β-Catenin is involved in alterations in mitochondrial activity in non-transformed intestinal epithelial and colon cancer cells (original) (raw)
Main
Leukotriene D4 (LTD4) is a powerful proinflammatory mediator derived from arachidonic acid through the 5-lipoxygenase pathway. Arachidonic acid is the common precursor of a group of mediators collectively called eicosanoids (Funk, 2001). Leukotriene D4 is the most potent of the cysteinyl leukotrienes (LTC4, LTD4, and LTE4). Leukotriene D4 is known to mediate its effects through specific cell surface receptors belonging to the G protein-coupled receptor family. The CysLT1 and CysLT2 receptors has been most studied (Heise et al, 2000; Lynch et al, 1999; Sarau et al, 1999) of which, the CysLT1 receptor has been proven to have a much higher affinity for LTD4 (Heise et al, 2000). Leukotriene D4 signalling has been implicated in chronic inflammatory conditions such as asthma and inflammatory bowel diseases (IBDs) (Drazen, 2002).
Inflammatory bowel diseases are associated with an increased incidence of neoplastic transformation (Ekbom et al, 1990), suggesting that there is a link between inflammation and cancer (Sheng et al, 1997). Furthermore, earlier studies have shown that colon cancer is underrepresented in a population of patients with ulcerative colitis, who where treated with non-steroidal anti-inflammatory drugs (Smalley and DuBois, 1997). This suggests that inflammatory mediators, such as LTD4, could be essential factors in mediating the coupling between IBD and colon cancer.
In 1930, Warburg showed that some types of cancer cells are capable of constitutive upregulation of glucose metabolism, even in the presence of the abundant oxygen. This phenomenon is known as the Warburg effect (Warburg, 1956). Cancer cells synthesise ATP mainly through ‘anaerobic glycolysis’, a metabolic state that is linked to high glucose uptake and local acidification due to lactate production, even in the presence of oxygen. Cancer cells often upregulate glycolysis enzymes as a result of constitutive signalling through the Akt pathway, or because of the expression of oncogenes such as Ras or Src (Elstrom et al, 2004; Pelicano et al, 2006). Earlier we have shown that LTD4 induces Akt phosphorylation in Int 407 cells (Paruchuri et al, 2005).
A major finding in understanding increases in respiration is based on the well-established fact that there is an increase in the average of ATP/ADP ratio (Matsunaga et al, 1996). The stimulation of both respiration (oxidative phosphorylation) and glycolysis is presumably responsible for the rise in the ATP/ADP ratio, which begins almost simultaneously with the increase in respiration.
Certain types of chronic inflammation such as ulcerative colitis have long been associated with a high risk of cancer development (Coussens and Werb, 2002; Hussain et al, 2003). It is believed that increased free radical generation is one important mechanism that promotes the progression of chronic inflammation into malignant transformation (Hussain et al, 2003). Cancer patients commonly have decreased glucose clearance capacity, high glycolytic activity and raised lactate production. Therefore, it has been suggested that the observed pro-oxidative shift is mediated by an increased availability of mitochondrial energy substrate. The ‘inflammatory oxidative conditions’ are typically associated with an excessive stimulation of NAD(P)H oxidase by cytokines and other inflammatory mediators (Kohli et al, 2007). Increased reactive oxygen species (ROS) production or changes in intracellular glutathione levels are often involved with pathological changes, both of which are indicative of signal cascade, or gene expression dysregulation (Waris and Ahsan, 2006).
_β_-Catenin is a multifunctional protein, its function is altered in most colon cancers. In non-transformed cells it is present at the cell membrane, where together with E-cadherin forms part of the adherent-type junction (Conacci-Sorrell et al, 2002; Cullen et al, 2004). In the presence of a Wnt signal, _β_-catenin translocates to the nucleus in which it activates transcription factors of the TCF/LEF family (Korinek et al, 1997). _β_-Catenin has an essential role in normal cell physiology, which makes it an unsuitable target for antagonising with specific inhibitors or siRNA. We have shown earlier that _β_-catenin translocates to the nuclei of Int 407 cells after exposure to the pro-inflammatory mediator LTD4 (Mezhybovska et al, 2006). We also found that after LTD4 stimulation, _β_-catenin was present in mitochondria where it was associated with the anti-apoptotic protein, Bcl-2 (Mezhybovska et al, 2006). Here we further examine the effects of _β_-catenin on mitochondrial activity.
Materials and methods
Constructs
HA-wt-_β_-catenin (_β_-cat wt, wild type) and HA-S33Y-_β_-catenin (_β_-cat S33Y, constitutively active mutant) constructs were generously provided by Dr Ben-Zeev (Weizmann Institute of Science, Rehovot, Israel). The NF_κ_B-RE luciferase construct was from Promega (Madison, WI, USA).
Cell culture
The non-transformed human intestinal epithelial cell line (Int 407) which shows typical epithelial morphology and growth, was isolated from jejunum and ileum of a human embryo of 2 months gestation (Henle and Deinhardt, 1957). Int 407 cells were cultured as a monolayer in Eagle's basal medium supplemented with 15% newborn calf serum. The colon cancer cell line Caco-2 was grown in Dulbecco's modified Eagle medium (Sigma Chemicals Co, St Louis, MO, USA) with 10% fetal bovine serum (Wikström et al, 2003). All media was supplemented with 2 mM L-glutamine, 55 IU ml–1 penicillin and 55 _μ_g ml–1 streptomycin. The cell lines were cultured at 37°C in a humidified atmosphere with 5% CO2. The cells were regularly tested to ensure the absence of mycoplasma contamination.
Transfection
Transfection was performed in complete medium for 24 h using PolyFect transfection reagent (Qiagen GmbH, Hilden, Germany), according to the manufacturer's instructions. In all transfection experiments it was routinely confirmed that empty vector had no effect.
Reverse transcription–PCR
Messenger RNA (mRNA) for the p65 NF-_κ_B subunit was synthesised using the forward primer: GCGAGAGGAGCACAGATACCACCAA and the reverse primer: GGCAGATCTTGAGCTCGGCAGTGTT.
mRNA isolation cDNA synthesis and quantitative real-time PCR
Messenger RNA was isolated using the RNeasy Plus Mini Kit from Qiagen following the manufacturer's instructions. Complementary DNA (cDNA) was synthesised using the Fermentas Revert Aid H Minus First Strand cDNA Synthesis kit following the manufacturer's instructions, or with random hexamer primers and superscript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA), according to the standard procedures. Complementary DNA was used as a template in quantitative real-time PCR reactions with TaqMan Gene Expression Assay (Applied Biosystems, Cambridge, UK) and Maxima Probe qPCR Mastermix (Fermentas Life Sciences, Vilnius, Lithuania). For relative quantification of expression levels, the comparative _C_t method was used. The point at which the fluorescence crosses the threshold was taken as the [_C_t] value. Expression levels of the different genes of interest were normalised to the expression level of the housekeeping gene, _β_-actin or GAPDH.
NADPH activity determination (MTS assay)
Cells were grown to 60% confluency. They were then stimulated or not with 40 nM LTD4 for the indicated period of times. Assays were performed using the Cell Titer 96 assay kit from Promega, following the manufacture's instructions. Conversion of MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) into aqueous, soluble formazan was measured by recording absorbance at 490 nm using a 96-well plate reader.
ATP/ADP ratio determination
The transfected and untransfected cells were grown to 60% confluency, and thereafter stimulated or not with 40 nM LTD4. Cells were collected in 20% TCA and frozen on dry ice for ATP determination. ATP was determined using the ATP Kit SL (luminescent assays) from BioThema (Handen, Sweden). Untreated samples were diluted 40-fold and measured bioluminometrically. Thereafter, ATP was irreversibly converted to AMP with ATP sulfurylase in the presence of molybdate. In all, 20 _μ_l of sample were mixed with 180 _μ_l of Buffer I (50 mM/5 mM Tris-HCl/MgCl2, pH 8.0, 10 mM Na2MoO4, 2.5 mM GMP, and 0.5 U ATP sulfurylase), incubated for 20 min at 30°C, boiled for 2.5 min and chilled on ice. Samples were diluted with 800 _μ_l milliQ-water and ATP was measured bioluminometrically. ADP was converted to ATP by pyruvate kinase. In total, 100 _μ_l of the diluted sample from the previous step was mixed with 100 _μ_l of the Buffer II (50 mM/5 mM Tris-HCl/MgCl2, pH 8.0, 38 mM KCl, 0.5 mM phosphopyruvate, and 50 U pyruvate kinase), samples were incubated at room temperature for 30 min. To terminate the reaction 300 _μ_l of H2O was added and ATP was measured bioluminometricaly (Lundin, 2000). ADP was calculated as the difference between the ATP measured after incubation with pyruvate kinase, and the residual ATP measured after treatment with sulfurylase alone.
Transient transfection and luciferase assays
Luciferase assays were carried out using the Dual Luciferase Reporter Assay System from Promega. Each plasmid was used at a final concentration of 1 _μ_g ml–1, except for the control Renilla luciferase vector, which was always used at 0.2 _μ_g ml–1 to standardise for transfection efficiency. Vector DNA was allowed to form complexes with PolyFect. Cells in 12-well plates were washed once in serum-free medium, and the DNA-Polyfect mixture was added. Cells were transfected at 37°C for 24 h in complete medium, after which the medium was changed to normal growth medium, and the cells allowed to recover for 24 h. Before any further stimulation, the cells were left in serum-free medium for 1 h. The cells were then pre-treated or not with 30 μ M NAC (N-acetyl-L-cysteine, Sigma Chemicals Co, an antioxidant capable of neutralising ROS) for 15 min and then incubated in the absence or presence of 40 nM LTD4 for the indicated period of time. After stimulation with LTD4, cells were washed in PBS and lysed using 250 _μ_l well–1 of the DLR passive lysis buffer provided in the assay. Lysed samples were collected and briefly centrifuged to precipitate the debris. A 20 _μ_l volume of each lysate was used to measure luciferase activity with 50 _μ_l luciferase assay substrate using a MiniLumat LB 9506 (Berthold Technologies, Hannover, Germany) luminometer. The control Renilla luciferase signal was recorded after the subsequent addition of 50 _μ_l of Stop&Glow buffer, and the level of expression given as a ratio. In every experiment, triplicate samples were prepared and analysed for each condition.
Dihydroethidium staining
The oxidative fluorescent dihydroethidium (DHE) dye was used to evaluate the intracellular production of O2−• (Kitada et al, 2003). Dihydroethidium is cell permeable and reacts with O2−• to form ethidium, which in turn intercalates with DNA, providing nuclear fluorescence at an excitation wavelength of 520 nm and emission wavelength of 610 nm. The cells were grown to 60% confluency, and were transfected with HA-S33Y-•_β_-catenin or HA-wt-•_β_-catenin. After 24 h, the cells were serum starved overnight and the following day incubated with 50 μ M DHE at 37°C for 30 min. Then the cells were washed with PBS and serum-free culture media was added before fluorescent microscopy (Kohli et al, 2007). Exogenous addition of H2O2 was used as a positive control. All images shown are of living cells maintained under physiological conditions by fluorescent microscopy using an Olympus microscope IX81 (Hamburg, Germany), objective × 20.
Western blot
The cells were lysed and scraped loose with ice-cold lysis buffer (50 mM Tris, pH 7.5, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 1% Triton X-100, 50 mM NaF, 5 mM sodium pyrophosphate, 10 mM sodium glycerophosphate, 4 _μ_g ml–1 leupeptin, and 30 _μ_g ml–1 phenylmethanesulfonyl fluoride (PMSF)). The resultant lysate was boiled with sample buffer (62 mM Tris pH 6.8, 1.0% SDS, 10% glycerol, 15 mg ml–1 dithiothreitol, and 0.05% bromphenol blue) for 10 min. Equal amounts of protein (30–50 _μ_g protein well–1) were loaded and subjected to electrophoresis on 8% homogeneous polyacrylamide gels. The separated proteins were electrophoretically transferred to PVDF membranes, which were then blocked for 1 h at ambient temperature with 3% BSA/PBS. The membranes were then incubated overnight at 4°C with the primary antibody (_β_-catenin diluted 1 : 500). The membranes were washed and incubated for 1 h with HRP-conjugated secondary antibody, diluted 1 : 5000 in 3% BSA/PBS with 0.1% Tween-20. Membranes were incubated with ECL western blot detection reagents, and exposed to Hyperfilm-ECL to visualise immunoreactive proteins (Mezhybovska et al, 2006).
Cell fractionation
Treatments were terminated by the addition of ice-cold lysis buffer (20 mM NaHepes pH 8.0, 2 mM MgCl2, 1 mM EDTA, 5 mM orthovandate, 60 _μ_g ml–1 PMSF and 4 _μ_g ml–1 leupeptin) and the cells were placed on ice. After N2-decompression at 1000 psi for 10 min using a cell disruption bomb (Parr Instrument Company, Moline, IL, USA) and centrifugation at 200 g, the supernatant was centrifuged at 10 000 g for 10 min, and further centrifugation at 200 000 g for 1 h, to obtain an isolated cytosolic fraction.
Results
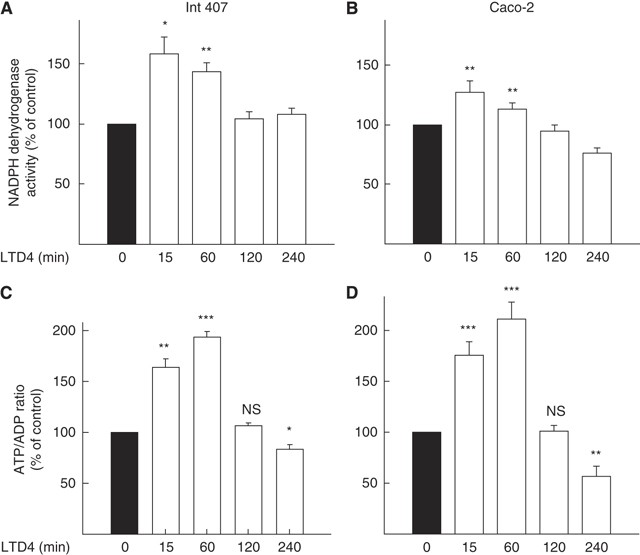
Leukotriene D4 increases metabolic activity in mitochondria
We have shown earlier that LTD4 increases cell survival and causes translocation of _β_-catenin to mitochondria. Therefore, we analysed whether LTD4 could affect mitochondrial activity. We observed a significant increase in the NADPH dehydrogenase activity in both the non-transformed epithelial cell line Int 407 and the cancer cell line Caco-2 (Figures 1A and B) in response to the LTD4 stimulation (40 nM). The maximum effect was reached after 15 min of stimulation with a significant increase after 1 h. The activity declined again after 2 h of stimulation. As a consequence of increased metabolism in the cell, an increase in the ATP/ADP ratio occurs, which contributes to plasma membrane depolarisation followed by activation of calcium channels leading to an increase in intracellular calcium (Fridlyand et al, 2005). Leukotriene D4 increased intracellular calcium levels in both Int 407 and Caco-2 cells (Nielsen et al, 2005). We also found an increase in the ATP/ADP ratio that was delayed compared with NADPH dehydrogenase activity, with a maximum effect after 1 h. The change was statistically significant at time points between 15 min and 1 h of LTD4 stimulation in both cell lines (Figures 1C and D).
Figure 1
Leukotriene D4 (LTD4)-induced increase in mitochondrial activity. Int 407 (A, C) and Caco-2 (B, D) cells were pre-incubated for indicated periods of time with 40 nM LTD4. (A, B) Cells cultured in complete medium in 96-well plates were subjected to a MTS assay (NADPH dehydrogenase activity) according to the manufacturer's instructions. Conversion of MTS into aqueous soluble formazan was measured by recording the absorbance at 490 nm using a 96-well plate reader. (C, D) Cells were collected in 20% TCA in PBS, and fast frozen. Thereafter, ATP and ADP amounts were measured as described in the Materials and Methods section. The presented data are given as means±s.e. of three separate experiments. *P<0.05; **P<0.01; ***P<0.005.
Leukotriene D4 increases mitochondrial gene transcription
Next, we examined whether LTD4 had any effect on mitochondrial gene transcription. The mitochondrial DNA (mtDNA) is present at a high copy number per cell. It contains genes encoding 13 polypeptides, essential for protein synthesis in mitochondria, and a non-coding region called the displacement loop (D-loop). The D-loop is involved in the control of replication and transcription of mtDNA. The two mtDNA strands differ in their G+T content and can therefore be separated into a heavy strand (H-strand), and a light strand (L-strand). In human cells each strand contains one single promoter for transcriptional initiation (Clayton, 1991; Ojala et al, 1981), and an additional initiation site for heavy strand transcription (Montoya et al, 1983). We chose to measure mRNA of one gene from each transcript of the two strands of the D-loop that control replication and transcription of mtDNA, in order to detect changes in mitochondrial-encoded gene activity. We observed a significant increase in mRNA for ND2 (NADH dehydrogenase subunit 2) both in Int 407 and Caco-2 cells (Figures 2A and B). This increase in transcription level occurred after 15 min of LTD4 stimulation, with the maximum three-fold increase observed after 2 h in Int 407 cells and after 1 h in Caco-2 cells. The same was seen in both cell lines for the other gene transcripts, ND6 (NADH dehydrogenase subunit 6) and 16 s, with the maximum effect after 2 h stimulation with 40 nM LTD4 in Int 407 cells and 1 h for the Caco-2 cells (Figures 2C-E and F). The maximum increase (three-fold) was found for 16 s mRNA in Int 407 cells after 2 h of LTD4 treatment (Figure 2E).
Figure 2
Expression of the mitochondrial-encoded genes upon leukotriene D4 (LTD4) stimulation. Levels of ND2 (A, B), ND6 (C, D), and 16 s (E, F) messenger RNA (mRNA) in Int 407 (A, C, and E) and Caco-2 (B, D, and F) cells, were measured by real-time PCR after stimulation with 40 nM LTD4 for the indicated periods of time. Expression levels of the genes of interest were normalised to the expression of the reference gene _β_-actin. The presented data are given as means±s.e. of three separate experiments. *P<0.05; **P<0.01; ***P<0.005.
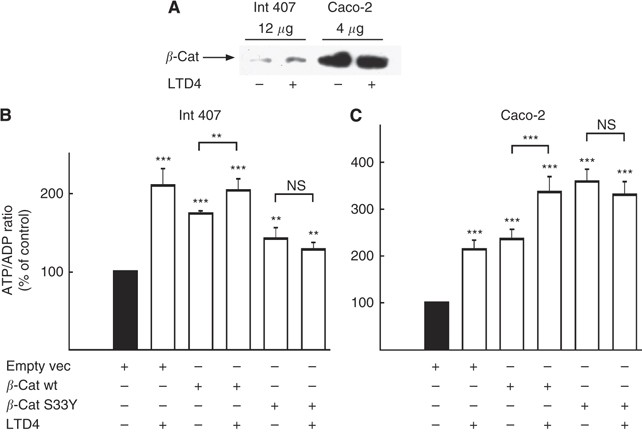
_β_-Catenin affects mitochondrial activity
Int 407 is a non-transformed cell line showing epithelial cell morphology, in which _β_-catenin is mainly present at the sites of cell–cell junctions (i.e., at the plasma membrane) and follows a normal degradation pattern (Mezhybovska et al, 2005). The Caco-2 cell line, however, has a mutation in the APC gene. APC is a part of the _β_-catenin degradation complex. Caco-2 cells have a non-functional _β_-catenin degradation pathway, leading to accumulation of _β_-catenin in the cells. As seen in Figure 3A, the endogenous level of _β_-catenin in the Caco-2 cells is significantly higher than in Int 407 cells (12 _μ_g of total protein from Int 407 cells and 4 _μ_g from Caco-2 cells were loaded on the gel). Next we examined whether _β_-catenin affects mitochondrial activity. We have shown that _β_-catenin translocates to the mitochondria and interacts with the survival protein Bcl-2 (Mezhybovska et al, 2006). We overexpressed the following _β_-catenin constructs: _β_-cat wt and _β_-cat S33Y, and measured ATP/ADP ratio in the cells. It is worth mentioning that both _β_-catenin constructs are expressed equally in the cells (data not shown). Overexpression of both wild-type _β_-cat wt and mutant _β_-cat S33Y gave at least a 1.5-fold increase in ATP/ADP ratio in Int 407 cells (Figure 3B) and a 2.5-fold increase in Caco-2 cells (Figure 3C). This effect was almost totally dependent on the _β_-catenin-mediated stimulation of the mitochondrial activity and not on the increase of number of the mitochondria, as seen from Figure 1A. The increase in viable cell number after wt or S33Y _β_-catenin overexpression were <0.2-fold after 24 h (118±11% (wt) and 122±10% (S33Y) of that seen after transfection with empty vector), which is <15% of the total increase in ATP/ADP ratio. Interestingly, LTD4 stimulation of cells overexpressing the _β_-cat wt construct caused a further increase in ATP/ADP ratio compared with unstimulated cells. However, LTD4 stimulation in cells overexpressing the _β_-cat S33Y construct did not cause any significant changes in mitochondrial activity (Figures 3B and C). The empty vector control had no effect.
Figure 3
Overexpression of _β_-catenin increases ATP/ADP ratio. (A) The endogenous levels of _β_-catenin in Int 407 and Caco-2 cells cytosolic fractions. For the Caco-2 cells only one-third of total protein was added compared with the Int 407 cells, in order to evaluate the amount of _β_-catenin present in the cytosol. (B) Levels of overexpressed _β_-catenin HA-wt-_β_-catenin (_β_-cat wt) and HA-S33Y-_β_-catenin (_β_-cat S33Y) mutant in Int 407 cells. Int 407 (C) and Caco-2 (D) cells were transiently transfected with empty vector (empty vec), _β_-cat wt or _β_-cat S33Y, and treated or not treated with 40 nM LTD4 for 1 h. Thereafter, cells were collected in 20% TCA in PBS, and fast frozen. ATP and ADP amounts were measured as described in the Materials and methods section. The presented data are given as means±s.e. of four separate experiments. *P<0.05; **P<0.01; ***P<0.005.
The basal expression levels of mitochondrial-encoded genes were different between Int 407 and Caco-2 cells. The basal levels of mitochondrial gene expression were significantly higher in Caco-2 cells (Table 1A). The difference in _β_-catenin levels in the two cell lines obviously correlated with the basal expression profiles of their mitochondrial-encoded genes.
Table 1 Overexpression of _β_-catenin increases mitochondria-encoded genes activity
In Int 407 cells, there was a dramatic increase in the expression levels of all three genes ND2, ND6 and 16 s after transfection with _β_-cat wt or _β_-cat S33Y (Table 1B). These stimulated levels in Int 407 cells were compatible to basal expression levels of Caco-2 cells. After the same treatment in Caco-2 cells, the effect of LTD4 on gene activity was less prominent. Activity of the ND2 and ND6 genes was not significantly different from cells transfected with empty vector, but 16 s transcription was increased 1.5-fold (Table 1C). In the Int 407 cells that had been transfected with wild-type or mutant _β_-catenin, LTD4 stimulation induced a significant increase in ND2, ND6 and 16 s transcription. Stimulation of Caco-2 cells overexpressing wild-type _β_-catenin was not significantly different from the unstimulated cells for ND2, ND6 and 16 s expression. Stimulation of Caco-2 cells that overexpressed constitutively active _β_-catenin, resulted in a significant increase compared with unstimulated cells (the effect was 1.5-fold for all analysed genes). The difference in the response between Int 407 and Caco-2 cells to LTD4 stimulation might be explained by the substantial difference in basal levels of gene expression between the two cell lines (Table 1A).
_β_-Catenin-mediated increase in mitochondria activity leads to NF-_κ_B activation through ROS
Oxidative phosphorylation is the main source of ROS production. Increased mitochondrial activity is expected to induce ROS production. As we observed earlier that LTD4 stimulation leads to an accumulation of _β_-catenin in mitochondria, we next examined whether LTD4 stimulation and _β_-catenin overexpression resulted in elevated levels of ROS. The mean fluorescence intensity value per cells for unstimulated empty vector was 11.2 relative units (RU), calculated by the image program ImageJ (W Burger & MJ Burge, NIH, USA). Extracellular addition of H2O2 was used as a positive control (mean fluorescent intensity per cell was 26.5 RU). Leukotriene D4 increased ROS production in Int 407 cells, although the effect was less prominent than in the positive control (mean fluorescence per cell 16.1 RU; Figure 4). Overexpression of _β_-catenin constructs also caused an increase in ROS production. The downstream target for ROS in many cell types is the NF-_κ_B family of transcription factors (Bubici et al, 2006). We found that overexpression of _β_-catenin resulted in the activation of the p65 subunit of NF-_κ_B (Figures 5A and B). Furthermore, the p65 subunit mRNA of NF-_κ_B is increased in LTD4-stimulated cells. A similar increase was observed after overexpression of the _β_-cat wt or _β_-cat S33Y constructs. Leukotriene D4 stimulation of cells overexpressing _β_-cat wt or _β_-cat S33Y construct did not significantly change the level of the p65 mRNA (Figures 5A and B).
Figure 4
Detection of O2−• production by dihydroethidium (DHE) staining in Int 407 cells. Representative results of DHE staining are shown from three independent experiments Int 407 cells (with or without overexpression of HA-wt-_β_-catenin (_β_-cat wt) or HA-S33Y-_β_-catenin) stimulated or not with 40 nM LTD4 for 1 h. The mean fluorescent intensity per cell in empty vector transfected cells was 11.2 RU, the mean value per cell for H2O2 positive control 26.5 RU, and for the LTD4 stimulation in Int 407 cells was 16.1 RU, mean values for other treatments were not different from LTD4 stimulation. Before imaging, the cells were preincubated with DHE for 30 min. The fluorescent micrographs show living cells under physiological conditions with 20 × objective.
Figure 5
Activation of the p65 subunit of NF-_κ_B and the NF-_κ_B response element in cells overexpressing _β_-catenin. Int 407 (A, C) and Caco-2 (B, D) cells were transiently transfected with empty vector (empty vec), HA-wt-_β_-catenin (_β_-cat wt) or HA-S33Y-_β_-catenin (_β_-cat S33Y), and treated or not treated with 40 nM leukotriene D4 (LTD4) for 1 h. PCR was performed to estimate levels of p65 messenger RNA (mRNA) (A, B). The results shown are representative of three separate experiments. In (C) and (D) cells were transiently co-transfected with NF_κ_B-luc and Renilla, plus one of the following vectors: empty vector (empty vec), _β_-cat wt or _β_-cat S33Y. As indicated, the cells were the pre-treated or not with NAC for 15 min before incubation in the absence or presence of 40 nM LTD4 for 1 h. The luciferase values were measured and normalised to the Renilla values. The present data are given as means±s.e. of three separate experiments. *P<0.05; **P<0.01; ***P<0.005.
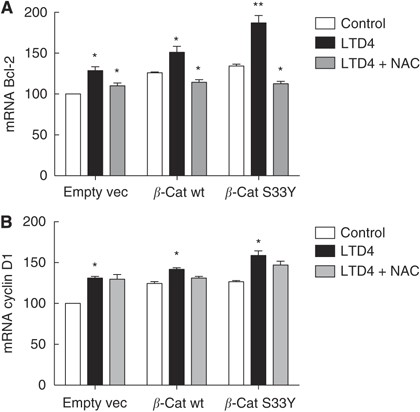
We next analysed whether LTD4 or _β_-catenin could affect the activity of NF-_κ_B. After LTD4 stimulation, we detected a three-fold increase in the activity of NF-_κ_B responsive elements in Int 407 cells and a 1.5-fold increase in Caco-2 cells (Figures 5C and D). Overexpression of _β_-cat wt or _β_-cat S33Y significantly increased activity of NF-_κ_B responsive elements in Int 407 (two-fold), and Caco-2 cells (1.4-fold). To analyse whether the increase in NF-_κ_B activity was ROS mediated, we used an antioxidant to block ROS formation. Preincubation with N-acetylcysteine (NAC) significantly reduced the effect of LTD4 and _β_-catenin overexpression in both cell lines (Figures 5C and D). We also analysed how NF-_κ_B activation affected its downstream target gene Bcl-2. Leukotriene D4 induced a significant increase in Bcl-2 expression that was further increased in cells transfected with either wt _β_-catenin or the S33Y−_β_-catenin mutant (Figures 6A). These effects of LTD4 were totally dependent on the LTD4-induced ROS production as shown by the use of the ROS inhibitor NAC that totally blocked the LTD4-induced increase in Bcl-2 expression (Figure 6A). To show the specificity of ROS/NF-_κ_B activation we also analysed the well-known target gene of _β_-catenin/TCF activity Cyclin D1. We found that both LTD4 and _β_-catenin overexpression significantly increased Cyclin D1 expression (Figure 6B). In contrast to the effects on Bcl-2 expression, the effects on Cyclin D1 expression were independent of ROS formation, as shown by preincubating the cells with the ROS inhibitor NAC (Figure 6B).
Figure 6
Effects of leukotriene D4 (LTD4) stimulation and _β_-catenin overexpression on Bcl-2 and Cyclin D1 expression. Caco-2 cells were transiently transfected with empty vector (empty vec), HA-wt-_β_-catenin (_β_-cat wt), or HA-S33Y-_β_-catenin (_β_-cat S33Y). As indicated, the cells were the pre-treated or not with NAC for 15 min before incubation in the absence or presence of 40 nM LTD4 for 18 h. Levels of Bcl-2 (A), and Cyclin D1 (B) messenger RNA (mRNA) in Caco-2 cells, were then measured by quantitative real-time PCR. The expression levels of the genes of interest were normalised to the expression of the reference gene _β_-actin and GAPDH. The presented data are given as means±s.e. of three separate experiments. *P<0.05; **P<0.01.
Discussion
We have earlier observed that exposure of Int 407 cells to LTD4 causes translocation of _β_-catenin to the mitochondria, and association with the anti-apoptotic protein Bcl-2, thereby enhancing cell survival (Mezhybovska et al, 2006; Paruchuri et al, 2006). Here we suggest a possible mechanism linking _β_-catenin to survival signalling in the inflammatory environment. We observed an increase in complex I activity and an increase in ATP/ADP ratio in Int 407 and Caco-2 cells. It is worth mentioning that both Lef-1 and mitochondria transcription factor TFAM1 bears the similar HMG domain that is not only responsible for DNA binding but for protein–protein interaction as well. It has earlier been shown that ATP/ADP ratio is crucial for the decision of the cell's fate (Leist et al, 1997; Richter et al, 1996). Furthermore, Vrbacky et al (2003), showed that a decrease in ATP/ADP ratio in BSC-40 cells is a hallmark of apoptosis. It has been shown in tumour cells (HeLa, MCF-7, and HL-60) that mitochondrial ATP supported increased cellular proliferation rates (Guppy et al, 2002; Sweet and Singh, 1995). In agreement with earlier findings our data strongly indicate that an increase in ATP/ADP ratio favours survival signalling in the cell. Leukotriene D4 increased mitochondrial gene activity in both Int 407 and Caco-2 cells as the activity of the mtDNA encoding for complex I was also increased transiently in both Int 407 cells and Caco-2 cells.
Changes in mitochondria gene activity as well as mutations in the mitochondrial genome are reported for many cancers (Pelicano et al, 2004; Polyak et al, 1998; Waris and Ahsan, 2006). ND2 is overexpressed in human acute myeloid leukaemia cells (Matsunaga et al, 1996). An increased level of 16 s RNA was shown in polyps of familial polyposis coli patients (Yamamoto et al, 1989). Mutations in 16 s RNA has been reported for colorectal tumours as well (Polyak et al, 1998). _β_-Catenin overexpression in the non-transformed cell line, Int 407, had a dramatic effect on mitochondrial gene activity, but in cancer cells _β_-catenin overexpression did not show any significant differences. Caco-2 cells have higher levels of _β_-catenin compared with Int 407 cells and as a result, higher activity of the mitochondrial genome. Therefore, the effect of _β_-catenin overexpression on mitochondrial gene activity in Caco-2 cells was not as prominent as in Int 407 cells.
Reactive oxygen species are formed predominantly through oxidative phosphorylation. Formation of ROS is often increased in cancer in order to maintain cell growth and proliferation (Chen et al, 2007; Chen et al, 1998; Irani et al, 1997). Therefore, it was expected that ROS production would be elevated in this system. We observed increased ROS production in Int 407 cells (but not to the extent of the positive control, H2O2), upon LTD4 stimulation and after _β_-catenin overexpression. The cell fate decision for suicide or survival is dependent on signalling pathways activated upon oxidative stress. High doses lead to cell death while a moderate increase favours cell survival signalling (Martindale and Holbrook, 2002). The observed change in ROS was not as significant as in H2O2 stimulation, leading to the conclusion that a moderate increase in ROS levels in this system mediates survival signalling.
Earlier we showed that LTD4 facilitates survival signalling through both PI-3 kinase/Akt/GSK-3_β_- and PKC/Erk1/2-dependent mechanisms (Mezhybovska et al, 2006; Paruchuri et al, 2002). Erk activation as a result of oxidant injury was reported in 1996 (Guyton et al, 1996), resulting in cell survival and tumour growth. Akt activation in response to oxidant injury contributes to survival signalling in peroxide-induced apoptosis in a human glioblastoma cell line (Sonoda et al, 1999). Both Erk and Akt signalling has been shown to be upregulated upon LTD4 stimulation in the non-transformed intestinal cell line Int 407 (Mezhybovska et al, 2006; Paruchuri et al, 2002). In the Caco-2 cancer cell line, we observed ROS formation in untreated cells, in which the treatment with LTD4 did not have any additional effect (data not shown). This is not surprising since the elevated levels of ROS have been connected to cancer development for several decades (Pelicano et al, 2004). It was shown that extracellular addition of O2 resulted in activation of NF-_κ_B, and that this effect was ROS mediated (Cazals et al, 1999; Suzuki et al, 2000). We found an increase in activity of the p65 subunit of NF-_κ_B after LTD4 stimulation for 1 h in Int 407 and Caco-2 cells. Earlier reports from our group did not find any significant effect on NF-kB activation in Int 407 cells after 8 h or longer stimulation (Bengtsson et al, 2008). Overexpression of wild-type _β_-catenin- and _β_−catenin-S33Y constructs also activated the p65 subunit of NF-_κ_B. We also checked whether the activation of p65 subunit resulted in its increased activity. We used a luciferase assay to test activity of the p65 response element. The p65 responsive element activity was elevated after LTD4 stimulation as well as after _β_-catenin overexpression in both cell lines. There is still some confusion in the literature regarding possible cross-talk of NF-_κ_B and _β_-catenin signalling. _β_-Catenin has been reported to regulate NF-_κ_B in tumour cells (Amit and Ben-Neriah, 2003). Other reports suggest that NF-_κ_B is a regulator of the _β_-catenin pathway (Wang et al, 2007), whereas some studies reported that ROS activity is subject to negative feedback regulation by NF-_κ_B (Bubici et al, 2006). We used the antioxidant (NAC) to validate whether ROS is upstream or downstream of NF-_κ_B. p65 activation seems to be ROS mediated as the LTD4 mediated increase in ROS was reduced after antioxidant treatment. We also found that the well-known downstream target gene of NF-_κ_B activity Bcl-2 was induced by LTD4 stimulation or _β_-catenin overexpression. These effects were mediated through ROS production, which is in contrast to the effects of LTD4 stimulation and _β_-catenin overexpression on _β_-catenin/Tcf-mediated Cyclin D1 expression.
In conclusion, we have shown earlier that LTD4 induces _β_-catenin translocation to the mitochondria and increases survival by interacting with the cell survival protein Bcl-2. Here we describe for the first time that _β_-catenin is involved in signal transduction leading to increased activity of the respiratory chain, which in turn leads to an increased ROS production and as a consequence, NF-_κ_B activation. NF-_κ_B is one of the key players in inflammation, linking chronic inflammation and cancer development. Consequently, the present data add further support to the idea that NF-_κ_B activation might be crucial for LTD4-induced cell survival.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
- Amit S, Ben-Neriah Y (2003) NF-kappaB activation in cancer: a challenge for ubiquitination- and proteasome-based therapeutic approach. Semin Cancer Biol 13: 15–28
Article CAS PubMed Google Scholar - Bengtsson AM, Massoumi R, Sjölander A (2008) Leukotriene D(4) induces AP-1 but not NFkappaB signaling in intestinal epithelial cells. Prostaglandins Other Lipid Mediat 85: 100–106
Article CAS PubMed Google Scholar - Bubici C, Papa S, Dean K, Franzoso G (2006) Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene 25: 6731–6748
Article CAS PubMed Google Scholar - Cazals V, Nabeyrat E, Corroyer S, de Keyzer Y, Clement A (1999) Role for NF-kappa B in mediating the effects of hyperoxia on IGF-binding protein 2 promoter activity in lung alveolar epithelial cells. Biochim Biophys Acta 1448: 349–362
Article CAS PubMed Google Scholar - Chen H, Yang S, Yang Z, Ma L, Jiang D, Mao J, Jiao B, Cai Z (2007) Inhibition of GSK-3beta decreases NF-kappaB-dependent gene expression and impairs the rat liver regeneration. J Cell Biochem 102: 1281–1289
Article CAS PubMed Google Scholar - Chen QM, Bartholomew JC, Campisi J, Acosta M, Reagan JD, Ames BN (1998) Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J 332 (Pt 1): 43–50
CAS PubMed PubMed Central Google Scholar - Clayton DA (1991) Replication and transcription of vertebrate mitochondrial DNA. Annu Rev Cell Biol 7: 453–478
Article CAS PubMed Google Scholar - Conacci-Sorrell M, Zhurinsky J, Ben-Ze’ev A (2002) The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest 109: 987–991
Article CAS PubMed PubMed Central Google Scholar - Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420: 860–867
Article CAS PubMed PubMed Central Google Scholar - Cullen DA, Killick R, Leigh PN, Gallo JM (2004) The effect of polyglutamine expansion in the human androgen receptor on its ability to suppress beta-catenin-Tcf/Lef dependent transcription. Neurosci Lett 354: 54–58
Article CAS PubMed Google Scholar - Drazen JM (2002) Anti-leukotrienes as novel anti-inflammatory treatments in asthma. Adv Exp Med Biol 507: 217–221
Article CAS PubMed Google Scholar - Ekbom A, Helmick C, Zack M, Adami HO (1990) Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med 323: 1228–1233
Article CAS PubMed Google Scholar - Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB (2004) Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 64: 3892–3899
Article CAS PubMed Google Scholar - Fridlyand LE, Ma L, Philipson LH (2005) Adenine nucleotide regulation in pancreatic beta-cells: modeling of ATP/ADP-Ca2+ interactions. Am J Physiol Endocrinol Metab 289: E839–E848
Article CAS PubMed Google Scholar - Funk CD (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294: 1871–1875
Article CAS PubMed Google Scholar - Guppy M, Leedman P, Zu X, Russell V (2002) Contribution by different fuels and metabolic pathways to the total ATP turnover of proliferating MCF-7 breast cancer cells. Biochem J 364: 309–315
Article CAS PubMed PubMed Central Google Scholar - Guyton KZ, Gorospe M, Kensler TW, Holbrook NJ (1996) Mitogen-activated protein kinase (MAPK) activation by butylated hydroxytoluene hydroperoxide: implications for cellular survival and tumor promotion. Cancer Res 56: 3480–3485
CAS PubMed Google Scholar - Heise CE, O’Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, Stocco R, Bellefeuille JN, Abramovitz M, Cheng R, Williams Jr DL, Zeng Z, Liu Q, Ma L, Clements MK, Coulombe N, Liu Y, Austin CP, George SR, O’Neill GP, Metters KM, Lynch KR, Evans JF (2000) Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem 275: 30531–30536
Article CAS PubMed Google Scholar - Henle G, Deinhardt F (1957) The establishment of strains of human cells in tissue culture. J Immunol 79: 54–59
CAS PubMed Google Scholar - Hussain T, Gupta S, Mukhtar H (2003) Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett 191: 125–135
Article CAS PubMed Google Scholar - Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ (1997) Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 275: 1649–1652
Article CAS PubMed Google Scholar - Kitada M, Koya D, Sugimoto T, Isono M, Araki S, Kashiwagi A, Haneda M (2003) Translocation of glomerular p47phox and p67phox by protein kinase C-beta activation is required for oxidative stress in diabetic nephropathy. Diabetes 52: 2603–2614
Article CAS PubMed Google Scholar - Kohli R, Pan X, Malladi P, Wainwright MS, Whitington PF (2007) Mitochondrial reactive oxygen species signal hepatocyte steatosis by regulating the phosphatidylinositol 3-kinase cell survival pathway. J Biol Chem 282: 21327–21336
Article CAS PubMed Google Scholar - Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science 275: 1784–1787
Article CAS PubMed Google Scholar - Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P (1997) Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med 185: 1481–1486
Article CAS PubMed PubMed Central Google Scholar - Lundin A (2000) Use of firefly luciferase in ATP-related assays of biomass, enzymes, and metabolites. Methods Enzymol 305: 346–370
Article CAS PubMed Google Scholar - Lynch KR, O’Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N, Abramovitz M, Figueroa DJ, Zeng Z, Connolly BM, Bai C, Austin CP, Chateauneuf A, Stocco R, Greig GM, Kargman S, Hooks SB, Hosfield E, Williams Jr DL, Ford-Hutchinson AW, Caskey CT, Evans JF (1999) Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature 399: 789–793
Article CAS PubMed Google Scholar - Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192: 1–15
Article CAS PubMed Google Scholar - Matsunaga T, Kudo J, Takahashi K, Dohmen K, Hayashida K, Okamura S, Ishibashi H, Niho Y (1996) Rotenone, a mitochondrial NADH dehydrogenase inhibitor, induces cell surface expression of CD13 and CD38 and apoptosis in HL-60 cells. Leuk Lymphoma 20: 487–494
Article CAS PubMed Google Scholar - Mezhybovska M, Wikstrom K, Ohd JF, Sjölander A (2005) Pro-inflammatory mediator leukotriene D4 induces transcriptional activity of potentially oncogenic genes. Biochem Soc Trans 33: 698–700
Article CAS PubMed Google Scholar - Mezhybovska M, Wikstrom K, Ohd JF, Sjölander A (2006) The inflammatory mediator leukotriene D4 induces beta-catenin signaling and its association with antiapoptotic Bcl-2 in intestinal epithelial cells. J Biol Chem 281: 6776–6784
Article CAS PubMed Google Scholar - Montoya J, Gaines GL, Attardi G (1983) The pattern of transcription of the human mitochondrial rRNA genes reveals two overlapping transcription units. Cell 34: 151–159
Article CAS PubMed Google Scholar - Nielsen CK, Massoumi R, Sonnerlind M, Sjölander A (2005) Leukotriene D4 activates distinct G-proteins in intestinal epithelial cells to regulate stress fibre formation and to generate intracellular Ca2+ mobilisation and ERK1/2 activation. Exp Cell Res 302: 31–39
Article CAS PubMed Google Scholar - Ojala D, Montoya J, Attardi G (1981) tRNA punctuation model of RNA processing in human mitochondria. Nature 290: 470–474
Article CAS PubMed Google Scholar - Paruchuri S, Broom O, Dib K, Sjölander A (2005) The pro-inflammatory mediator leukotriene D4 induces phosphatidylinositol 3-kinase and Rac-dependent migration of intestinal epithelial cells. J Biol Chem 280: 13538–13544
Article CAS PubMed Google Scholar - Paruchuri S, Hallberg B, Juhas M, Larsson C, Sjölander A (2002) Leukotriene D(4) activates MAPK through a Ras-independent but PKCepsilon-dependent pathway in intestinal epithelial cells. J Cell Sci 115: 1883–1893
CAS PubMed Google Scholar - Paruchuri S, Mezhybovska M, Juhas M, Sjölander A (2006) Endogenous production of leukotriene D4 mediates autocrine survival and proliferation via CysLT1 receptor signalling in intestinal epithelial cells. Oncogene 25: 6660–6665
Article CAS PubMed Google Scholar - Pelicano H, Carney D, Huang P (2004) ROS stress in cancer cells and therapeutic implications. Drug Resist Updat 7: 97–110
Article CAS PubMed Google Scholar - Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS, Hu Y, Ramdas L, Hu L, Keating MJ, Zhang W, Plunkett W, Huang P (2006) Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J Cell Biol 175: 913–923
Article CAS PubMed PubMed Central Google Scholar - Polyak K, Li Y, Zhu H, Lengauer C, Willson JK, Markowitz SD, Trush MA, Kinzler KW, Vogelstein B (1998) Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat Genet 20: 291–293
Article CAS PubMed Google Scholar - Richter C, Schweizer M, Cossarizza A, Franceschi C (1996) Control of apoptosis by the cellular ATP level. FEBS Lett 378: 107–110
Article CAS PubMed Google Scholar - Sarau HM, Ames RS, Chambers J, Ellis C, Elshourbagy N, Foley JJ, Schmidt DB, Muccitelli RM, Jenkins O, Murdock PR, Herrity NC, Halsey W, Sathe G, Muir AI, Nuthulaganti P, Dytko GM, Buckley PT, Wilson S, Bergsma DJ, Hay DW (1999) Identification, molecular cloning, expression, and characterization of a cysteinyl leukotriene receptor. Mol Pharmacol 56: 657–663
Article CAS PubMed Google Scholar - Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD, DuBois RN (1997) Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest 99: 2254–2259
Article CAS PubMed PubMed Central Google Scholar - Smalley WE, DuBois R (1997) Colorectal cancer and nonsteroidal anti-inflammatory drugs. Adv Pharmacol 39: 1–20
Article CAS PubMed Google Scholar - Sonoda Y, Watanabe S, Matsumoto Y, Aizu-Yokota E, Kasahara T (1999) FAK is the upstream signal protein of the phosphatidylinositol 3-kinase-Akt survival pathway in hydrogen peroxide-induced apoptosis of a human glioblastoma cell line. J Biol Chem 274: 10566–10570
Article CAS PubMed Google Scholar - Suzuki Y, Nishio K, Takeshita K, Takeuchi O, Watanabe K, Sato N, Naoki K, Kudo H, Aoki T, Yamaguchi K (2000) Effect of steroid on hyperoxia-induced ICAM-1 expression in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 278: L245–L252
Article CAS PubMed Google Scholar - Sweet S, Singh G (1995) Accumulation of human promyelocytic leukemic (HL-60) cells at two energetic cell cycle checkpoints. Cancer Res 55: 5164–5167
CAS PubMed Google Scholar - Wang Y, Kreisberg JI, Ghosh PM (2007) Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Curr Cancer Drug Targets 7: 591–604
Article CAS PubMed Google Scholar - Warburg O (1956) On the origin of cancer cells. Science 123: 309–314
Article CAS PubMed Google Scholar - Waris G, Ahsan H (2006) Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog 5: 14
Article PubMed PubMed Central Google Scholar - Wikström K, Öhd JF, Sjölander A (2003) Regulation of leukotriene-dependent induction of cyclooxygenase-2 and Bcl-2. Biochem Biophys Res Commun 302: 330–335
Article PubMed Google Scholar - Vrbacky M, Krijt J, Drahota Z, Melkova Z (2003) Inhibitory effects of Bcl-2 on mitochondrial respiration. Physiol Res 52: 545–554
CAS PubMed Google Scholar - Yamamoto A, Horai S, Yuasa Y (1989) Increased level of mitochondrial gene expression in polyps of familial polyposis coli patients. Biochem Biophys Res Commun 159: 1100–1106
Article CAS PubMed Google Scholar
Acknowledgements
We thank Maria Julias for invaluable to chemical assistance. This work was supported by grants awarded to the authors: AS from the Swedish Cancer Foundation, the Swedish Medical Research Council, the Foundations at Malmö University Hospital, the Julin Foundation, Gunnar Nilsson Foundation, Österlund Foundation, and MM from Royal Physiographic Society in Lund.
Author information
Authors and Affiliations
- Department of Laboratory Medicine, Cell and Experimental Pathology, Malmö University Hospital, Lund University, Malmö, SE-205 02, Sweden
M Mezhybovska, Y Yudina & A Sjölander - Department of Clinical Sciences, Clinical Research Centre, Malmö University Hospital, Lund University, Malmö, SE-205 02, Sweden
A Abhyankar
Authors
- M Mezhybovska
You can also search for this author inPubMed Google Scholar - Y Yudina
You can also search for this author inPubMed Google Scholar - A Abhyankar
You can also search for this author inPubMed Google Scholar - A Sjölander
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toA Sjölander.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Mezhybovska, M., Yudina, Y., Abhyankar, A. et al. _β_-Catenin is involved in alterations in mitochondrial activity in non-transformed intestinal epithelial and colon cancer cells.Br J Cancer 101, 1596–1605 (2009). https://doi.org/10.1038/sj.bjc.6605342
- Received: 26 January 2009
- Revised: 17 July 2009
- Accepted: 03 September 2009
- Published: 13 October 2009
- Issue Date: 03 November 2009
- DOI: https://doi.org/10.1038/sj.bjc.6605342