A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas (original) (raw)
Main
Neuroendocrine carcinomas (NEC) comprise a family of neoplasms derived from the diffuse neuroendocrine system with a wide range of morphologic, functional and behavioural characteristics (Jensen and Doherty, 2005). Conventional chemotherapy has not shown a significant activity in advanced NEC, except for islet cell carcinomas (ICC) where streptozocin-based combinations with either 5-fluorouracil or doxorubicin have produced partial remissions in 40–60% of selected patients (Moertel et al, 1982; Moertel et al, 1992). However, carcinoid tumours (CT) seem to be quite chemo-resistant, with response rates of <10% and a median 5-year survival of 18% (Oberg, 2001). Alternative treatments including _α_-interferon 2b and local hepatic therapies for isolated metastases, such as hepatic arterial bland or chemo-embolisation, resection and radiofrequency ablation, have not demonstrated significant impacts on overall survival (OS).
The mammalian target of rapamycin (mTOR) is a serine–threonine kinase that participates in the regulation of cell growth, proliferation and apoptosis through modulation of cell cycle progression (Vignot et al, 2005). Mammalian target of rapamycin regulates initiation of cap-dependent translation by phosphorylating 4E-binding protein 1, releasing eukaryotic initiation factor 4E (eIF4E) to bind with eIF4G of the 4E initiation protein complex. Mammalian target of rapamycin also modulates ribosomal function by phosphorylating p70S6 kinase which activates the ribosomal protein S6 (Podsypanina et al, 2001). Signalling through the PI3K/AKT/mTOR pathway leads to an increase in translation, particularly of proteins regulating cell cycle progression and metabolism. In cancer cells, aberrant activation of the pathway may occur through increased signalling via growth factor receptors, activating mutations/amplification of the pathway kinases or by loss of the tumour suppressor protein PTEN. The latter has been described in NEC (Wang et al, 2002). Temsirolimus (sirolimus 42-ester 2,2-bis hydroxymethyl propionic-acid; CCI-779) is a more water-soluble ester derivative of its parent compound sirolimus, selected for development as an anticancer agent based on its more favourable pharmaceutical characteristics and superior therapeutic index. Temsirolimus has already been tested in phase I and II trials with promising activity and good safety profile (Atkins et al, 2004; Baselga et al, 2004; Raymond et al, 2004; Chan et al, 2005). In phase I studies, rash and mucositis were dose-limiting, and other adverse events (AE) observed include eczematous reactions, dry skin, herpes-type lesions, mild myelosupression, hypercholesterolaemia and hypertriglyceridemia (Atkins et al, 2004; Baselga et al, 2004; Raymond et al, 2004).
The primary objective of this study was to examine the objective response rate (ORR) of temsirolimus in patients with recurrent or metastatic NEC. Secondary objectives were: (i) to assess drug toxicity; (ii) to determine through pharmacodynamic evaluations whether temsirolimus downregulates mTOR and modulates elements of the PI3K/AKT/mTOR pathway and (iii) to associate pretreatment molecular characteristics, and molecular changes between paired tumour biopsies, with clinical outcome.
Patients and methods
Eligibility
Patients were eligible if they were 18 years of age or older and had histologically or cytologically confirmed NEC of either carcinoid or pancreatic ICC pathologies. Patients had to have documented progressive metastatic disease within 6 months of study entry. Previous chemotherapy, investigational agents, radioactive therapies and/or radiation were allowed if completed >4 weeks before study entry. Previous local therapy (e.g. bland or chemo-embolisation) was allowed if completed >6 weeks before study entry. Patients were required to have measurable disease, an ECOG performance status ⩽2, normal serum cholesterol and triglyceride, adequate haematologic, hepatic, renal and cardiac functions and a life expectancy of >3 months. Patients had to have tumour lesions accessible for core biopsy, and must agree to undergo tumour biopsy before and 2 weeks after initiation of temsirolimus.
Treatment
Temsirolimus at 25 mg was administered as a 30-min intravenous infusion on a weekly schedule. Four weeks of treatment were considered as one cycle.
Assessment of toxicity
Adverse events were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events v3.0.
Dose modifications
Dose modifications of temsirolimus were based on haematologic and non-haematologic toxicities at the time of every weekly dose. Upon recovery of toxicity within a maximum delay of 3 weeks, temsirolimus may be re-started with a dose reduction. Stepwise dose modifications from 25 to 20, 15 and 10 mg were allowed, but doses once reduced cannot be re-escalated.
Response assessment
Radiological imaging was repeated every 8 weeks to assess for tumour response until disease progression, completion of study treatment or discharge of patient from study. Tumour responses were evaluated according to standard RECIST criteria (Therasse et al, 2000). Objective responses were confirmed by central independent radiological review.
Correlative studies
Archival tissues
Archival paraffin slides were stained for PTEN, p53, pAKT, pS6 and pmTOR (phosphorylated mTOR) by immunohistochemistry. Slides were pretreated and incubated with primary antibody (Appendix 1), followed by biotin-conjugated secondaries and HRP-Streptavidin labelling reagent (ID Labs Inc., London, Ontario, Canada). Two pathologists, who were blinded to clinical outcome (M-ST. and SA-R), evaluated independently for each marker: (i) the percentage of stained cells, which was converted to a four-tiered system (0=0; 1=⩽10%; 2=11–50% and 3=⩾51%), and (ii) the intensity of staining (0–3). The sum of both values was the specimen's score (range 0–6). The scores from the two pathologists were then averaged. PTEN was scored as either ‘lost’ or ‘retained’.
Computerised image analysis for paired tumour biopsies
Tumour biopsies taken before and after 2 weeks post-treatment with temsirolimus were collected into 10% neutral buffered formalin, fixed overnight and transferred in 70% ethanol for processing into paraffin blocks. Four micrometre thick sections were cut onto Surgipath x-tra™ slides. Slides were pretreated by either pepsin digestion or microwave retrieval and then incubated in primary antibody overnight inside a moist chamber (Appendix 1). This was followed with Cy3- or Cy5-conjugated secondaries (Jackson ImmunoResearch, West Grove, PA, USA) for immunofluorescence. Paired biopsies were stained for pAKT, pS6, pmTOR and peIF4G. Biopsy slides were imaged with a laser scanning TISSUEscope (Biomedical Photometrics, Waterloo, Ontario, Canada) using 2 _μ_m per pixel resolution. All images were analysed blinded using MCID Elite software (Imaging Research Inc., St Catharines, Ontario, Canada). A threshold was set to select for positive staining of a specific marker of interest. Results were expressed as the percentage of positively stained areas in square microns within the tumour regions, and the staining intensity reported as mean optical density (IOD) in grey levels.
Statistical analysis
The primary endpoint was objective tumour response rate (complete response (CR) or partial response (PR)). Secondary endpoints included toxicity, stable disease (SD) rate, response and SD duration, time to progression (TTP) and OS. A two-stage phase II design was used. The treatment combination would be assumed to be inactive if the objective response was at most 5% and active if it was at least 25%, with _α_=0.05 and _β_=0.10. After completion of accrual of 15 evaluable patients to stage I, while no CR or PR was observed, there were 10 patients who fulfilled the criteria for SD. After plotting out patient's pretreatment and post-treatment tumour progression rate, it was observed that at least three out of these 10 patients had a significant decrease in this parameter after starting temsirolimus, along with improvement in their disease-related symptoms. As a result of these findings, it was hypothesised that temsirolimus may have antitumour activity in this tumour type but possibly not reliably evaluated by conventional RECIST criteria. The protocol was amended and additional patients accrued to stage II.
The Kaplan–Meier method was used to estimate survival outcomes. Time to progression was measured from the first date a patient received study medication until the date of tumour progression. Progression-free survival (PFS) was estimated from the first date a patient received study medication until the date of progression, or death; OS was measured from the first date a patient received study medication until the date of death or last date the patient was known to be alive.
Molecular marker levels before temsirolimus treatment and their changes measured on study were investigated as predictors of objective tumour response, TTP and OS, using Spearman rank correlation coefficients and Cox proportional hazards regression. All tests were two-sided and _P_-values of 0.05 or less were considered statistically significant.
This study was a collaborative effort between three consortia, led by the Princess Margaret Hospital Phase II Consortium. Local institutional review board approvals were obtained at all participating centres.
Results
Patients
A total of 37 patients were accrued to the study from January 2004 to July 2005. One patient did not receive any treatment owing to progressive disease before treatment initiation and was considerable ineligible. Thirty-six patients received at least one dose of temsirolimus and were evaluated for safety. Patient characteristics are listed in Table 1.
Table 1 Patient characteristics
Efficacy
Tumour response
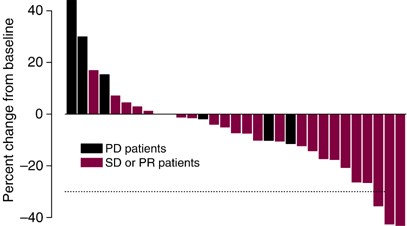
Two patients, one with CT and one with ICC, achieved a confirmed PR. One of them progressed after 18 cycles (11.4 months after first observation of PR) and the other came off study owing to unrelated cardiac disease (4.5 months after first observation of PR). A third patient had an unconfirmed PR at the end of cycle 8 and discontinued therapy not owing to toxicity. Twenty additional patients had SD of at least 2 months' duration and among these, 10 patients continued treatment beyond six cycles. Ten patients progressed on temsirolimus without ever achieving an objective response. Eight of these patients had radiological evidence of disease progression, one had symptomatic progression during cycle 1, and one patient died of disease before end of cycle 2. Figure 1 shows the maximum percentage of target tumour lesion(s) reduction compared to baseline as assessed by the RECIST criteria, listed by individual study patients.
Figure 1
Maximal percentages of tumour reduction for target lesion(s) by RECIST criteria (Note: some patients with PD progressed owing to new or increasing non-target lesions, or by symptomatic progression).
As serum markers such as chromogranin A or 5HIAA were not mandated in this protocol and not collected in all patients, the biochemical response could not be assessed.
The intent-to-treat response rate for the entire study cohort is 2/36=5.6% (95% CI 0.6–18.7%) and tumour control (SD+PR) rate is 23/36=63.9% (95% CI 46.2–79.2%). Response outcomes were similar between the CT and ICC histologies, with PR rates of 4.8 and 6.7%, respectively.
TTP
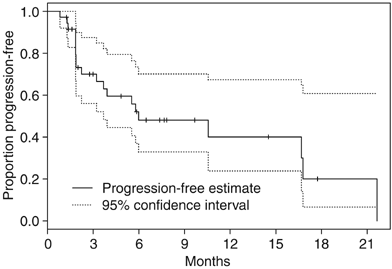
Five patients remain on study and continue to receive treatment as of January 2006. Of the 31 patients who have come off treatment, the reasons for discontinuation were: PD (15), symptomatic PD (four), death (one), physician discretion (two), AE (seven) and patient withdrawal (two). Median TTP is estimated to be 6.0 months (95% CI 3.7-not reached); 6-month progression-free rate is estimated to be 48.1% (95% CI 33.0–70.1%) and 1-year progression-free rate is estimated to be 40.1% (95% CI 23.8–67.4%) (Figure 2).
Figure 2
Time to progression for entire study cohort.
Survival
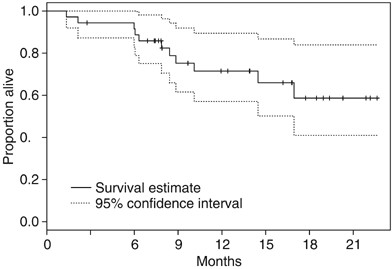
At the time of reporting, 11 patients have died. Median follow-up on the 25 patients alive at last follow-up is 13.9 months (range 2.8–22.6 months), minimum follow-up is 6.9 months. Median OS has not been reached; 6-month survival rate is estimated to be 91.6% (95% CI 82.9–100.0%) and 1-year survival rate is estimated to be 71.5% (95% CI 57.1–89.5%) (Figure 3). Patients with ICC appeared to have slightly better TTP and OS but statistical comparisons were not made for this subgroup analysis (Table 2).
Figure 3
Overall survival for entire study cohort.
Table 2 Efficacy outcomes by histology
Toxicity
Safety and tolerability data are available for 213 treatment cycles, with a median number of four cycles delivered per patient (range 1–21), AE deemed by the investigator as at least possibly related to study drug and that have occurred in more than 10% of the cycles are shown in Table 3. Overall, treatment with temsirolimus was well tolerated. The most frequent AE of all grades, at least possibly related to study treatment, were: fatigue (78% of patients; 53% of cycles), hyperglycaemia (69% of patients; 54% of cycles) and rash/desquamation (64% of patients; 50% of cycles). Severe AE that were grade ⩾3 and at least possible attribution were uncommon. Seven patients developed pneumonitis considered as possibly related to temsirolimus, three of whom required drug discontinuation. Other observed AE include two patients who had grade 5 events. One died from a pneumothorax and bronchospasm in cycle 2 unlikely related to temsirolimus and the other died from pulmonary embolism 5 days after removal from study, deemed to be possibly drug related.
Table 3 Drug-related adverse events occurring in >10% of treatment cycles
Pharmacodynamic analysis
Twenty-two patients had archival specimens evaluable for analysis of baseline molecular markers. No significant association was seen between any of the pretreatment markers tested (PTEN, p53, pS6, pmTOR and pAKT) and tumour response, TTP or survival. Despite being nonstatistically significant, the loss of PTEN expression was associated with a trend towards a shorter TTP (_P_=0.07).
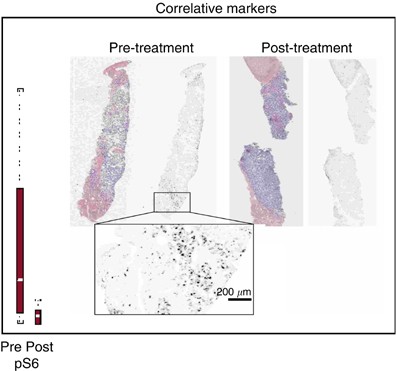
Paired baseline and post-treatment biopsies were obtained from 23 patients, and 13 paired-samples were evaluable. The best responses of these 13 patients were one PR, eight SD, three PD and one nonevaluable. Baseline expression levels of pAKT, pS6, pmTOR and peIF4G were determined and compared with expression levels following 2 weeks of treatment. Temsirolimus effectively inhibited the phosphorylation of S6 (_P_=0.02) (Figure 4) and higher baseline levels of pS6 showed a trend towards being predictive of a better response (_P_=0.097). Higher baseline levels of pmTOR were predictive of tumour response (_P_=0.01). Increases in the expression of pAKT (_P_=0.041), and decreases in pmTOR (_P_=0.048) after 2 weeks on treatment, were associated with an increased TTP.
Figure 4
Pre- and post-treatment liver biopsies. Tissue sections were first immunofluorescence-labelled for S235/236-S6 ribosomal protein, imaged, and then restained with H&E. The grey scale images of pS6 are unenhanced, at original resolution.
A discrepancy was noted in the predictive abilities of pS6 and pmTOR between freshly procured pretreatment specimens vs paraffin-embedded archival specimens. However, only seven patients had both archival specimens and pretreatment tumour biopsies that were evaluable. Hence, statistical comparisons were not performed.
Discussion
Neuroendocrine carcinomas, generally subcategorised into CT and ICC, often pursue an indolent clinical course. However, patients ultimately will become symptomatic either as a result of increasing tumour bulk or hormonal hypersecretion. Somatostatin analogues have proven successful in ameliorating symptoms of the carcinoid syndrome but its benefit in survival is unclear (Saltz et al, 1993). Streptozocin and DTIC-based regimens have been tested with only modest activity and may also be associated with significant toxicity (Moertel and Hanley, 1979; Engstrom et al, 1984; Moertel et al, 1992; Bukowski et al, 1994; Rivera and Ajani, 1998; Cheng and Saltz, 1999; Ramanathan et al, 2001; McCollum et al, 2004; Sun et al, 2005). As conventional systemic approaches remain insufficient and highly toxic, there is an obvious need for novel therapies in this tumour population.
The intent-to-treat response rate of 5.6% and median TTP of 6.0 months observed in our study compares favourably with other targeted therapies tested in this tumour population. A recently reported phase II study of gefitinib in 96 patients with progressive NEC (55 with CT and 42 with ICC) revealed a 6-month-PFS of 51% for the former and 28% for the latter. However, objective responses were only seen in one out of 40 patients (2.5%) in the CT group (Hobday et al, 2005). A phase II study with the multi-targeted tyrosine kinase inhibitor sunitinib involving 112 patients (41 CT and 61 ICC), reported an ORR of 8.8%, with a median TTP of 40 weeks and a high percentage of SD (Kulke et al, 2005). Efficacy varied by tumour histology with an ORR of 2% (1/41) among CT vs 13% (8/61) among ICC. In another phase II study where 44 patients with CT were randomised to bevazucimab or pegylated interferon, the former showed superior activity (4/22 vs 0/22 confirmed PR), reduction in tumour perfusion and improvement in PFS at 18 weeks (96% vs 68%) (Yao et al, 2005). However, neither of these two latter trials required evidence of progressive disease before study entry, therefore the patient populations are likely different from ours.
Regarding histology, in our study temsirolimus appears to be slightly more active in ICC than in CT, as reported with other therapies (Moertel and Hanley, 1979; Engstrom et al, 1984; Sun et al, 2005; Kulke et al, 2006). While our small sample size precludes any definitive conclusions, it is possible that CT and ICC will manifest different sensitivities to different targeted agents, as is the situation with cytotoxic agents.
Pharmacodynamic evaluations in paired biopsies, confirmed, for the first time in this patient population, that temsirolimus effectively downregulates the phosphorylation of S6, and that higher baseline levels of pS6 and pmTOR seem to predict for a better response. These results are consistent with those reported by Cho et al (2005) which analysed predictive markers of temsirolimus in advanced renal cell carcinomas, confirming the value of pS6 (Cho et al, 2005). In this study, we have selected an antibody against pmTOR (ser2448) with high specificity, and this may explain the finding of pmTOR as a predictive marker of response.
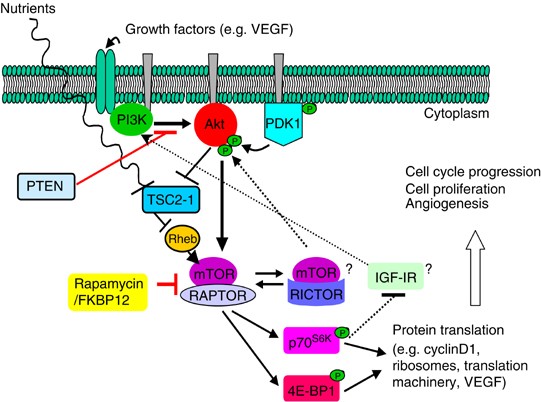
Other interesting pharmacodynamic findings include the rising trend in AKT phosphorylation noted after treatment with temsirolimus. Two alternative pathways that induce AKT activation may explain this finding (Figure 5). RAPTOR (regulatory associated protein of mTOR) and RICTOR (rapamycin-insensitive companion of mTOR) are key partnering proteins which complex with mTOR and modulate its functions. Activation of AKT through the mTOR–RICTOR complex could explain our observation (Hresko and Mueckler, 2005; Sarbassov et al, 2005). Additionally, AKT phosphorylation has been described through a feedback loop of the PI3K/AKT/mTOR pathway from the insulin-like growth factor I receptor (IGF-IR) (O'Reilly et al, 2006). Further pharmacodynamic analysis revealed a positive association between increases in pAKT and decreases in pmTOR with a more prolonged TTP. These exploratory findings require confirmation with larger series. The evaluation of archival specimens searching for prognostic factors only showed a trend towards shorter TTP in those patients with loss of PTEN. The discordance between the results from the archival and the freshly procured pretreatment specimens in our study could be due to differences in the genetic profile of primary and recurrent/metastatic tumours; tumour heterogeneity; and different protocols for specimen handling. Differences in time and speed of tissue fixation after biopsy or resection may significantly affect the phosphorylated states of signalling molecules.
Figure 5
PI3K/AKT/mTOR pathway showing the mTOR protein complexes, mTOR/RAPTOR and mTOR/RICTOR, and the feedback loop involving IGF-IR. Arrows indicate activation; bars indicate inhibition.
In conclusion, temsirolimus appears to have only modest activity with a manageable toxicity profile in advanced NEC. The results of this study do not warrant further investigation of this drug as a single agent in this patient population. Evaluation of temsirolimus, in combination with other targeted agents, such as a multi-kinase inhibitor or an antiangiogenic compound, should be considered. The loss of PTEN expression could represent a poor prognostic marker for NEC. Pharmacodynamic analysis in paired tumour biopsies reflected effective mTOR pathway downregulation and identified possible predictive factors. Evaluations in larger populations are needed to confirm these findings.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
- Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR, Park Y, Liou SH, Marshall B, Boni JP, Dukart G, Sherman ML (2004) Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 22: 909–918
CAS PubMed Google Scholar - Baselga J, Fumoleau P, Gil M, Colomer R, Roche H, Cortes-Funes H, Burstein H, Kaufman P, Kong S, Moore L (2004) Phase II, 3-arm study of CCI-779 in combination with letrozole in postmenopausal women with locally advanced or metastatic breast cancer: preliminary results. Proc Am Soc Clin Oncol 23: 13
Google Scholar - Bukowski RM, Tangen CM, Peterson RF, Taylor SA, Rinehart JJ, Eyre HJ, Rivkin SE, Fleming TR, Macdonald JS (1994) Phase II trial of dimethyltriazenoimidazole carboxamide in patients with metastatic carcinoid. A Southwest Oncology Group study. Cancer 73: 1505–1508
Article CAS PubMed Google Scholar - Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, Eiermann W, Hess D, Morant R, Semiglazov V, Borner M, Salzberg M, Ostapenko V, Illiger HJ, Behringer D, Bardy-Bouxin N, Boni J, Kong S, Cincotta M, Moore L (2005) Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol 23: 5314–5322
Article CAS PubMed Google Scholar - Cheng PN, Saltz LB (1999) Failure to confirm major objective antitumor activity for streptozocin and doxorubicin in the treatment of patients with advanced islet cell carcinoma. Cancer 86: 944–948
Article CAS PubMed Google Scholar - Cho DC, Signoretti S, Regan M, Mc Dermott D, Polivy A, Youmans A, Stanbridge E, Atkins M (2005) Low expression of surrogates for mTOR pathway activation predicts resistance to CCI-779 in patients with advanced renal cell carcinoma. Clin Cancer Res 11 (24 Suppl): 9133s
Google Scholar - Engstrom PF, Lavin PT, Moertel CG, Folsch E, Douglass Jr HO (1984) Streptozocin plus fluorouracil versus doxorubicin therapy for metastatic carcinoid tumor. J Clin Oncol 2: 1255–1259
Article CAS PubMed Google Scholar - Hobday TJ, Mahoney M, Erlichman C, Lloyd R, Kim G, Mulkerin D, Picus J, Fitch T, Donehower R (2005) Preliminary results of a phase II trial of Gefitinib in progressive metastatic neuroendocrine tumors (NET): a phase II Consortium (PC2) study. Proc Am Soc Clin Oncol 23: 328s
Google Scholar - Hresko RC, Mueckler M (2005) MTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem 280: 40406–40416
Article CAS PubMed Google Scholar - Jensen RT, Doherty GM (2005) Carcinoid tumors and the carcinoid syndrome. In Cancer: Principles and Practice of Oncology, De Vita VT Jr, Hellman S, Rosenberg SA (eds). Philadelphia: Lippincott Williams & Wilkins
Google Scholar - Kulke M, Henz J, Meropolo NJ, Posey J, Ryan DP, Picus J, Bergsland E, Stuart K, Baurn CM, Fuchs CS (2005) A phase 2 study to evaluate the efficacy and safety of SU11248 in patients (pts) with unresectable neuroendocrine tumors (NETs). In: Proc Am Soc Clin Oncol (Orlando) 23: 310s
Google Scholar - Kulke MH, Stuart K, Enzinger PC, Ryan DP, Clark JW, Muzikansky A, Vincitore M, Michelini A, Fuchs CS (2006) Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol 24: 401–406
Article CAS PubMed Google Scholar - McCollum AD, Kulke MH, Ryan DP, Clark JW, Shulman LN, Mayer RJ, Bartel S, Fuchs CS (2004) Lack of efficacy of streptozocin and doxorubicin in patients with advanced pancreatic endocrine tumors. Am J Clin Oncol 27: 485–488
Article CAS PubMed Google Scholar - Moertel CG, Hanley JA (1979) Combination chemotherapy trials in metastatic carcinoid tumor and the malignant carcinoid syndrome. Cancer Clin Trials 2: 327–334
CAS PubMed Google Scholar - Moertel CG, Lavin PT, Hahn RG (1982) Phase II trial of doxorubicin therapy for advanced islet cell carcinoma. Cancer Treat Rep 66: 1567–1569
CAS PubMed Google Scholar - Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D (1992) Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 326: 519–523
Article CAS PubMed Google Scholar - O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66: 1500–1508
Article CAS PubMed PubMed Central Google Scholar - Oberg K (2001) Chemotherapy and biotherapy in the treatment of neuroendocrine tumours. Ann Oncol 12 (Suppl 2): S111–S114
Article PubMed Google Scholar - Podsypanina K, Lee RT, Politis C, Hennessy I, Crane A, Puc J, Neshat M, Wang H, Yang L, Gibbons J, Frost P, Dreisbach V, Blenis J, Gaciong Z, Fisher P, Sawyers C, Hedrick-Ellenson L, Parsons R (2001) An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/- mice. Proc Natl Acad Sci USA 98: 10320–10325
Article CAS PubMed PubMed Central Google Scholar - Ramanathan RK, Cnaan A, Hahn RG, Carbone PP, Haller DG (2001) Phase II trial of dacarbazine (DTIC) in advanced pancreatic islet cell carcinoma. Study of the Eastern Cooperative Oncology Group-E6282. Ann Oncol 12: 1139–1143
Article CAS PubMed Google Scholar - Raymond E, Alexandre J, Faivre S, Vera K, Materman E, Boni J, Leister C, Korth-Bradley J, Hanauske A, Armand JP (2004) Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol 22: 2336–2347
Article CAS PubMed Google Scholar - Rivera E, Ajani JA (1998) Doxorubicin, streptozocin, and 5-fluorouracil chemotherapy for patients with metastatic islet-cell carcinoma. Am J Clin Oncol 21: 36–38
Article CAS PubMed Google Scholar - Saltz L, Trochanowski B, Buckley M, Heffernan B, Niedzwiecki D, Tao Y, Kelsen D (1993) Octreotide as an antineoplastic agent in the treatment of functional and nonfunctional neuroendocrine tumors. Cancer 72: 244–248
Article CAS PubMed Google Scholar - Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101
Article CAS PubMed Google Scholar - Sun W, Lipsitz S, Catalano P, Mailliard JA, Haller DG (2005) Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol 23: 4897–4904
Article CAS PubMed Google Scholar - Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216
Article CAS PubMed Google Scholar - Vignot S, Faivre S, Aguirre D, Raymond E (2005) mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol 16: 525–537
Article CAS PubMed Google Scholar - Wang L, Ignat A, Axiotis CA (2002) Differential expression of the PTEN tumor suppressor protein in fetal and adult neuroendocrine tissues and tumors: progressive loss of PTEN expression in poorly differentiated neuroendocrine neoplasms. Appl Immunohistochem Mol Morphol 10: 139–146
CAS PubMed Google Scholar - Yao JC, N C, Hoff PM, Phan AT, Hess K, Chen H, Wang X, Abbruzzese JL, Ajani JA (2005) Improved progression free survival (PFS), and rapid, sustained decrease in tumor perfusion among patients with advanced carcinoid treated with bevacizumab. In: Proc Am Soc Clin Oncol (Orlando) 23: 309s
Google Scholar
Acknowledgements
SAR is a fellow of the CIHR training program for clinician scientist in molecular oncology pathology [ICR/CIHR Training Grant STP-53912]. The authors would like to thank Drs Mark Minden and Karen Yee for their assistance with the manuscript.
Author information
Authors and Affiliations
- Department of Medical Oncology and Hematology, Princess Margaret Hospital Phase II Consortium, 610 University Avenue, Suite 5-718, Toronto, M5G 2M9, ON, Canada
I Duran, H Hirte, W Kocha, G Goss, L Le, A Oza, T Nicklee, J Ho, D Birle, G R Pond, D Arboine, S Aviel-Ronen, M-S Tsao, D Hedley & L L Siu - Memorial Sloan Kettering Cancer Center, New York, USA
J Kortmansky - University of Chicago, Chicago, USA
D Singh - National Cancer Institute, Bethesda, USA
J Dancey
Authors
- I Duran
- J Kortmansky
- D Singh
- H Hirte
- W Kocha
- G Goss
- L Le
- A Oza
- T Nicklee
- J Ho
- D Birle
- G R Pond
- D Arboine
- J Dancey
- S Aviel-Ronen
- M-S Tsao
- D Hedley
- L L Siu
Corresponding author
Correspondence toL L Siu.
Additional information
Supported by clinical trial contracts from the US National Cancer Institute N01-CM-17107 and translational research fund contract number N01-CO-124001, subcontract number 22XS208-T08/P6171.
This study was presented in part at the AACR-NCI-EORTC International Conference, Philadelphia, USA, November 2005.
Appendix 1
Appendix 1
Table 4 Antibodies used for immunofluorescence and immunohistochemistry analysis.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Duran, I., Kortmansky, J., Singh, D. et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas.Br J Cancer 95, 1148–1154 (2006). https://doi.org/10.1038/sj.bjc.6603419
- Received: 03 August 2006
- Revised: 11 September 2006
- Accepted: 11 September 2006
- Published: 10 October 2006
- Issue Date: 06 November 2006
- DOI: https://doi.org/10.1038/sj.bjc.6603419