γ-Tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple-signalling pathways (original) (raw)
Main
Prostate cancer (PCa) is the most common type of cancer in developed countries. It is responsible for more male deaths than any other cancers, except for lung and bronchial cancer (Ries et al, 2006). Most PCas present themselves as mixtures of androgen-dependent and androgen-independent cells during clinical diagnosis. They initially respond to androgen ablation therapy by undergoing programmed cell death (apoptosis) (Craft et al, 1999). However, patients with advanced PCa develop hormonal refractory disease that results in a fatal effect because of the growth of androgen-independent PCa cells (Feldman and Feldman, 2001). Until recently, the only chemotherapeutic drug that shows improvement in survival of patients, Docetaxel, can only extend the overall survival by 2 1/2 months (Chowdhury et al, 2007). Furthermore, the treatment is associated with significant side effects. Therefore, an alternative methodology to enhance the apoptotic response is necessary to develop new therapeutic drugs for the treatment of PCa.
Natural products have historically been a rich source of biologically active compounds for drug discovery (Schiff et al, 1979). Tocotrienols (T3) are important plant vitamin-E constituents found in palm oil. Together with tocopherols (T), they provide a significant source of antioxidant activity to all living cells (Ahmad et al, 2005; Mazlan et al, 2006). This common antioxidant attribute reflects the similarity in chemical structures of the tocotrienols and the tocopherols, which differ only in their structural side chain (contains farnesyl for tocotrienol or saturated phytyl side chain for tocopherol). The common hydrogen atom from the hydroxyl group on the chromanol ring acts to scavenge the chain-propagating peroxyl-free radicals. Depending on the locations of methyl groups on their chromanol ring, tocopherols and tocotrienols can be distinguished into four isomeric forms: alpha (α), beta (β), gamma (γ), and delta (δ).
Apart from tocotrienol's antioxidant, anti-inflammatory, antiangiogenic, antineurodegeneration, antihypercholesterolemic and antimicrobial properties, emerging in vitro and in vivo evidences have manifested the anticancer activity of tocotrienol on numerous human cancer cells including prostate, breast, colon, liver and gastric (Agarwal et al, 2004; Nesaretnam et al, 2004; Sylvester and Shah, 2005; Eitsuka et al, 2006; Kumar et al, 2006; McAnally et al, 2007) (reviewed in (Sen et al, 2007)). For example, a recent study (Srivastava and Gupta, 2006) has demonstrated that treatment of PCa cell lines (LNCaP, DU145, PC-3) with tocotrienol-rich fraction (TRF) resulted in significant decreases in cell viability and colony formation capability. More interestingly, TRF was able to selectively spare the normal human prostate epithelial cells, an important characteristic of effective chemoprevention strategy. However, it should be noted here that the TRF used in the study was a mixture of various vitamin-E isomers. Thus, it was not possible to determine which isomer possesses the highest potency in eliminating proliferating prostate cancer cells selectively, let alone the discovery of molecular pathways responsible for the inhibitory effect. In other studies (McAnally et al, 2003; Conte et al, 2004; Vraka et al, 2006) _γ_-T3 isomer was reported to suppress prostate cancer cell growth with IC50 values for PC-3 between 25 and 51 μ M, although how the cell responds to the other isomers was not investigated.
Although scientific evidences evoking suppression of cancer cells by T3 are increasing, the exact pathways involved in T3-induced apoptosis remain elusive. Initially, T3 treatment was shown to induce apoptosis through a p53-dependent mechanism (Agarwal et al, 2004). Using p53+ human colon carcinoma RKO cells, researchers demonstrated that TRF-induced activation of the Bax gene through upregulation of the p53 protein. This was associated with the release of cytochrome c from nucleus to cytosol, induction of Apaf1 oligomerisation as well as activation of caspase 9. Meanwhile, the T3-induced p53 expression was also found to result in downregulation of Bcl-2 level, which eventually triggers apoptotic-signalling cascade by increasing the bax/bcl-2 ratio. In addition to p53 pathway, phosphatidylinositol-3-kinase-dependent kinase (PI3K)/PI3K-dependent (PDK1) mitogenic-signalling cascade (Samant and Sylvester, 2006) and NF-_κ_B (Ahn et al, 2007) pathways have both been suggested to contribute to T3-induced apoptosis. For example, in _γ_-T3-treated cancer cells, inactivation of PDK1 and Akt as well as downregulation of their downstream effectors such as FLICE-inhibitory protein (FLIP) and phospho-NF-_κ_B were observed. FLIP reduction was shown to promote the cleavage of caspases 3 and 8, which results in growth arrest and apoptosis in T3-treated cells. Taken together, it seems that T3-induced apoptosis in cancer cells may involve activation/inactivation of multiple-signalling pathways.
Materials and methods
Prostate cancer cell lines, cell culture conditions and chemicals
The human androgen-dependent PCa cells (LNCaP), human androgen-independent PCa cells (PC-3) (ATCC, Rockville, MD, USA) were maintained in their respective medium recommended by ATCC (Invitrogen, Carlsbad, CA, USA) supplemented with 2 mmol l−1 L-glutamine, 10% fetal calf serum (FCS) and 2% penicillin streptomycin at 37°C in 5% CO2. The immortalised human prostate epithelial cells (PZ-HPV-7) (ATCC, Rockville, MD, USA) were maintained in keratinocyte serum-free medium (K-SFM) supplemented with bovine pituitary extract (BPE, 0.05 mg ml−1) and human recombinant epidermal growth factor (EGF, 5 ng ml−1). Docetaxel (Calbiochem, San Diego, CA, USA) and JNK inhibitor, SP600125 (Sigma-Aldrich, St Louis, MO, USA), were dissolved in dimethylsulphoxide (DMSO). The treatment solutions were diluted in culture medium to obtain the desired concentrations.
Tocotrienol and tocopherol isomers
Tocotrienol and tocopherol isomers were extracted and purified from palm oil using Davos separation technology. The extraction facility is located at Tuas Singapore. Crude palm oil feed was purchased from Kuala Lumpur Kepong Berhad. Using the corresponding tocotrienol isomers as the reference standard, the purity of T3 and T isomers was verified to be ⩾97% by high performance liquid chromatography percentage area (% area).
Cell viability study and time course experiment
For cell viability study, 5 × 103 cells resuspended in 100 _μ_l medium were plated into each well of a 96-well plate. The cells were then treated with different concentrations (20, 40, 60, 80, 100 μ M) of the vitamin-E isomers for 24- and 48-h. After the treatment, 20 _μ_l of MTT solution was added into each well and the cells were incubated at 37°C for 2 h. The formazan crystals were then resuspended in 200 _μ_l of DMSO and the intensity at 595 nm were measured. For JNK inhibitor study, cells were pre-treated with 20 μ M of SP600125 for 8 h prior to the addition of vitamin-E isomers. For time course study, 5 × 103 cells (LNCaP and PC3) were treated with IC50 concentrations of the vitamin-E isomers and were subjected to the MTT assay at the indicated time point. If IC50 for the isomer is >100 μ M, 100 μ M will be used as treatment dosage. Each experiment was repeated three times in triplicate wells and the growth curves showed the means and s.d.
To test the effect of _γ_-T3 on the cytotoxicity of Docetaxel, cells were pre-incubated with _γ_-T3 for 3 h before addition of 20 and 100 nM of Docetaxel. After 24 h, cells were subjected to western blotting and MTT assays respectively.
Flow cytometry
Cell cycle distribution was examined using flow cytometry. Briefly, cells were harvested by trypsinisation, fixed in 70% ethanol at 4°C overnight, and then resuspended in PBS. After incubation at 4°C overnight, 2 × 106 cells were incubated with 20 _μ_g ml−1 propidium iodide and 2 mg RNase A for 15 min at 37°C. Cells were then examined by BD SLRII cytometer and the results were analysed using ModFit software (Becton Dickinson, Mountain View, CA, USA). Data were expressed as the percentage of cell cycle distribution in the entire population.
Matrigel-invasion assay
Matrigel-invasion assay was performed according to a method published earlier with modifications (Albini et al, 1987). Briefly, the invasive androgen-independent PCa cells (PC-3) were pre-incubated in a serum-free RPMI-1640 medium with or without _γ_-T3 isomers for 24 h. The PC-3 cells, (2.5 × 105) resuspended in 500 _μ_l of serum-free RPMI-1640 containing 0.1% bovine serum albumin, were then added to the upper chamber of an 8-_μ_m pore size insert (Millipore, Bedford, MA, USA) manually coated with Matrigel (0.5 mg ml−1) (BD Bioscience, Bedford, MA, USA). Five hundred microliters of invasion buffer containing fibronectin (10 _μ_g ml−1) and RPMI-1640 supplemented with 10% FCS were added in the lower chamber as a chemo-attractant. The PC-3 cells were incubated at 37°C for 24 h in 5% CO2-humidified conditions. At the end of incubation period, inserts were stained with Diff-Quick staining solution (Fischer Scientific). Non-invaded PC-3 cells on the inside of the insert were scraped off with a cotton swab. The PC-3 cell invasions were then examined by a phase-contrast microscope. The invaded cells were extracted using extraction buffer (Millipore, Bedford, MA, USA) and the cell number was estimated based on absorbance at 595 nm.
Western blotting
Detailed protocols have been described earlier (Chu et al, 2006). Briefly, cell lysates were prepared by suspending cell pellets in lysis buffer (50 mmol l−1 Tris-HCl (pH 8.0), 150 mmol l−1 NaCl, 1% NP40, 0.5% deoxycholate, 0.1% SDS, 1 mg ml−1 aprotinin, 1 _μ_g ml−1 leupeptin and 1 mmol l−1 phenylmethylsulphonyl fluoride). For nuclear protein extraction, NucBuster™ protein extraction kit was used. Protein concentration was measured using the DC Protein Assay kit (Bio-Rad, Hercules, CA, USA). Equal amount of protein (30 _μ_g) was loaded onto a 10% SDS polyacrylamide gel for electrophoresis and then transferred onto a polyvinylidene difluoride membrane (Amersham, Piscataway, NJ, USA). The membrane was then incubated with primary antibodies for 1 h at room temperature against E-cadherin (BD Biosciences, Bedford, MA, USA), _α_-catenin, _β_-catenin, _γ_-catenin, Id-1, Id-3, EGF-R, phosphor-c-jun, phospho-ATF2, cleaved PARP, vimentin, _α_-smooth muscle actin, twist (Santa Cruz Biotechnology, CA, USA), Phospho-i_κ_B-α (Ser32/36), Phospho-IKK α (Ser180)/IKK β (Ser181), Phospho-SAPK/JNK (Thr183/Tyr185) G9, SAPK/JNK, NF-_κ_B p65 (5A5) (Cell Signaling Technology Inc., Beverly, MA, USA), Snail (Abcam, Cambridge, UK). After incubation with appropriate secondary antibodies, signals were visualised by ECL western blotting system (Amersham, Piscataway, NJ, USA). Expression of _β_-actin and histone H1 were assessed as an internal loading control for total cell lysate and nuclear protein lysate, respectively.
Results
Antiproliferation effect of vitamin-E isomers
PCa cells were treated with vitamin-E isomers for 24- and 48-h at increasing dosage (low: 20 μ M, medium: 40 μ M and high: 80 μ M) and for varying time points. Our results showed that vitamin-E isomers did not affect significantly the proliferation rate of normal prostate epithelial cells (PZ-HPV-7), but significantly suppressed the proliferation of LNCaP and PC-3 (Table 1A). The dose to suppress 50% cell growth (IC50) in LNCaP and PC-3 was inversely proportional to the length of incubation time. Surprisingly, PC-3 cells were more sensitive to the growth inhibition of the vitamin-E isomers than LNCaP cells. The inhibition of cell proliferation was significantly stronger for T3 isomers in PC-3, particularly for _γ_-T3, which showed a dose- and time-dependent inhibition (Table 1B). Although _δ_-T3 was more potent in suppressing cell growth in LNCaP (Table 1A), the IC50 value was significantly higher than that for _γ_-T3 in PC-3. Separately, _γ_-T was also found to induce apoptosis in LNCaP cells (Jiang et al, 2004) at a dose similar to _γ_-T3. Based on the IC50 values in PC-3 cells incubated with various isomers for 24-h, the order of inhibitory effect is _γ_-T3>_δ_-T3>_β_-T3>_γ_-T>_δ_-T≈_α_-T3≈_α_-T≈_β_-T. For the subsequent experiments, we investigated the most potent isomer for PC-3 (_γ_-T3) as they are in general considered more invasive and resistant to chemotherapeutic agents compared with LNCaP cells (Coppe et al, 2004; Ghosh et al, 2005).
Table 1 Effect of vitamin-E isomers on prostate cells
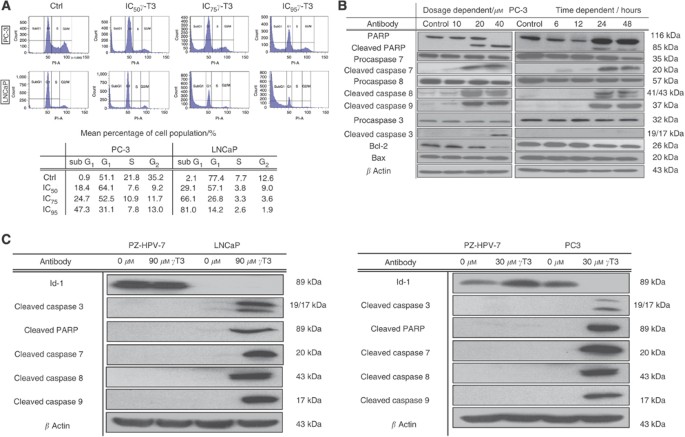
To study the mechanism responsible for _γ_-T3-induced growth inhibition, cell cycle distribution of the cells with or without _γ_-T3 treatment for 24 h were analysed by flow cytometry. Consequently, treatment of cells with _γ_-T3 (IC50–95) resulted in an induction of sub-G1 cell population, indicating the presence of apoptotic cells after the treatment (Figure 1A). The proportion of apoptotic cells (sub-G1 fraction) increased in a dose-dependent manner. It is noteworthy that although _γ_-T3 was previously reported to induce G1 arrest in some cell lines (Mo and Elson, 1999), we did not observe a significant increase of G1 population in prostate cancer cells that were treated with _γ_-T3. Consistent with the induction of sub-G1 cell population in flow cytometry, activation of procaspase 3, 7, 8, 9 as well as PARP, as evidenced by the appearance of the cleaved products, were observed in PC-3 cells treated with different _γ_-T3 dosage for 24 h. Downregulation of bcl-2 was also detected after the treatment, although bax expression was not affected, which is likely because of the lack of p53 expression in PC-3 cells (Figure 1B). Meanwhile, these _γ_-T3-mediated activation of the proapoptotic proteins as well as the change of bcl-2/Bax ratio were in a dose- and time-dependent manner (Figure 1B), consistent with the effect of _γ_-T3 treatment on inhibition of cell proliferation. In addition, activation of these pro-apoptotic genes after IC50 _γ_-T3 treatment (Figure 1C) were only observed in PC-3 and LNCaP cells, but not in PZ-HPV-7, indicating that _γ_-T3 specifically induced apoptosis of androgen-independent prostate cancer cells.
Figure 1
Induction of apoptosis by _γ_-T3 treatment. (A) Cell cycle analysis by flow cytometry. Control cells and treated cells incubated with _γ_-T3 at IC50 for 24-h were subjected to flow cytometry analysis. Note that the sub-G1 population appears after treatment. (B) IC50 time-dependent and 24-h dose-dependent activation (in hrs and μ M respectively) of the pro-apoptosis pathway in PC-3. Note that _γ_-T3 induces activation of the critical molecules (cleaved caspase 3, 7, 8, 9, PARP) and modulate the ratio between the amounts of bcl-2 and bax in a cell dose- and time-dependent fashion. (C) IC50 _γ_-T3 activates pro-apoptotic genes and suppresses pro-survival genes expression on LNCaP and PC-3 but not on non-tumorigenic prostate epithelial cells (PZ-HPV) for 24-h incubation period.
_γ_-T3 downregulates the pro-survival signalling pathways
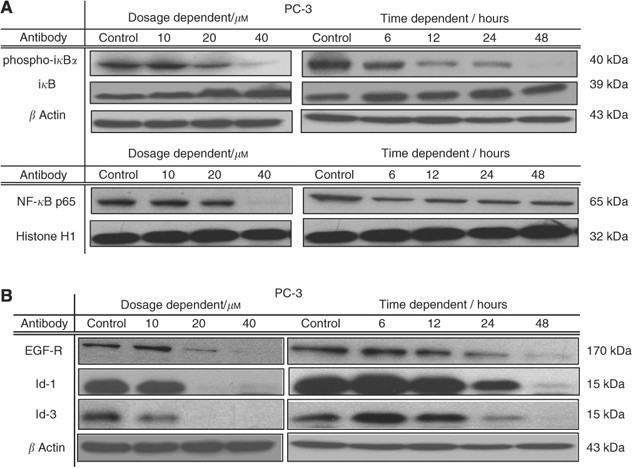
Although NF-_κ_B was reported to be constitutively activated in PC-3 (Dhanalakshmi et al, 2002), the possibility that _γ_-T3-induced cell apoptosis attributable to the suppression of NF-_κ_B activation was considered. The NF-_κ_B activities of PC-3 treated with _γ_-T3 at either different dosages or at IC50 for different periods were measured by examining the translocation of NF-_κ_B subunit p65. As illustrated in Figure 2A, _γ_-T3 treatment suppressed constitutive NF-κ_B p65 activity in a dose-dependent and time-dependent manner. The effect of γ_-T3 on NF-κ_B signalling was further explored by examining the expression of other upstream regulators, such as phospho-i_κ_B_α/β and i_κ_B_α/β. In γ_-T3-treated PC-3 cells, a time- and dose-dependent decrease in the level of the phosphorylated I_κ_B_α/β were observed (Figure 2A). This is associated with the increase in the level of I_κ_B_α/β, as well as an inhibition of NF-_κ_B p65 nuclear translocation. These results indicate that _γ_-T3 suppressed NF-κ_B activity through the dephosphorylation and accumulation of I_κ_B_α/β.
Figure 2
Inactivation of pro-survival pathways by _γ_-T3. (A) Effect of _γ_-T3 on the activity of NF-_κ_B pathway was examined by IC50 time-dependent and 24-h dose-dependent western blotting (in hours and μ M respectively). Note that nuclear translocation of NF-_κ_B p65 and phosphorylated i_κ_B were inhibited by _γ_-T3 treatment. (B) Treatment of _γ_-T3 also resulted in downregulation of Id family proteins and EGF-R in PC-3 cells.
Surprisingly, we found that _γ_-T3 treatment also downregulate a number of the key proteins that are involved in the development and progression of prostate cancer. As shown in Figure 2B, EGF-R expression was significantly suppressed to almost an undetectable level by treatment with increasing dosages of _γ_-T3. Similar effect on Id-1 and Id-3 protein level was observed. As EGF-R and Id protein family are essential for cancer cell growth and survival, their downregulation may be associated with the _γ_-T3-induced growth arrest and apoptosis.
Activation of pro-apoptotic pathway by _γ_-T3 treatment
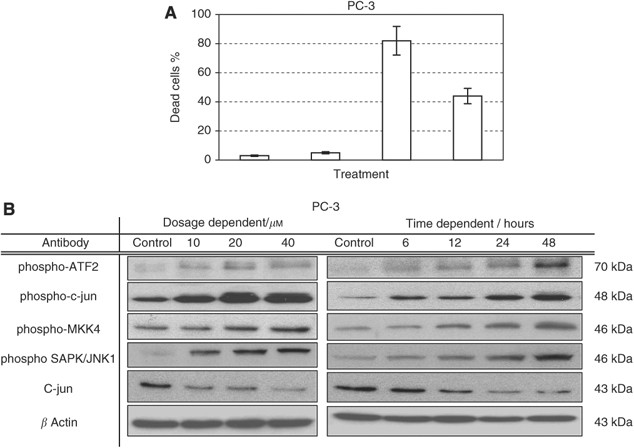
The c-Jun N-terminal kinase is an evolutionarily conserved serine/threonine protein kinase that is activated by stress and genotoxic agents. JNK phosphorylates the amino terminal of all three Jun transcription factors and ATF-2 members of the AP-1 family. The activated transcription factors modulate gene expression to generate appropriate biological responses, including cell migration and cell death. When PC-3 cells were treated with various doses of _γ_-T3, a dosage- and time-dependent increase in JNK phosphorylation activities were detected (Figure 3B). Meanwhile, phosphorylation of the JNK downstream effectors such as ATF-2 or c-jun were all upregulated by _γ_-T3, supporting that JNK-signalling pathway was activated by the _γ_-T3.
Figure 3
Jun N-terminal kinase (JNK) activation is involved in _γ_-T3-induced apoptosis. (A) Cell viability, after incubation with _γ_-T3 and JNK inhibitor (SP600125) for 24-h, was examined by an MTT assay. Note that the addition of JNK inhibitor alleviates the cytotoxicity of _γ_-T3 in PC-3, suggesting that JNK mediates the antiproliferation effect of _γ_-T3. (B) JNK activity after 24-h dose-dependent and IC50 time-dependent _γ_-T3 treatment (in μ M and hours respectively) and was found to be elevated by measuring the phosphorylation levels of MKK4, SAPK/JNK, c-jun and ATF-2. Thus, confirming the involvement of JNK in _γ_-T3 anticancer property.
To further confirm the importance of JNK activation in _γ_-T3-induced apoptosis in PCa cells, we investigated whether inactivation of JNK with a specific inhibitor, SP600125, could protect cells from _γ_-T3. As shown in Figure 3A, co-treatment of _γ_-T3 together with 20 μ M of SP600125, a dose that was previously determined to inhibit JNK activity in the same cell lines (Uzgare and Isaacs, 2004), decreased the percentage of apoptotic cells compared with that treated with _γ_-T3 alone, confirming that JNK activation may be required for _γ_-T3-induced apoptosis.
Effect of _γ_-T3 on inhibition of cell invasion
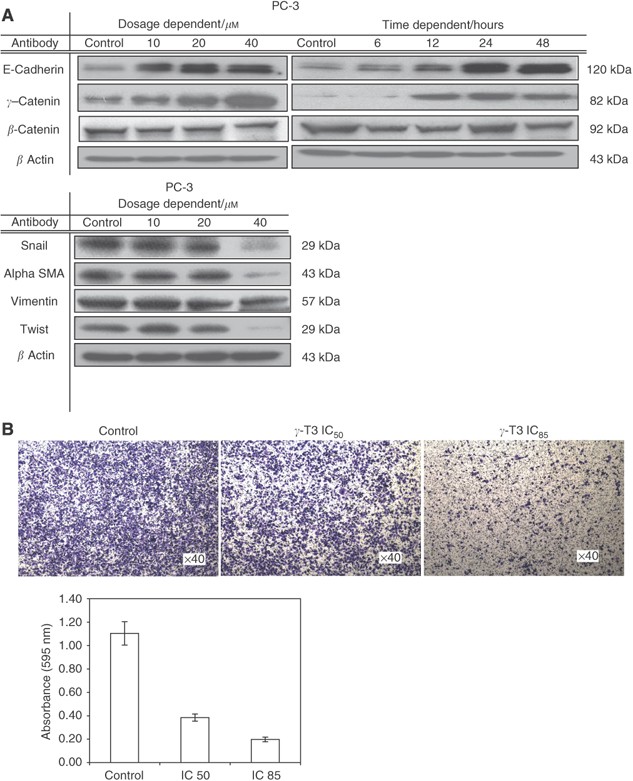
Although _γ_-T3 has been shown to have antiproliferation effect on many cancers, it is not clear if it affects cancer metastasis. Therefore, we examined whether _γ_-T3 could suppress the invasive ability of the prostate cancer cells. As shown in Figure 4B, using matrigel-invasion assay, we found that _γ_-T3-treated (IC50) PC-3 cells for 24 h showed an at least 2.5-time lower invasion capability compared with the untreated control as evidenced by a decrease in the number of cells invaded through the matrigel layer. This inhibitory effect on cell invasion was not the result of cell growth inhibition induced by _γ_-T3 as the number of viable cells added into the invasion chamber were the same. These results indicate that _γ_-T3 is able to inhibit the invasion ability of PCa cells, independent to their cytotoxic effects.
Figure 4
Inhibition of cell invasion by _γ_-T3 treatment. (A) 24-h dose-dependent and IC50 time-dependent _γ_-T3 treatment induces the expression of epithelial markers (E-cadherin, _γ_-catenin), but suppresses the expression of mesenchymal markers (vimentin, twist and _α_-SMA) and E-cadherin's repressor (snail). (B) The invasive androgen-independent PCa cells (PC-3) treated with the indicated dosage of _γ_-T3 was harvested and then plated into the Matrigel-coated (0.5 mg ml−1) insert. Cells invaded through the membrane were stained with crystal violet and the images were photographed under a microscope. After being lysed with extraction buffer, intensity at 595 nm was measured.
Downregulation of E-cadherin expression is one of the most frequently reported characteristics of metastatic cancers. Restoration of E-cadherin expression in cancer cells leads to the suppression of metastatic ability (Morton et al, 1993; Chu et al, 2006). In PCa, downregulation of E-cadherin expression is correlated with high-grade tumours and poor prognosis (van Oort et al, 2007), indicating their roles in PCa progression. Interestingly, we found that E-cadherin and _γ_-catenin protein expression were upregulated whereas E-cadherin's repressor (snail) (Cano et al, 2000) were downregulated after treatment with _γ_-T3 (Figure 4A), although the expression for _β_-catenin remained constant at all treatment dosages and time points. Owing to the deletion of the _α_-catenin in PC-3 cells (Morton et al, 1993), there was no expression detected. Separately, the mesenchymal markers (vimentin, _α_-SMA and twist) were all downregulated after treatment with _γ_-T3 for 24 h.
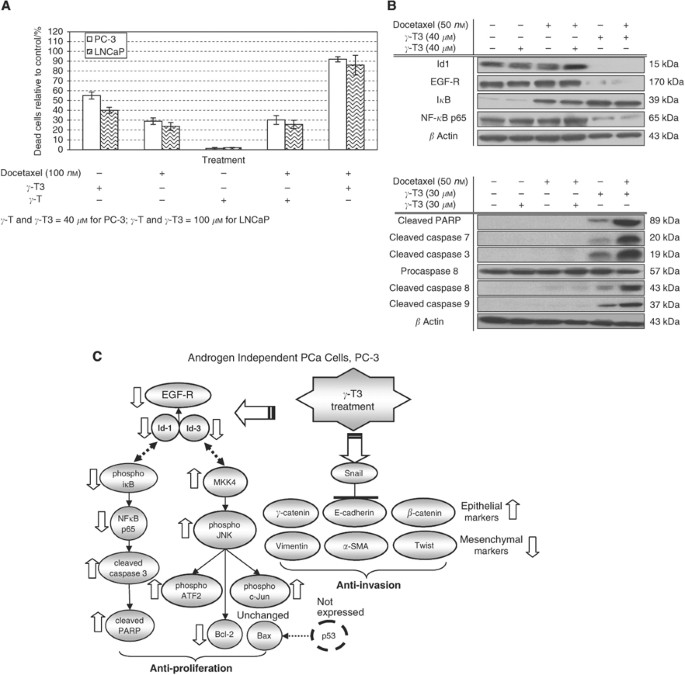
Effect of _γ_-T3 treatment on Docetaxel-induced apoptosis
Many of the natural products, such as aged garlic extract (Howard et al, 2007) or lupeol (Prasad et al, 2008) which are extracted from fruit or plant have been shown to have a chemosensitisation effect. Although it is not clear if _γ_-T3 may affect the sensitivity of cancer cells to chemotherapy, previous study has shown that it did enhance the effectiveness of radiation treatment against prostate tumour (Kumar et al, 2006). To test if _γ_-T3 can act synergistically with a chemotherapeutic agent, we have compared the effect of _γ_-T3 alone or in combination with Docetaxel. As shown in Figure 5A, the percentage of apoptotic cells in PC-3 and LNCaP cell lines following co-treatment of Docetaxel with _γ_-T3 for 24 h was significantly higher than that treated with _γ_-T3 or Docetaxel alone. Using western blotting, we further demonstrated that _γ_-T3 co-treatment with Docetaxel enhances cell apoptosis through activation of pro-apoptotic proteins (cleaved PARP, caspases 3, 7, 8, 9) and downregulation of pro-survival proteins (Id-1, EGF-R, i_κ_B and NF-_κ_B p65) (Figure 5B). The level of apoptotic cells is in stark contrast to the _γ_-T co-treatment with Docetaxel. These results suggested that _γ_-T3 and Docetaxel might have a synergistic effect against prostate cancer cells.
Figure 5
Synergistic effect of _γ_-T3 on Docetaxel-induced apoptosis. (A) Effect of Docetaxel and _γ_-T3 co-treatment for 24-h. Cells were incubated with different dosages of _γ_-T3 and 100 nM of Docetaxel for 24 h. Cell viability was examined by MTT assay. The percentage of apoptotic PC-3 and LNCaP cells following co-treatment of Docetaxel and _γ_-T3 was significantly higher than that treated with either agent alone. (B) Using western blotting, we further demonstrated that _γ_-T3 co-treatment with Docetaxel for 24-h enhances PC-3 cell apoptosis through activation of pro-apoptotic molecules (cleaved PARP, caspases 3, 7, 8, 9). Additional suppression of proliferation genes were also confirmed for Id-1, EGF-R, i_κ_B, and NF-_κ_B p65. (C) Proposed T3 anticancer pathway in PCa cells.
Discussion
Tocotrienol isomers have been earlier shown to inhibit cancer cell proliferation, promote cell cycle arrest, and decrease angiogenesis (reviewed in (Sen et al, 2007)). The present report shows that, among eight vitamin-E isomers, _γ_-T3 inhibits PCa cell proliferation through modulation of pro-survival (Id-1, Id-3, EGF-R and NF-_κ_B) and pro-apoptotic (JNK) pathways. Meanwhile, we demonstrated for the first time that _γ_-T3 inhibits cell invasion by restoration of the E-cadherin, _γ_-catenin expression and suppression of mesenchymal markers. Together with the finding that _γ_-T3 enhanced the anticancer effect of Docetaxel, our study provides strong evidences that _γ_-T3 may be developed as a safe and effective anticancer agent for the treatment of prostate cancer.
It is worth noting that published reports (McAnally et al, 2003; Conte et al, 2004; Vraka et al, 2006) indicated earlier that _γ_-T3 isomer suppressed prostate cancer cell growth. In these reports, _γ_-T3's IC50 value for PC-3 cells varied between 25–51 μ M. The difference between our result and those reported previously could be due to several reasons. Firstly, the IC50 values reported earlier were for cells supplemented with _γ_-T3 for 72-h incubation period. Secondly, the _γ_-T3 isomer used in those studies may have different purity than that used in this study (⩾97%). Thirdly, the difference in the proliferation assay used may result in data variation. Although _γ_-T3 treatment caused significant apoptosis in prostate cancer cells, breast cancer cells (Guthrie et al, 1997), gastric cancer cells (Sun et al, 2008) and human myeloid cells (Ahn et al, 2007), several published reports have also indicated that _δ_-T3 is equally potent for inducing apoptosis in other cancer types (He et al, 1997; Qureshi et al, 2000; Wada et al, 2005). For example, HepG2 and B16 melanoma cells treated with _δ_-T3 showed a significant reduction in cell viability with IC50 9.6 and 10 μ M, respectively. In our experiments with the androgen-dependent LNCaP cell line, _δ_-T3 is more potent in suppressing the cell proliferation of this cell type among the eight vitamin-E isomers investigated (Table 1A). Taken together, it seems likely that _γ_- and _δ_-T3 may possess tumour-suppressing activities with different cell type specificity and potency.
In this study, we demonstrated that _γ_-T3 suppressed constitutive NF-_κ_B activity through inhibition of i_κ_B kinase activation, leading to apoptosis in PCa cells. This is in agreement with the previous study which showed that _γ_-T3 can interfere with the TNF-induced NF-_κ_B activation pathway in human myeloid KMB-5 cells and several other cancer cell lines (Ahn et al, 2007). In addition to their findings, we have demonstrated that _γ_-T3-induced NF-_κ_B inactivation also downregulates the level of bcl-2 in a dosage-dependent and time-dependent fashion. Consequently, this induced apoptosis through activation of caspases 3, 7, 8, 9 and PARP. Consistent with previous results obtained with diverse cell lines differing in p53 status (Mo and Elson, 1999), our results showed that p53 is not required for _γ_-T3-induced apoptosis, as the p53-null cell lines (PC-3 and HL-60 (Mo and Elson, 1999)) are still responsive to _γ_-T3 treatment. It is worth noting that _γ_-T3 was previously demonstrated to abolish NF-_κ_B activation induced by epidermal growth factor (EGF) and other pro-inflammatory cytokines (Ahn et al, 2007). Although the molecular mechanism involved was not clear at that time, the authors proposed that _γ_-T3 may act through a common step in the suppression of NF-_κ_B. Our result revealed that downregulation of EGF receptor (EGF-R) was correlated to _γ_-T3-induced NF-_κ_B inactivation (Figure 2B). This finding may explain why _γ_-T3 was able to suppress NF-_κ_B activation by EGF treatment in KBM-5 cells (Ahn et al, 2007). Interestingly, the androgen-independent prostate cancer cell line PC-3 was found to be more sensitive to _γ_-T3 treatment than the androgen-dependent LNCaP cells. PC-3 cells were found to have constitutive NF-_κ_B activation and are in general more resistant to chemotherapeutic drug-induced apoptosis than the LNCaP cells. Although the exact reason for this observation is unclear, but based on the fact that non-tumorigenic prostate epithelial cells are highly resistant to _γ_-T3 as well, it is possible that _γ_-T3 may preferentially target the cells with higher malignant phenotype.
We believed that one possible mechanism by which _γ_-T3 could mediate its effects on the NF-_κ_B pathway is through the suppression of Id-1 and EGF-R (Ling et al, 2003). We have previously demonstrated that ectopic Id-1 expression in LNCaP cells resulted in increase of NF-_κ_B transactivation activity and nuclear translocation of the p65 and p50 proteins, which was accompanied by upregulation of their downstream effectors Bcl-xL and ICAM-1. In addition, inactivation of Id-1 by its antisense oligo-nucleotide and retroviral construct in DU145 cells resulted in the decrease of nuclear level of p65 and p50 proteins, which was associated with increased sensitivity to TNF-induced apoptosis. Considering these findings, our results strongly suggest that Id gene family may be one of the upstream regulators of NF-_κ_B that is targeted directly by _γ_-T3, and inhibition of NF-_κ_B-signalling pathway may be responsible for _γ_-T3-induced antiproliferation.
In this study, we also showed that c-Jun N-terminal kinase participates in _γ_-T3-induced apoptosis. When PCa cells were treated with _γ_-T3, a series of molecules associated with JNK pathway, such as c-Jun and ATF-2 (Figure 3A), were activated simultaneously. Meanwhile, we demonstrated that treatment of JNK inhibitor (SP600125) protects the PCa cells from _γ_-T3-induced apoptosis (Figure 3A). This further confirms the involvement of the JNK pathway in _γ_-T3-induced apoptosis in PCa cells. It is worth noting that the JNK pathway is also known to be involved in cell apoptosis induced by the chemotherapeutic drug, Docetaxel (Zhang et al, 2006). Taking these findings into consideration, we therefore question whether _γ_-T3 possesses synergistic interaction with Docetaxel as a result of activation of the JNK pathway. To this end, we compared the antiproliferation capability of Docetaxel treatment alone, and co-treatment with _γ_-T3. Remarkably, we found that combined treatment of Docetaxel and _γ_-T3, but not _γ_-T, resulted in higher proportion of apoptotic cells (Figure 5A). This finding suggests a possible synergistic role of _γ_-T3 with the chemotherapeutic agent.
In this study, we determined that restoration of E-cadherin and _γ_-catenin expression, together with suppression of snail, _α_-SMA, vimentin and twist, may account for _γ_-T3's inhibitory effect on PCa cell invasion capability. Although the antiproliferation effect of _γ_-T3 has been reported in several cancer types (reviewed in (Sen et al, 2007)), our results provide first evidence to suggest that it may also be a potential agent for suppression of cancer invasion. Downregulation of E-cadherin and upregulation of mesenchymal markers (_α_-SMA, vimentin and twist) are some of the most frequently reported phenomena in metastatic cancers (Morton et al, 1993). It is suggested that loss of E-cadherin expression is able to promote epithelial–mesenchymal transition (EMT), which plays a key role in the progression of cancer cells to metastatic stage. Although the precise mechanism responsible for E-cadherin inactivation in cancer cells is not clear, alterations at transcriptional level due to its repressor Snail seem to be one of the mechanisms responsible for its decreased expression in several cancer types. In this study, we found that the _γ_-T3-treated PCa cells showed increased E-cadherin expression (Figure 4A), which was associated with reduced Snail protein expression and invasion ability (Figure 4B). Catenins (α,γ), a family of cytoplasmic cadherin-binding proteins, link E-cadherin to the actin cytoskeleton and are thought to be essential for normal E-cadherin function. In our study, we found that _γ_-T3 only upregulated the expression of E-cadherin and _γ_-catenin, but not _α_-catenin. The expression for _β_-catenin remains static, similar to our previous experiments using garlic derivatives (Chu et al, 2006). Although PC-3 cells do not express _α_-catenin, a key molecule for functional E-cadherin expression, _γ_-T3 might restore the function of E-cadherin through other molecules such as vinculin, which has been reported to play a role in the establishment of the E-cadherin-based cell adhesion complex (Hazan et al, 1997). Taken together, our results suggest that _γ_-T3 may suppress cancer metastasis through induction of mesenchymal–epithelial transition (MET).
As summarised in Figure 5C, our results demonstrated that _γ_-T3 is a potent and specific inhibitor of PCa cell proliferation and invasion which acts through multiple molecular pathways. As no side effect can be observed after long term intake of natural T3 extract (Patel et al, 2006; McAnally et al, 2007) (LD50⩾2000 mg kg−1, data not shown), _γ_-T3 may be used alone or in combination with chemotherapy for treating advanced stage PCa.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
- Agarwal MK, Agarwal ML, Athar M, Gupta S (2004) Tocotrienol-rich fraction of palm oil activates p53, modulates Bax/Bcl2 ratio and induces apoptosis independent of cell cycle association. Cell Cycle 3: 205–211
Article CAS Google Scholar - Ahmad NS, Khalid BA, Luke DA, Ima Nirwana S (2005) Tocotrienol offers better protection than tocopherol from free radical-induced damage of rat bone. Clin Exp Pharmacol Physiol 32: 761–770
Article CAS Google Scholar - Ahn KS, Sethi G, Krishnan K, Aggarwal BB (2007) Gamma-tocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J Biol Chem 282: 809–820
Article CAS Google Scholar - Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN (1987) A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 47: 3239–3245
CAS Google Scholar - Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2: 76–83
Article CAS Google Scholar - Chowdhury S, Burbridge S, Harper PG (2007) Chemotherapy for the treatment of hormone-refractory prostate cancer. Int J Clin Pract 61: 2064–2070
Article CAS Google Scholar - Chu Q, Ling MT, Feng H, Cheung HW, Tsao SW, Wang X, Wong YC (2006) A novel anticancer effect of garlic derivatives: inhibition of cancer cell invasion through restoration of E-cadherin expression. Carcinogenesis 27: 2180–2189
Article CAS Google Scholar - Conte C, Floridi A, Aisa C, Piroddi M, Floridi A, Galli F (2004) Gamma-tocotrienol metabolism and antiproliferative effect in prostate cancer cells. Ann NY Acad Sci 1031: 391–394
Article CAS Google Scholar - Coppe JP, Itahana Y, Moore DH, Bennington JL, Desprez PY (2004) Id-1 and Id-2 proteins as molecular markers for human prostate cancer progression. Clin Cancer Res 10: 2044–2051
Article CAS Google Scholar - Craft N, Chhor C, Tran C, Belldegrun A, DeKernion J, Witte ON, Said J, Reiter RE, Sawyers CL (1999) Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res 59: 5030–5036
CAS PubMed Google Scholar - Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R (2002) Silibinin inhibits constitutive and TNFalpha-induced activation of NF-kappaB and sensitizes human prostate carcinoma DU145 cells to TNFalpha-induced apoptosis. Oncogene 21: 1759–1767
Article CAS Google Scholar - Eitsuka T, Nakagawa K, Miyazawa T (2006) Down-regulation of telomerase activity in DLD-1 human colorectal adenocarcinoma cells by tocotrienol. Biochem Biophys Res Commun 348: 170–175
Article CAS Google Scholar - Feldman BJ, Feldman D (2001) The development of androgen-independent prostate cancer. Nat Rev Cancer 1: 34–45
Article CAS Google Scholar - Ghosh A, Wang X, Klein E, Heston WD (2005) Novel role of prostate-specific membrane antigen in suppressing prostate cancer invasiveness. Cancer Res 65: 727–731
Article CAS Google Scholar - Guthrie N, Gapor A, Chambers AF, Carroll KK (1997) Inhibition of proliferation of estrogen receptor-negative MDA-MB-435 and -positive MCF-7 human breast cancer cells by palm oil tocotrienols and tamoxifen, alone and in combination. J Nutr 127: 544S–548S
Article CAS Google Scholar - Hazan RB, Kang L, Roe S, Borgen PI, Rimm DL (1997) Vinculin is associated with the E-cadherin adhesion complex. J Biol Chem 272: 32448–32453
Article CAS Google Scholar - He L, Mo H, Hadisusilo S, Qureshi AA, Elson CE (1997) Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr 127: 668–674
Article CAS Google Scholar - Howard EW, Ling MT, Chua CW, Cheung HW, Wang X, Wong YC (2007) Garlic-derived S-allylmercaptocysteine is a novel in vivo antimetastatic agent for androgen-independent prostate cancer. Clin Cancer Res 13: 1847–1856
Article CAS Google Scholar - Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN (2004) gamma-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci USA 101: 17825–17830
Article CAS Google Scholar - Kumar KS, Raghavan M, Hieber K, Ege C, Mog S, Parra N, Hildabrand A, Singh V, Srinivasan V, Toles R, Karikari P, Petrovics G, Seed T, Srivastava S, Papas A (2006) Preferential radiation sensitization of prostate cancer in nude mice by nutraceutical antioxidant gamma-tocotrienol. Life Sci 78: 2099–2104
Article CAS Google Scholar - Ling MT, Wang X, Ouyang XS, Xu K, Tsao SW, Wong YC (2003) Id-1 expression promotes cell survival through activation of NF-kappaB signalling pathway in prostate cancer cells. Oncogene 22: 4498–4508
Article CAS Google Scholar - Mazlan M, Sue Mian T, Mat Top G, Zurinah Wan Ngah W (2006) Comparative effects of alpha-tocopherol and gamma-tocotrienol against hydrogen peroxide induced apoptosis on primary-cultured astrocytes. J Neurol Sci 243: 5–12
Article CAS Google Scholar - McAnally JA, Gupta J, Sodhani S, Bravo L, Mo H (2007) Tocotrienols potentiate lovastatin-mediated growth suppression in vitro and in vivo. Exp Biol Med (Maywood) 232: 523–531
CAS Google Scholar - McAnally JA, Jung M, Mo H (2003) Farnesyl-O-acetylhydroquinone and geranyl-_O_-acetylhydroquinone suppress the proliferation of murine B16 melanoma cells, human prostate and colon adenocarcinoma cells, human lung carcinoma cells, and human leukemia cells. Cancer Lett 202: 181–192
Article CAS Google Scholar - Mo H, Elson CE (1999) Apoptosis and cell-cycle arrest in human and murine tumor cells are initiated by isoprenoids. J Nutr 129: 804–813
Article CAS Google Scholar - Morton RA, Ewing CM, Nagafuchi A, Tsukita S, Isaacs WB (1993) Reduction of E-cadherin levels and deletion of the alpha-catenin gene in human prostate cancer cells. Cancer Res 53: 3585–3590
CAS PubMed Google Scholar - Nesaretnam K, Ambra R, Selvaduray KR, Radhakrishnan A, Reimann K, Razak G, Virgili F (2004) Tocotrienol-rich fraction from palm oil affects gene expression in tumors resulting from MCF-7 cell inoculation in athymic mice. Lipids 39: 459–467
Article CAS Google Scholar - Patel V, Khanna S, Roy S, Ezziddin O, Sen CK (2006) Natural vitamin E alpha-tocotrienol: retention in vital organs in response to long-term oral supplementation and withdrawal. Free Radic Res 40: 763–771
Article CAS Google Scholar - Prasad S, Nigam N, Kalra N, Shukla Y (2008) Regulation of signaling pathways involved in lupeol induced inhibition of proliferation and induction of apoptosis in human prostate cancer cells. Mol Carcinog [e-pub ahead of print]
- Qureshi AA, Mo H, Packer L, Peterson DM (2000) Isolation and identification of novel tocotrienols from rice bran with hypocholesterolemic, antioxidant, and antitumor properties. J Agric Food Chem 48: 3130–3140
Article CAS Google Scholar - Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner M, Howlader N, Eisner M, Reichman M, Edwards B (2006) SEER Cancer Statistics Review, 1975–2004, Vol. 2007. National Cancer Institute: Bethesda, MD
Google Scholar - Samant GV, Sylvester PW (2006) gamma-Tocotrienol inhibits ErbB3-dependent PI3K/Akt mitogenic signalling in neoplastic mammary epithelial cells. Cell Prolif 39: 563–574
Article CAS Google Scholar - Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277: 665–667
Article CAS Google Scholar - Sen CK, Khanna S, Rink C, Roy S (2007) Tocotrienols: the emerging face of natural vitamin E. Vitam Horm 76: 203–261
Article CAS Google Scholar - Srivastava JK, Gupta S (2006) Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem Biophys Res Commun 346: 447–453
Article CAS Google Scholar - Sun W, Wang Q, Chen B, Liu J, Liu H, Xu W (2008) Gamma-tocotrienol-induced apoptosis in human gastric cancer SGC-7901 cells is associated with a suppression in mitogen-activated protein kinase signalling. Br J Nutr 99: 1247–1254
Article CAS Google Scholar - Sylvester PW, Shah S (2005) Intracellular mechanisms mediating tocotrienol-induced apoptosis in neoplastic mammary epithelial cells. Asia Pac J Clin Nutr 14: 366–373
CAS PubMed Google Scholar - Uzgare AR, Isaacs JT (2004) Enhanced redundancy in Akt and mitogen-activated protein kinase-induced survival of malignant versus normal prostate epithelial cells. Cancer Res 64: 6190–6199
Article CAS Google Scholar - van Oort IM, Tomita K, van Bokhoven A, Bussemakers MJ, Kiemeney LA, Karthaus HF, Witjes JA, Schalken JA (2007) The prognostic value of E-cadherin and the cadherin-associated molecules alpha-, beta-, gamma-catenin and p120ctn in prostate cancer specific survival: a long-term follow-up study. Prostate 67: 1432–1438
Article CAS Google Scholar - Vraka PS, Drouza C, Rikkou MP, Odysseos AD, Keramidas AD (2006) Synthesis and study of the cancer cell growth inhibitory properties of alpha-, gamma-tocopheryl and gamma-tocotrienyl 2-phenylselenyl succinates. Bioorg Med Chem 14: 2684–2696
Article CAS Google Scholar - Wada S, Satomi Y, Murakoshi M, Noguchi N, Yoshikawa T, Nishino H (2005) Tumor suppressive effects of tocotrienol in vivo and in vitro. Cancer Lett 229: 181–191
Article CAS Google Scholar - Zhang X, Ling MT, Wang X, Wong YC (2006) Inactivation of Id-1 in prostate cancer cells: a potential therapeutic target in inducing chemosensitization to taxol through activation of JNK pathway. Int J Cancer 118: 2072–2081
Article CAS Google Scholar
Acknowledgements
This work was supported by Research Incentive Scheme for Companies (RISC) to Davos Life Science Pte. Ltd. and grants to YCW (HKU 7314/01M, HKU7490/03M and 7470/04M). The 3-week (10 February –1 March 2008) work attachment of Miss Yap Wei Ney at YCW's laboratory (Department of Anatomy, HKU) was sponsored by Davos Life Science Pte. Ltd. and YCW's grants.
Author information
Authors and Affiliations
- Davos Life Science Pte. Ltd., Cancer Research Laboratory, 11 Biopolis way, #07-03, The Helios, 138667, Singapore
W N Yap, P N Chang & Y L Yap - Department of Anatomy, Cancer Biology Lab, Li Ka Shing Faculty of Medicine, The University of Hong Kong (HKU), 1/F, Laboratory Block, 21 Sassoon Road, Hong Kong SAR
H Y Han, D T W Lee, M T Ling & Y C Wong
Authors
- W N Yap
- P N Chang
- H Y Han
- D T W Lee
- M T Ling
- Y C Wong
- Y L Yap
Corresponding authors
Correspondence toM T Ling, Y C Wong or Y L Yap.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Yap, W., Chang, P., Han, H. et al. _γ_-Tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple-signalling pathways.Br J Cancer 99, 1832–1841 (2008). https://doi.org/10.1038/sj.bjc.6604763
- Received: 09 September 2008
- Revised: 30 September 2008
- Accepted: 06 October 2008
- Published: 11 November 2008
- Issue date: 02 December 2008
- DOI: https://doi.org/10.1038/sj.bjc.6604763