The risk of febrile neutropenia in patients with non-small-cell lung cancer treated with docetaxel: a systematic review and meta-analysis (original) (raw)
Main
Chemotherapy-induced febrile neutropenia (FN) is a serious adverse event caused by cancer therapies that can have a significant impact on mortality, morbidity and health care costs. FN impacts on quality of life both directly and indirectly, because it may lead to serious and potentially fatal infections and also because chemotherapy treatments can only be delayed or reduced with potentially detrimental clinical consequences . In addition, patients almost always require hospitalisation and treatment with antibiotics.
To reduce the risk of FN, recombinant human granulocyte colony-stimulating factors (G-CSFs) or granulocyte–macrophage colony-stimulating factors (GM-CSFs) may be considered. These agents can be used to prevent FN in all patients receiving chemotherapy, but they are costly. Consequently, they have been recommended for use in treating and preventing FN only in high-risk patients (Aapro et al, 2006; NCCN, 2006; Smith et al, 2006), in whom the evidence for their effectiveness is strongest. Antibiotic prophylaxis is an alternative prevention strategy that has been considered in several studies, although concerns about widespread antibiotic resistance have limited the use of this strategy in practice (NCCN, 2006).
In considering alternative preventative strategies, the differential risks, costs and potential benefits must be weighed up. Therefore, it is essential to accurately identify the risk of FN associated with any particular chemotherapy regimen. The European Organisation for Research and Treatment of Cancer guidelines (Aapro et al, 2006) and American Society of Clinical Oncology guidelines (Smith et al, 2006) both state that where the risk of FN with a chemotherapy is in excess of 20%, G-CSF prophylaxis is recommended in that patient group, and that where the risk associated with a specific regimen is lower than 20% there should be consideration of other patient factors such as age and co-morbidities.
Yet information on the risk of FN is lacking in this area. In patients with non-small-cell lung cancer (NSCLC), three second-line treatments are currently licensed in Europe: pemetrexed (Alimta®, Eli Lilly and Company, Indianapolis, USA), docetaxel (Taxotere®, Sanofi-Aventis, Paris, France) and erlotinib (Tarceva®, Roche, Basel, Switzerland). Pemetrexed is rarely used in practice. In the UK National Health Service (NHS), pemetrexed is not recommended for use in this patient group by the National Institute for Health and Clinical Excellence ([NICE, 2007a](/articles/6604863#ref-CR13 "NICE – National Institute for Health and Clinical Excellence (2007a) Technology appraisal guidance 124 pemetrexed for the treatment of non small cell lung cancer. http://www.nice.org.uk/guidance/index.jsp?action=download&o=36170
(accessed 23 April 2008)")). On the other hand, docetaxel is widely used in practice and is recommended by NICE ([NCCAC, 2005](/articles/6604863#ref-CR11 "NCCAC – National Collaborating Centre for Acute Care (2005) The diagnosis and treatment of lung cancer. Methods, evidence and guidance.
http://www.nice.org.uk/nicemedia/pdf/cg024fullguideline.pdf
(accessed 23 April 2008)")). A third treatment, erlotinib, is a newer oral treatment that has been the subject of a recent NICE appraisal. As a part of that appraisal, the cost effectiveness of erlotinib compared with docetaxel was estimated. As erlotinib does not have haematological toxicity, a crucially important component of the cost effectiveness estimate is the probability of FN in patients treated with docetaxel. Results are particularly sensitive to this probability ([DSU, 2007](/articles/6604863#ref-CR5 "Decision Support Unit (2007) The risk and costs of febrile neutropenia in patients with non small cell lung cancer treated with docetaxel. Available at
http://www.nice.org.uk/nicemedia/pdf/LungCancerErlotinibACDDSUReport2.pdf
(accessed 8 August 2008)")) yet data on this parameter proved controversial ([NICE, 2007b](/articles/6604863#ref-CR14 "NICE – National Institute for Health and Clinical Excellence (2007b) Guidance on erlotinib for the treatment of non-small cell lung cancer. Decision of the appeal panel
http://www.nice.org.uk/nicemedia/pdf/LungCancerErlotinibAppealDecision.pdf
(accessed 20 May 2008)")). The European Organisation for Research and Treatment of Cancer cite percentages of patients experiencing one or more episodes of FN of 26% for docetaxel in combination with carboplatin, and between 5 and 11% in combination with cisplastin. However, these estimates are based on only a small number of studies of varying quality, and as much of the evidence relates to first-line treatments, they may not be applicable for second-line NSCLC patients. The American Society of Clinical Oncology guidelines report that 12.7% of second-line NSCLC patients experienced FN but this is based on a single study ([Hanna et al, 2004](/articles/6604863#ref-CR10 "Hanna N, Shepherd FA, Fossella FV, Pereira JR, de Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar, Manegold C, Paul S, Paoletti P, Einhorn L, Bunn Pl (2004) Randomized phase III trial of pemetrexed vs docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22: 1589–1597")). We therefore conducted a systematic review and meta-analysis of docetaxel studies in NSCLC to identify the risk of FN, specifically for these patients.Methods
Search strategy
A comprehensive search was undertaken to identify the literature on docetaxel use in NSCLC. The databases searched (for all years that were indexed) were Medline, Medline in Process, EMBASE and The Cochrane Library, including the Cochrane Database of Systematic Reviews, Cochrane Controlled Trials Register (CENTRAL), DARE, NHS EED and HTA databases. Searches were not restricted by language or publication type. The search strategy for EMBASE was modified to include additional terms around ‘Neutropenia’, as omitting these terms resulted in an unmanageable result set of limited specificity.
Earlier systematic reviews considering docetaxel were also considered so that a manual search of their reference lists could be conducted to ensure that all relevant studies had been identified. Studies that met the inclusion criteria mentioned above but were only published as abstracts or as conference presentations were not included in the review unless a full paper could be obtained that related to the abstract.
Inclusion criteria
Studies were included if they assessed the use of docetaxel as monotherapy at the standard recommended dose (75 mg m−2 as a 1-h infusion every 3 weeks), in patients with NSCLC who had received one or more previous chemotherapy regimens for their disease and for which FN events were reported.
Data extraction
The primary outcome of the review was the rate of one or more episodes of FN among patients receiving docetaxel. We also sought to extract information on the grade of neutropenia and the proportion of patients receiving either G-CSFs or antibiotics to prevent or treat FN in those studies. Additional information regarding the baseline characteristics of the patient populations who took part in the studies and description of survival outcomes and treatment duration were also extracted and were reported.
Evidence synthesis
Meta-analysis was performed on the log odds scale and results were transformed back to the proportion scale for interpretation. Heterogeneity between the studies was assessed using the I 2 statistic and where it was greater than 0, a random effect meta-analysis model was used in preference over a fixed effect one. Where heterogeneity existed covariates were included in the analysis in an attempt to explain the between-study heterogeneity.
Results
A total of 950 studies were identified from the literature searches. Titles and abstracts were scanned for relevance and full copies of 40 studies were ordered. Of these, 13 studies were selected for inclusion, eight of which were phase III randomised controlled trials (RCTs) (Fossella et al, 2000; Shepherd et al, 2000; Gridelli et al, 2004; Hanna et al, 2004; Schuette et al, 2005; Camps et al, 2006; Chen et al, 2006; Ramlau et al, 2006) and five were phase II RCTs (Quoix et al, 2004; Gervais et al, 2005; Pectasides et al, 2005; Wachters et al, 2005; Cufer et al, 2006). The relevant data were contained in single trial arms, which consisted of a total of 1609 patients (see Table 1). The comparators to standard dose docetaxel included different doses of docetaxel, best supportive care or other active treatments (irinotecan, topotecan, vinorelbine, ifosfamide, gefitinib or pemetrexed).
Table 1 Characteristics of included studies
All studies report a mean patient age of around 60 years with a range of between the mid-30s and mid-70s in most studies. All patients have advanced NSCLC with the majority having metastatic disease (stage IV). The majority of patients in the trials have, however, good performance status (0 or 1); that is, they are ambulatory and are able to carry out work of a light or sedentary nature with few restrictions.
Seven of the 13 studies included described some use of G-CSF in their study patients (Table 1). Only one of these reported prophylactic use of G-CSF in all study patients (Wachters et al, 2005). The remainder reported that a minority of study patients received G-CSF. Two studies reported data separately for the proportions of patients receiving G-CSF for prophylaxis: 1.4% (Hanna et al, 2004), and for treatment of FN: 17% (Chen et al, 2006) and 12.1% (Hanna et al, 2004). Two studies reported combined prophylactic and treatment use of G-CSF: 28% (Pectasides et al, 2005) and 8% (Ramlau et al, 2006). One study reported that G-CSFs were used in 7% of cycles either prophylactically or as treatment for FN (Fossella et al, 2000) and one study simply reported that G-CSFs were used at the physician's discretion without providing actual data (Schuette et al, 2005).
In all trials FN events are presented as the percentage of patients experiencing one or more episode of FN. The final column of Table 1 summarises these percentages for the arms of the studies, in which the standard doses of docetaxel were administered; that is, 75 mg m−2 given intravenously over 1 h every 3 weeks. The trials do not generally report the number of events per person and almost all studies combined the grades of FN together. In studies that did not prescribe G-CSFs, FN rates for patients treated with the recommended standard dose of docetaxel ranged from 1.8 to 7.8%. In studies that prescribed G-CSFs, FN rates ranged from 2.0 to 12.7%. None of the studies reported using prophylactic antibiotics.
Meta-analysis
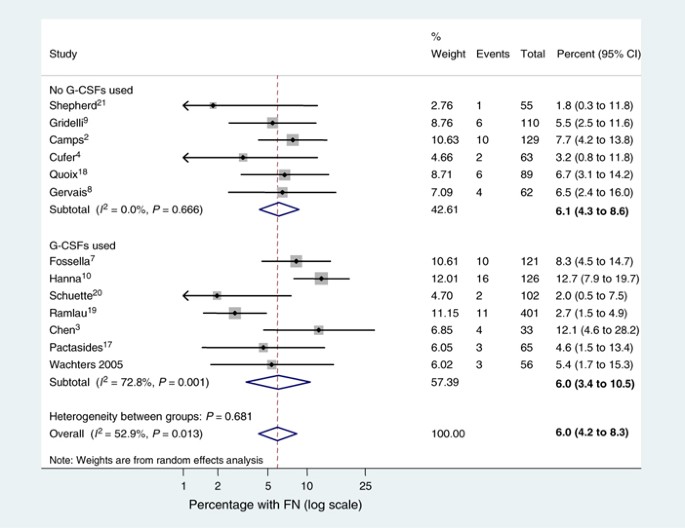
Meta-analysis was conducted on the 13 study arms described in Table 1. Some numerator data for the percentage of patients with FN were not reported but these were derived directly using the denominators and percentages reported in the papers. Only one figure was equivocal – the numerator for Gridelli et al (2004) – as five or six events would provide a percentage rounding up or down to 5%. Six events were imputed in this instance. Hence the data used in the meta-analysis are provided in Figure 1.
Figure 1
Forest plot for ln (odds) of experiencing FN on standard dose docetaxel (75 mg m−2 given intravenously once every 3 weeks).
Owing to between-study heterogeneity (I _2_=52.9%), a random effect meta-analysis was conducted. The pooled, random effects meta-analysis estimate for the proportion of patients who experience one or more episodes of FN on docetaxel is 5.95% (95% CI 4.22–8.31). We conducted a subgroup analysis comparing studies that reported any G-CSF use with those that did not report use of G-CSFs as well as an overall analysis (Figure 1). For studies in which no G-CSF use was reported, the proportion of patients experiencing FN was 6.07% (95% CI 4.27–8.63) compared with 6.01% (95% CI 3.36–8.32) for studies in which G-CSF use was reportedly permitted. Although effect sizes in both groups were almost identical, the majority of the observed heterogeneity was in the group, which had some G-CSF use (I _2_=72.8 and 0% for G-CSF and non-G-CSF groups, respectively).
A further subgroup analysis was carried out combining phase III and phase II trials separately. Pooled estimates were similar from both (phase II=5.54% (3.52–8.62) and phase III=6.08% (3.68–9.87)) with the majority of the heterogeneity being observed in the phase III studies (I _2_=70.6% for phase III and 0% for phase II).
Discussion
The meta-analysis results show that the incidence of FN associated with docetaxel as second-line therapy in trial patients with advanced NSCLC is approximately 6%. Individual patients and clinicians will want to consider this information as one element of the risks and benefits associated with alternative available chemotherapy regimens, which must be weighed up. Similarly, in deciding whether FN prophylaxis, such as antibiotics or G-CSFs, are appropriate for patients receiving docetaxel, the risk associated with the chemotherapy regimen must be considered alongside the risk factors related to the individual patient (for example, age, performance status) and the underlying disease. In relation to current European and American guidelines on the use of G-CSFs, the incidence of FN with docetaxel in NSCLC is relatively low.
This information is also a critical factor in policy-level decisions, such as those made by NICE, when considering the effectiveness and cost-effectiveness of available alternatives. In instances in which the figures for FN calculated in this report are used in assessing the cost effectiveness of erlotinib compared with docetaxel, it is considered unlikely that erlotinib is a cost-effective treatment ([NICE, 2008](/articles/6604863#ref-CR15 "NICE – National Institute for Health and Clinical Excellence (2008) Erlotinib for the treatment of non-small-cell lung cancer – appraisal consultation document. Available at http://www.nice.org.uk/guidance/index.jsp?action=article&o=41144
(accessed 11 August 2008)")), although this conclusion is obviously dependent on many other factors, including the price of erlotinib.It is important to note that in routine practice, FN rates might be different, as the clinical trial participants, although potentially more likely to have advanced disease, are often younger and have better performance status than those who do not choose to participate (Elting et al, 2006). In addition, the FN rates reported in this review are those associated with licensed use of docetaxel; that is, 75 mg m−2. FN rates associated with larger doses of docetaxel, for example, 100 mg m−2, tend to be higher: 12 vs 8% (Fossella et al, 2000) and 22.4 vs 1.8% (Shepherd et al, 2000), for 100 and 75 mg doses, respectively.
These estimates may be limited by the reporting of G-CSF use within the trials and the necessity to categorise studies somewhat crudely as either using G-CSFs or not. The analysis does not consider the proportion of patients being administered G-CSF within studies. It is perhaps surprising that no specific observational studies were identified for this review, although such studies would be appropriate to improve the evidence base and would also allow factors that can be obscured in multi-national trials to be addressed. For example, the degree of clinical experience in using docetaxel, as with any chemotherapy regimen, is likely to be an important factor in achieving good patient management of which low rates of FN would be considered an important element. For these reasons, future observational studies could add to this meta-analysis and allow health system-specific issues to be considered.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
- Aapro M, Cameron D, Pettengell R, Bohlius J, Crawford J, Ellis M (2006) EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer 42: 2433–2453
Article CAS Google Scholar - Camps C, Massuti B, Jimenez A, Maestu I, Gomez RG, Isla D, Gonzalez JL, Almenar D, Blasco A, Rosell R, Carrato A, Vinolas N, Batista N, Giron CG, Galan A, Lopez M, Blanco R, Provencio M, Diz P, Felip E (2006) Randomized phase III study of 3-weekly vs weekly docetaxel in pre-treated advanced non-small-cell lung cancer: a Spanish Lung Cancer Group trial. Ann Oncol 17: 467–472
Article CAS Google Scholar - Chen YM, Shih JF, Perng RP, Tsai C M, Whang-Peng J (2006) A randomized trial of different docetaxel schedules in non-small cell lung cancer patients who failed previous platinum-based chemotherapy. Chest 129: 1031–1038
Article CAS Google Scholar - Cufer T, Vrdoljak E, Gaafar R, Erensoy I, Pemberton K (2006) Phase II, open-label, randomized study (SIGN) of single-agent gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) non-small-cell lung cancer. Anticancer Drugs 17: 401–409
Article CAS Google Scholar - Decision Support Unit (2007) The risk and costs of febrile neutropenia in patients with non small cell lung cancer treated with docetaxel. Available at http://www.nice.org.uk/nicemedia/pdf/LungCancerErlotinibACDDSUReport2.pdf (accessed 8 August 2008)
- Elting LS, Cooksley C, Bekele BN, Frumovitz M, Elenir BC, Avritscher MB, Sun C, Bodurka DC (2006) Generalizability of cancer clinical trial results: prognostic differences between participants and non participants. Cancer 106: 2452–2458
Article Google Scholar - Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, Gandara D, Karp D, Vokes E, Kris M, Kim Y, Gamza F, Hammershaimb L (2000) Randomized phase III trial of docetaxel vs vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 18: 2354–2362
Article CAS Google Scholar - Gervais R, Ducolone A, Breton J-L (2005) Phase II randomised trial comparing docetaxel given every 3 weeks with weekly schedule as second-line therapy in patients with advanced non-small-cell lung cancer (NSCLC). Ann Oncol 16: 90–96
Article CAS Google Scholar - Gridelli C, Gallo C, Di Maio M, Barletta E, Illiano A, Maione P, Salvagni S, Piantedosi FV, Palazzolo G, Caffo O, Ceribelli A, Falcone A, Mazzanti P, Brancaccio L, Capuano MA, Isa L, Barbera S, Perrone F (2004) A randomised clinical trial of two docetaxel regimens (weekly vs 3 week) in the second-line treatment of non-small-cell lung cancer. The DISTAL 01 study. Br J Cancer 91: 1996–2004
Article CAS Google Scholar - Hanna N, Shepherd FA, Fossella FV, Pereira JR, de Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar, Manegold C, Paul S, Paoletti P, Einhorn L, Bunn Pl (2004) Randomized phase III trial of pemetrexed vs docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22: 1589–1597
Article CAS Google Scholar - NCCAC – National Collaborating Centre for Acute Care (2005) The diagnosis and treatment of lung cancer. Methods, evidence and guidance. http://www.nice.org.uk/nicemedia/pdf/cg024fullguideline.pdf (accessed 23 April 2008)
- National Comprehensive Cancer Network (NCCN) (2006) Clinical Practice Guidelines in Oncology: Myeloid Growth Factors. 2006
- NICE – National Institute for Health and Clinical Excellence (2007a) Technology appraisal guidance 124 pemetrexed for the treatment of non small cell lung cancer. http://www.nice.org.uk/guidance/index.jsp?action=download&o=36170 (accessed 23 April 2008)
- NICE – National Institute for Health and Clinical Excellence (2007b) Guidance on erlotinib for the treatment of non-small cell lung cancer. Decision of the appeal panel http://www.nice.org.uk/nicemedia/pdf/LungCancerErlotinibAppealDecision.pdf (accessed 20 May 2008)
- NICE – National Institute for Health and Clinical Excellence (2008) Erlotinib for the treatment of non-small-cell lung cancer – appraisal consultation document. Available at http://www.nice.org.uk/guidance/index.jsp?action=article&o=41144 (accessed 11 August 2008)
- Pectasides D, Pectasides M, Farmakis D, Kostopoulou V, Nikolaou M, Gaglia A, Koumpou M, Mylonakis N, Xiros N, Economopoulos T, Raptis SA (2005) Comparison of docetaxel and docetaxel-irinotecan combination as second-line chemotherapy in advanced non-small-cell lung cancer: a randomized phase II trial. Ann Oncol 16: 294–299
Article CAS Google Scholar - Quoix E, Lebeau B, Depierre A (2004) Randomised, multicentre phase II study assessing two doses of docetaxel (75 or 100 mg/m2) as second-line monotherapy for non-small-cell lung cancer. Ann Oncol 15: 38–44
Article CAS Google Scholar - Ramlau R, Gervais R, Krzakowski M, von Pawel J, Kaukel E, Abratt RP, Dharan B, Grotzinger KM, Ross G, Dane G, Shepherd FA (2006) Phase III study comparing oral topotecan to intravenous docetaxel in patients with pre-treated advanced non-small-cell lung cancer. J Clin Oncol 24: 2800–2807
Article CAS Google Scholar - Schuette W, Nagel S, Blankenburg T (2005) Phase III study of second-line chemotherapy for advanced non-small-cell lung cancer with weekly compared with 3-weekly docetaxel. J Clin Oncol 23: 8389–8395
Article CAS Google Scholar - Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, Coughlin S, Kim Y, Berille J (2000) Prospective randomized trial of docetaxel vs best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18: 2095–2103
Article CAS Google Scholar - Smith TJ, Khatcheressian J, Lyman GH (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24: 3187–3205
Article CAS Google Scholar - Wachters FM, Groen HJ, Biesma B, Schramel FM, Postmus PE, Stigt JA, Smit EF (2005) A randomised phase II trial of docetaxel vs docetaxel and irinotecan in patients with stage IIIb-IV non-small-cell lung cancer who failed first-line treatment. Br J Cancer 92: 15–20
Article CAS Google Scholar
Acknowledgements
This study was funded by the National Institute for Health and Clinical Excellence (NICE) Decision Support Unit. The funder had no involvement in study design, analysis or writing of this manuscript.
Author information
Authors and Affiliations
- Health Economics and Decision Science, School of Health and Related Research, University of Sheffield, Sheffield, UK
A Wailoo - Department of Health Sciences, University of Leicester, Leicester, UK
A Sutton - Health Services Research, Sheffield, UK
A Morgan
Authors
- A Wailoo
You can also search for this author inPubMed Google Scholar - A Sutton
You can also search for this author inPubMed Google Scholar - A Morgan
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toA Wailoo.
Appendix 1
Appendix 1
Search terms
A comprehensive search was undertaken to identify literature on docetaxel use in Lung cancer. The search strategy for EMBASE was modified to include additional terms around ‘Neutropenia’ as omitting these terms resulted in an unmanageable result set of limited specificity. Searches were not restricted by language, publication date or publication type. Databases searched were Medline, Medline in Process, EMBASE and The Cochrane Library, including the Cochrane Database of Systematic Reviews, Cochrane Controlled Trials Register (CENTRAL), DARE, NHS EED and HTA databases.
Search strategy for Medline and Cochrane Databases
- Carcinoma, Non-Small-Cell Lung/
- Lung neoplasms/
- docetax?l.tw.
- docetax?l.rn.
- docetax?l.nm.
- taxotere.mp.
- 1 or 2
- or/3–6
- 7 and 8
Search strategy for EMBASE
- Lung non Small Cell Cancer/
- Lung cancer/
- docetax?l.tw.
- docetax?l.rn.
- docetax?l.nm.
- taxotere.mp.
- 1 or 2
- or/3–6
- 7 and 8
- neutropen$.tw.
- neutropaen$.tw.
- 9 and 12
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Wailoo, A., Sutton, A. & Morgan, A. The risk of febrile neutropenia in patients with non-small-cell lung cancer treated with docetaxel: a systematic review and meta-analysis.Br J Cancer 100, 436–441 (2009). https://doi.org/10.1038/sj.bjc.6604863
- Received: 14 August 2008
- Revised: 03 November 2008
- Accepted: 04 December 2008
- Published: 03 February 2009
- Issue Date: 10 February 2009
- DOI: https://doi.org/10.1038/sj.bjc.6604863