br J Cancer 90, 2112–2117 (2004). https://doi.org/10.1038/sj.bjc.6601787
- Received: 09 October 2003
- Revised: 28 January 2004
- Accepted: 02 February 2004
- Published: 20 April 2004
- Issue Date: 01 June 2004
- DOI: https://doi.org/10.1038/sj.bjc.6601787