Bcl-xL promotes metastasis of breast cancer cells by induction of cytokines resistance (original) (raw)
Introduction
The molecular and cellular mechanisms responsible for the metastatic phenotype in breast cancer are poorly understood. This is partly due to the fact that metastatic cascade involves a series of interconnected events by means of which tumor cells withstand the severe pressures from host-cell components and their cytokines and growth factors. The ability of tumor cells to resist death signals may create favorable conditions for the successful development of metastatic foci. This is apparent in different stages, such as survival in the blood stream1 and in dormant state of micrometastasis2 among others, and these prevent tumor-cell loss in critical conditions. Furthermore, recent evidence suggests that the ability of tumor cells to acquire an aggressive phenotype may result from the accumulation of genetic alterations conferred by extended survival.3,4
Gene products controlling the balance between cell-death and survival arise from an expanding family of genes, of which Bcl-2 was the first to be clearly associated with apoptosis inhibition.5,6,7 The Bcl-xL gene, a member of the Bcl-2 gene family, exerts its antiapoptotic function by preventing the release of cytochrome c from the mitrochondria to the cytosol, like Bcl-2.8,9 Bcl-xL prevents the loss of mitochrondrial outer membrane integrity by blocking both membrane hyperpolarization and mitochondrial swelling in response to several sets of stimuli.10 Moreover, recent studies report that Bcl-xL protein interacts with Apaf-1 to inhibit the caspase activation involved in apoptosis.11 Overexpression of Bcl-2 or Bcl-xL can confer resistance to most apoptotic stimuli, suggesting that these genes do indeed function at a central convergence of many apoptotic pathways.5,6,7
In the breast, their overexpression could protect breast cancer cells from p53-mediated apoptosis.12 Increased levels of Bcl-xL expression were found in a subset of primary human breast carcinomas, mainly in undifferentiated histological grade (HG) III tumors.13,14 We also demonstrated that its expression can be modulated in breast tumors in vivo (data not shown). Recently, we identified loss of apoptosis and Bcl-2 overexpression in T1 ductal breast carcinomas and this phenotype was associated with the likelihood of lymph node metastasis in patients,15 and with accumulated oncogene alterations.16 We have also reported Bcl-xL overexpression in these carcinomas, but Bcl-xL and Bcl-2 in human breast cancer may not be functionally redundant because expression of Bcl-xL is higher in HGIII than in HGI or HGII, in which Bcl-2 is predominant.14 Importantly, those genes might be differentially expressed in the various stages of the metastatic cascade but the presence of death-regulatory proteins might be indicative of the aggressive nature of such tumors with no major genetic alterations providing a metastatic advantage.
The aim of this work was to explore the role of Bcl-xL in the metastatic progression of breast cancer. For this purpose we transfected human breast cancer cells MDA-MB 435 with Bcl-xL cDNA and then studied the tumor development, from intra-mammary fat pad injection (i.m.f.p.), and the spontaneous and experimental metastatic behavior of these transfectants in nude mice.
Results
Bcl-xL increases metastatic ability of cells
MDA-MB 435 cells transfected with Bcl-xL, an antiapoptotic gene, showed greater capacity to become metastatic than 435/Neo cells.
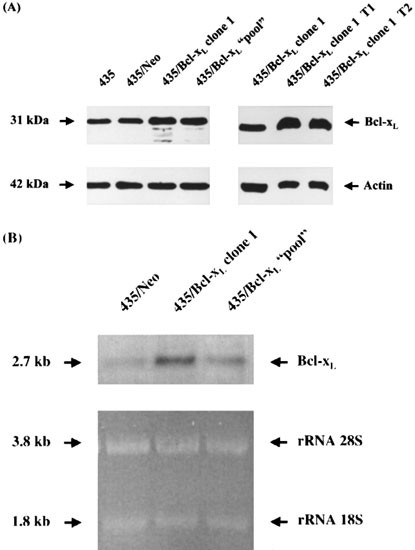
The parental clone used in this study expressed low levels of Bcl-xL and by transfection with Bcl-xL the expression increased in the two clones selected for the experiments in vivo, as shown in Figure 1A,B. Bcl-xL expression was 1.9-fold higher in 435/Bcl-xL1 and 1.6-fold higher in 435/Bcl-xLpool than in the control clone 435/Neo. The overexpression of Bcl-xL on cell clones and in tumors obtained by injecting cells i.m.f.p. was also analyzed by Western blot (Figure 1A) with specific antibodies, and was maintained in consecutive passages in vivo/in vitro. Bcl-xL expression was 2.07- and 1.19-fold higher in 435/Bcl-xL1 tumors (T1 and T2) than in 435/Bcl-xL1-in vitro.
Figure 1
Bcl-xL expression in MDA-MB 435 transfectant cells. (A) Western blot analysis of Bcl-xL protein from cell lysates of 435 parental, 435/Neo, 435/Bcl-xL1 and 435/Bcl-xLpool; and from tumors (T1 and T2) obtained by injecting 435/Bcl-xL1 cells i.m.f.p. Fifty μg of total protein from each sample were loaded and anti-Actin antibodies used to reprove each blot. The Bcl-xL expression levels among the cell clones measured in ratios to those observed in 435/Neo were 1.9 in 435/Bcl-xL1 and 1.6 in the 435/Bcl-xLpool; and in 435/Bcl-xL1 tumors measured in ratios to those observed in 435/Bcl-xL1-in vitro were 2.07 (T1) and 1.91 (T2). (B) Northern blot of total RNA (20 μg) extracted from 435/Bcl-xL clones, as indicated was hybridized with a human cDNA probe (0.8 kb _Eco_RI inserted in pSFFV-neo). Transcript sizes are noted on the left. RNA staining with ethidium bromide was used as a loading control
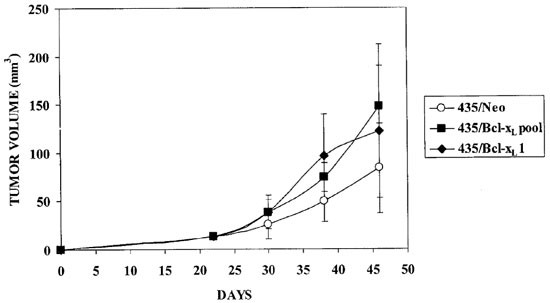
The weight of mice and tumor volume were monitored weekly (Figure 2). Tumor volume was calculated using the formula: Volume (mm3)=L×W2/2, where L and W are the major and minor diameters in millimeters, respectively. Tumors appeared 2 weeks after cells were injected i.m.f.p., and they were excised at 45 days. The tumor incidence was 100% in all cases. The mean tumor volume was 83.6±46.5 in 435/Neo (n =10), 147.8±64.2 in 435/Bcl-xLpool (n =12), and 121.5±68.8 in 435/Bcl-xL1 (n =14). These differences in tumor size were marginally insignificant (ANOVA, P =0.067).
Figure 2
In vivo growth of i.m.f.p tumors from 1×106 MDA-MB 435 transfectant cells. Tumor volume was calculated using the formula: Volume (mm3) = L×W2/2, where L and W are the major and minor diameters in millimeters, respectively
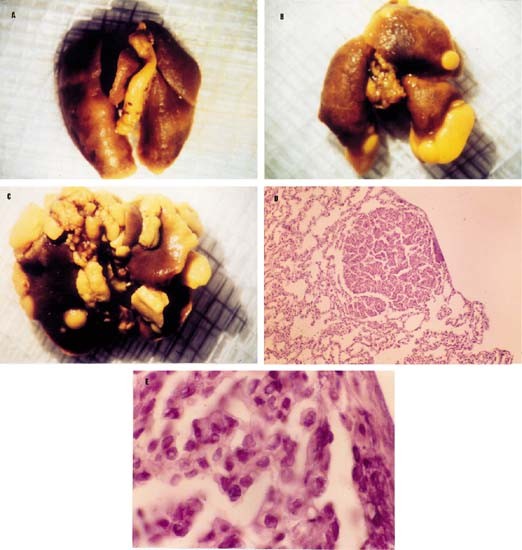
When mice were killed and analyzed for metastatis, lung metastatis from tumors overexpressing Bcl-xL reached over 3 mm in diameter, whereas only small metastases of ⩽3 mm were found in the lungs of 435/Neo-injected mice (Figure 3). As shown in Table 1, although the number of metastasis was heterogenous, the mean of metastasis in each mouse showed statistically significant differences between groups (ANOVA, P<0.005). Moreover, we analyzed the relationship between tumor volume and number of metastasis in each mouse but any correlation has been detected. Lymph node metastasis appeared in mice from Bcl-xL clones but not in mice from 435/Neo control cells.
Figure 3
Representative pictures of lung metastasis (×0.8) from 435/Neo (A), 435/Bcl-xLpool (B) and 435-Bcl-xL1 (C) at 4 months since cells were injected i.m.f.p Hematoxilin-eosin stain of lung metastasis from a 435/Bcl-xL1 tumor, visualized by light microscopy is shown in (D,×33) and (E,×200)
Table 1 Tumor volume and metastasis incidence induced by 435/Neo, 435/Bcl-xLpool and 435/Bcl-xL1 cells injected into nude mice
Resistance to cell-death-mediated by several cytokines induced in Bcl-xL tranfectants
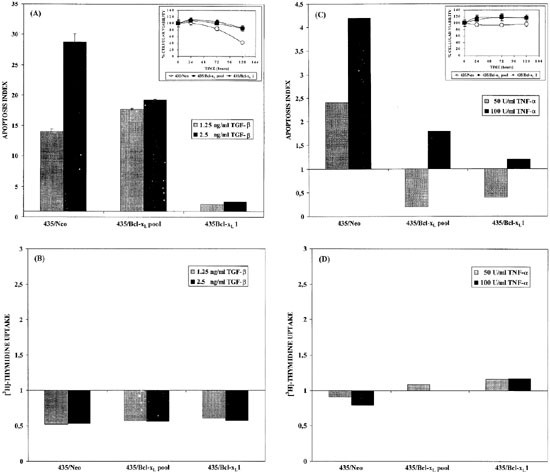
To further explore the biological role of Bcl-xL overexpression in breast cancer cells and its relationship to metastatic behavior, we analyzed apoptosis of 435-clones against different growth factors. Figure 4 shows the apoptosis of cells cultured with TGFβ and TNFα.
Figure 4
Proliferative and apoptotic status of 435/Neo and 435/Bcl-xL1 cells. This panel shows apoptosis measured by quantitative ELISA (A,C) and 3H-thymidine uptake (B,D) on 435/Neo and 435/Bcl-xL cells cultured without serum in presence of (h)TGFβ at 1.25–2.5 ng/ml (A,B) and r(h)TNFα at 50–100 U/ml (C,D). In (A) and (C), the upper panel shows viability of the transfectants when cultured for the indicating times with (h)TGFβ (2.5 ng/ml) and r(h)TNFα (100 U/ml), measured with MTT assay
435/Bcl-xL-clones stimulated with TGFβ exhibited less apoptosis than 435/Neo in several different experiments carried out between 48 and 96 h (Figure 4A). The most important effect was seen at 2.5 ng/ml concentration where apoptosis was stabilized in Bcl-xL clones while the apoptosis index reached 30 in 435/Neo cultures.
In the case of TNFα, 50 U/ml did not exert apoptotic activity in 435/Bcl-xL cultures; however, the apoptotic index of 435/Neo cells was twice that of untreated cultures (Figure 4C).
In order to assess the imbalance between cell proliferation and death as seen in tumors and metastasis in vitro, we also explored proliferation in response to these cytokines. As shown in Figure 4B, TGFβ had a cytostatic activity in 435/Neo and in 435/Bcl-xL cultures. Moreover, different cell activity was observed between 435/Neo and 435/Bcl-xL clones in cultures stimulated with TNFα (Figure 4D). This growth factor maintained basal proliferation of 435/Bcl-xL cells but had cytostatic activity in 435/Neo cells.
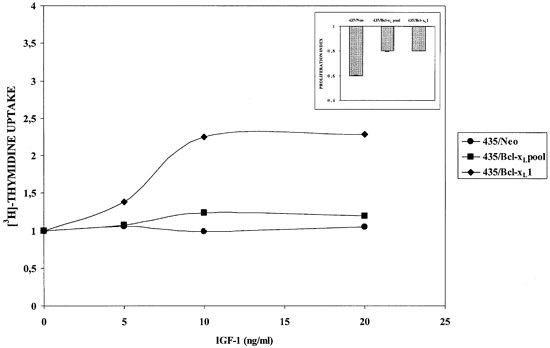
To contrast these observations we explored the response of cells against IGF-I, a common growth factor related to proliferation in breast cancer cells. This cytokine induced moderate proliferation to 435/Bcl-xL1 cell cultures in comparison to 435/Bcl-xLpool or 435/Neo cells. Moreover, as shown in the upper panel of Figure 5, experiments carried out at different concentrations of FCS showed that 435/Bcl-xL cells were less affected by low concentrations of FCS. The growth of 435-Bcl-xL1 cells was only 20% less at 1% of FCS than in standard culture conditions, in contrast to 435-Neo cells, which diminished by 40%.
Figure 5
435/Neo and 435/Bcl-xL cells cultured without serum in presence of r(h)IGF-I at 5–20 ng/ml as described in Material and Methods, and tested at 72 h for 3H-thymidine uptake. The upper panel indicates the growth-inhibitory effects in cells cultured at 1% of FCS concentration (ANOVA, P<0.0001)
435/Bcl-xL cells show decreased adhesion to extracellular matrix proteins (laminin, fibronectin and collagen IV), increased survival in suspension, and in the blood stream
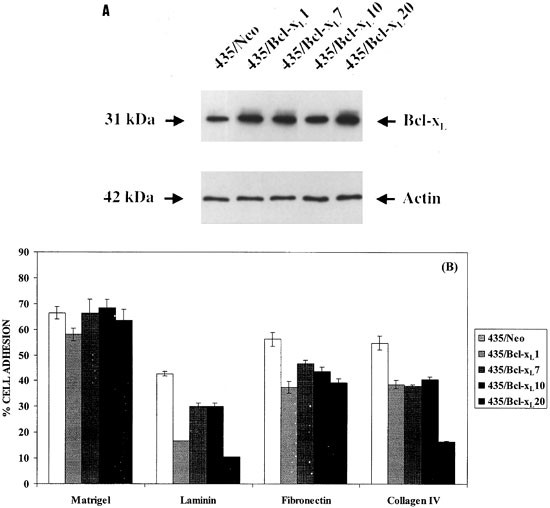
To analyze the influence of Bcl-xL in cell adhesion we measured the binding of tumor cells by coating culture wells with extracellular matrix proteins. Figure 6 shows that Bcl-xL cells bound less readily to LM, FN and CL IV than 435/Neo (ANOVA, P<0.0001). In contrast, there were no differences in cell clone binding to the matrigel.
Figure 6
Adhesion to extracellular matrix proteins of 435/Bcl-xL cells. (A) Western blot analysis of Bcl-xL protein from cell lysates of 435/Neo and different 435/Bcl-xL clones. Each sample was loaded and anti-Actin antibodies used to reprove each blot. (B) Binding to extracellular matrix components of 435/Bcl-xL cells labeled with 51Cr. The percentage of attached cells with regard to inoculum per well is represented. 435/Bcl-xL cells showed less adhesion to FN, LM and CL IV than 435/Neo cells (ANOVA, P<0.0001, for the three extracellular matrix compounds tested)
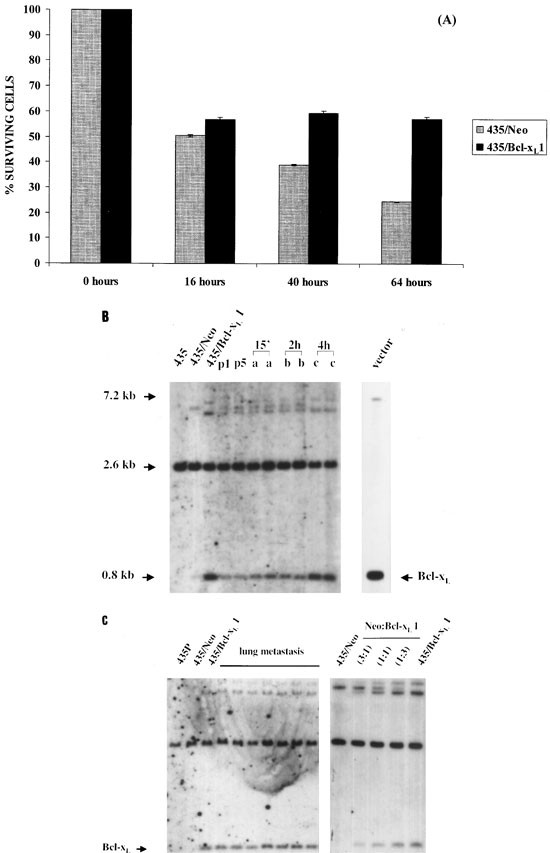
To assess the possibility that Bcl-xL cells could adapt to life without adhesion, we studied cell viability in suspension. As shown in Figure 7A, the percentage of Bcl-xL1-non-adherent cells that live longer in rocking conditions was higher than in 435/Neo cells. At this point, we explored survival in the blood stream with a competitive assay using a mixture of 435/Neo and 435/Bcl-xL cells, as described in Materials and Methods, which was then recovered from the lungs. Two of the injected mice were killed 15 min after injection, and at 2, 4 and 16 h. Survival of different cell populations in the lungs of intravenously injected mice was monitored by Southern blot. As shown in Figure 7B, this assay revealed the changes in the ratio between the two cell clones in the blood stream. A significant enrichment of 435/Bcl-xL cells vs 435/Neo was found at 15 min and reached a maximum at 4 h (Figure 7B). At this point the Bcl-xL-signal from cells recovered from the lungs was similar to 435/Bcl-xL in culture. Since at 16 h after injection only a few tumor cells were recovered, cell death of the vast majority of injected cells occurred within this time period. However, primary cultures from different lung metastasis revealed that metastasis development was preferentially originated from 435/Bcl-xL cells (Figure 7C).
Figure 7
(A) Comparison of viability dynamic of 435/Neo, 435/Bcl-xLpool and 435/Bcl-xL clones subjected to 16, 40 and 64 h floating, and plated for 4 h before quantitation, this was carried out in quatriplicate with an MTT assay. These results are representative of inhibition obtained in one of three experiments. (B) Survival of cells injected intravenously in mice. 3 : 1 mixture of control 435/Neo and Bcl-xL1, respectively. DNA was analyzed in mixture cells before injection (p1), in cell mixture after five passages in vitro (p5), in cells recovered from lungs 15 min (a), 2 h (b), and 4 h (c) after injection. Vector with Bcl-xL, 435/Neo and 435/Bcl-xL1 cells were used as a control. Transcript sizes are noted on the left. (C) On the right, we show DNA analyses from different mixtures of 435/Neo and 435/Bcl-xL1 cells to evaulate the changes in cell ratios at different period of time. On the left, we show DNA analyses of cells that were obtained in a primary culture from different lung metastatic nodules
Discussion
The Bcl-xL gene has not yet been implicated in breast cancer metastatic progression. In this report we have demonstrated that Bcl-xL expression increases the organotopic metastatic potential of breast cancer cells in vivo by offering resistance to growth factors and organ-derived cytokines.
On the other hand, we also show that Bcl-xL can improve cell survival without cellular adhesion and in the blood stream; therefore, it can enhance anchorage-independent growth, which may cause metastasis.
Several lines of evidence suggest that metastasis may be enhanced by an ability to resist apoptosis.17,18 Furthermore, highly metastatic cancer cells exhibit a greater survival ability and resistance to apoptosis than poorly metastatic cells.19 Moreover, we have reported previously that cytokines may extend the lifespan of metastatic cells by preventing apoptosis.20
In addition, we show in this study that Bcl-xL is able to protect 435 cells from apoptosis induced by TGFβ1. TGFβ acts as an anti-tumor promoter by inhibiting the progression of normal mammary epithelial cells, and those transformed epithelial cells still appear to be sensitive to TGFβ-mediated apoptosis.21,22 435/Neo cells were still vulnerable to TGFβ-mediated apoptosis, and this effect disappeared in 435/Bcl-xL cells. Thus, the abrogation of TGFβ responsiveness by Bcl-xL overexpression may prove to be an important element enabling metatasis in breast cancer. Furthermore, we argue that Bcl-xL can inhibit apoptosis induced by TNF-α in metastatic-435-breast cancer cells, and this mechanism could also be important in metastatic progression, leading to cell growth in the blood stream and in distal sites. In concordance with these results, it has been shown that Bcl-x could be a TNF-induced cell death inhibitor,23 probably because Bcl-xL functions downstream of caspase-8 to inhibit Fas- and TNFR1-induced apoptosis.24
Bcl-xL expression may contribute to metastasis by promoting cell survival, rather than by stimulating cellular proliferation. Overexpressing clones could be more likely to survive following a damage stimulus by overriding apoptosis. Since only a few G418-resistant cells could be recovered from lungs 16 h after injection, and finally Bcl-xL was detected in lung metastasis, we suggest that the majority of tumor cells die at the start of metastatic process. Thus, overexpression of the anti-apoptotic protein Bcl-xL might suppress apoptosis by increasing the survival of tumor cells in the circulation and/or in the organ-specific site, and thereby ultimately promoting metastasis. Indeed, tumor cells may undergo a period of ‘dormancy’, followed by faster growth when conditions are favorable, or growth factors/cytokines are present.2
Here we provide evidence that overexpression of Bcl-xL prevents adhesion to extracellular matrix proteins in vitro. Overexpression of Bcl-XL in 435 cells induced a diminished adhesion to LM, FN and CL IV, compared to the control clone. Moreover, 435/Bcl-xL cells had a survival advantage in suspension over 435/Neo cells. These results suggest that Bcl-xL could be important in regulating anoikis in breast cancer cells through an extracellular matrix adhesion independence, and are consistent with previously reported results which show that Bcl-xL protects against apoptosis depending on cell-substratum adhesion in vitro.25 Reports have stated that an overexpression of Bcl-2 resulted in blocked apoptosis in detached cells, and in increased tumor cell survival in the circulation.1 Moreover, Bcl-2 protects gastric cancer cells from anoikis prolonging survival in the absence of cell attachment to extracellular matrix proteins, and then increasing their ability to disseminate intraperitoneally.26 Thus, our results reinforce the idea that the action of apoptosis inhibitors may serve to rescue metastatic cells from anoikis, and from apoptosis in the blood or in a specific organ until the appropriate conditions for growth occur.
Recently, the metastatic role of Bcl-2 when it is transfected into MCF-7ADR breast cancer cells has been described;27 those cells overexpressing Bcl-2 showed increased chemotaxis and migration, without differences in cell-adhesion when compared with control cells in vitro. Bcl-2 and Bcl-xL might play a different role in breast cancer. Despite the apparent similarities between Bcl-2 and Bcl-xL functions we suggest that in breast cancer cells these two proteins control independent systems to inhibit cell death. As we reported previously,14 the expression of both proteins may not be functionally redundant in breast cancer, since in human tumors Bcl-xL is higher in HGIII than in HGI or HGII, in which Bcl-2 is predominant. Moreover, the overexpression of the anti-apoptotic proteins Bcl-2 and Bcl-xL, associated with the loss of apoptosis in breast-cancer cells in vivo, may account for an imbalance between the pro- and anti-apoptotic proteins, which might have decisive consequences for tumor progression when the lifespan of the cells is extended, suggesting that the loss or gain of apoptosis is tightly regulated in breast-cancer cells.16,28
In conclusion, breast cancer tumors that overexpress Bcl-xL have increased the metastatic potential by: overriding apoptosis, acquiring resistance against cytokines, modifying the relationship between cells and extracellular matrix, and probably by providing a mechanism for cells to adapt to a new environment. This may have an impact on the prognosis of breast cancer patients, and create an attractive target for gene-directed therapy.
Materials and Methods
Human breast carcinoma cell cultures and transfections
MDA-MB-435 cell cultures were maintained in 1 : 1 (v/v) mixture of DMEM and Ham F12 medium (DMEM/F12) supplemented with 10% fetal bovine serum, and 2 mM L-glutamine in 5% CO2-95% air at 37°C in a humidified incubator. PSFFVneo Bcl-xL and PSFFV neo (kindly provided by Dr JL Fernández-Luna from the University of Santander) was used. Transfections were carried out with Lipofectin (Life Technologies, Inc. Gibco BRL, Gaithersburg, MD, USA), and selection started 48 h later with 500 μg/ml of neomycin G418 (Life Technologies, Inc. Gibco BRL.). The expression of Bcl-xL in resistant clones was verified by immunoblotting and a Northern blot analysis.
In vivo selection of metastatic variants in Nude Balb/c mice
We selected for experiment a 435 Bcl-xL clone (435/Bcl-xL1), the pool of Bcl-xL populations (435/Bcl-xLpool) to avoid the possibility of clonal variation in cellular properties unrelated to the phenotypic effects, and a 435 clone transfected with the vector alone (435/Neo).
Four to five-week-old athymic Nude/Balbc female mice were used to grow tumors i.m.f.p.29 Cell suspension 1×106 in 0.05 ml was injected. Tumors were removed at 6 weeks, and 4 months later animals were killed by ether-anesthesia and lungs were examined for metastasis with a disection microscopy. Moreover, metastatic involvement was explored in paraffin sections by hematoxilin-eosin stain.
Bcl-xL expression: Northern and Western blot
Probes and mRNA analysis
We used 0.8-kb _Eco_RI (Boehringer Mannheim, Mannheim, Germany) fragment of plasmid pSFFVneo Bcl-xL, radiolabeled with α-32P-dCTP using DNA labelling beads c-dCTP (Pharmacia Biotech, UK) for Bcl-xL detection.
Total cellular RNA was isolated by the guanidinium thiocyanate-phenol-chloroform extraction method.30 Twenty μg of total RNA was electrophoresed in a 1.1% agarose-6% formaldehyde gel and then transferred to a positively-charged nylon membrane (Amersham, Life Science, Cleveland, OH, USA). Membranes were hybridized with a probe at 42°C in a hybridization oven and then washed in a SSC/0.1% SDS buffer. rRNA staining with ethidium bromide was used as a loading control.
Western blot analysis
Cells from exponential cultures were lysed in 200 μl buffer (50 mM Tris, 150 mM NaCl, 0.01% SDS, 1% NP-40, 0.5% sodium deoxycholate). Volumes for each cell line were adjusted to 50 μg protein as determined by BCA, Protein Assay Reagent (Pierce, Rockford, IL, USA). The proteins were transferred to nitrocellulose membrane Hybond ECL (Amersham). Non-specific protein-binding sites were blocked by a 5% solution of non-fat dried milk in PBS. Membranes were incubated with polyclonal rabbit antibodies specific to human Bcl-x (S-18) at 1 : 1000 dilution (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-rabbit antibody conjugated to peroxidase (Amersham) was used as a secondary antibody, and antigen reactivity was demonstrated by the chemiluminescence reaction ECL system (Amersham). Immunoreactive bands were visualised on Hyperfilm MP (Amersham). Molecular weights (MW) were established using pre-stained MW markers (Bio-Rad Laboratories S.A., Madrid, Spain).
Equal loading of the protein samples was determined by actin anti-human monoclonal antibody (Sigma, St. Louis, MO, USA). We scanned each sample on the X-ray films and analyzed each using the ‘Molecular Analyst Software’ (Bio Rad, Richmond, CA, USA).
Apoptosis assay
1×104 cells/well were seeded in a 24-well plate with complete medium. After 48 h, cells were rinsed twice and incubated with serum-free media added with purified growth factors: (h) TGFβ (Collaborative, Biomedical Products, Bedford, MA, USA) at 0.25–20 ng/ml, and r(h)TNFα (Boehringer Mannheim) at 12.5–200 U/ml.
The cultures were incubated for 48–96 h at 37°C in the conditions described above. Apoptosis was analyzed with a ‘cell death’ ELISA kit (Boehringer Mannheim) which measures cytoplasmic DNA-histone complexes generated during apoptotic DNA fragmentation.
Cell proliferation
Cell growth was meausred by [3H]thymidine uptake. Briefly, 3×103 cells/well were seeded in a 96-well microtiter plate and fed with complete medium. After 48 h, cells were rinsed twice and incubated with serum-free media with or without purified growth factors: (h)TGFβ (Collaborative) at 0.25–20 ng/ml, r(h)TNFα. (Boehringer Mannheim) at 12.5–200 U/ml, and r(h)IGF-I (Boehringer Mannheim) at 2.5–80 ng/ml.
Cells were cultured for 48–96 h and 0.2 μCi/well of [3H]-thymidine (Amersham) was added to each culture well 18 h before being counted. Radioactivity associated with proliferating cells was counted in an LKB Beta scintillation counter (Wallace Oy, Turky, Finland) and the results were expressed as a [3H]-thymidine uptake (c.p.m. of stimulated cells/c.p.m. of cells in a serum-free media). All determinations were performed in triplicate wells.
Tumor cell adhesion
435 transfected clones were assayed for adhesion. Briefly, tumor cells from exponential cultures were labeled with 51Cr (Amersham) 1 mCi/ml in the culture medium for 2 h. Radiolabeled cells were re-suspended in medium containing 2% BSA and seeded at concentrations ranging from 3×103 to 1×104 cells/well onto 96-well plates. The wells were incubated with medium containing 2% BSA or extracellular matrix proteins: matrigel, laminin (LM), 40 μg/ml, fibronectin (FN), 40 μg/ml, and collagen type-IV (CL IV), 40 μg/ml, for 2 h prior to addition of labeled cells. After 18 h, wells were washed with the same medium and the attached cells were lysed with 0.2% Triton X-100. The lysates were absorbed on cotton swabs and counted in an LKB gamma counter. All determinations were performed in triplicate wells. Results were expressed in terms of percentage of the radioactivity associated with attached cells with regard to the inoculum of cells per well.
Cell survival in suspension
2×104 cells per ml were re-suspended in 30 ml of media supplemented with 200 mM HEPES buffer in polypropylene tubes, where each cell clone was rocked gently at 37° for 16–72 h. These incubations cells were then centrifuged and cultured for an additional 4 h in fresh media before another assay to test cell viability by MTT tetrazolium that was carried out as described previously.23 All values were normalized to the values obtained in cells growing on plate for corresponding time intervals.
Cell survival in circulation
Clone survival in circulation was explored using a competition assay as described by Nikiforov et al.1 Briefly, a mixture of cells 3 : 1, 435/Neo and 435/Bcl-xL1, respectively, was injected into the tail vein of nude mice. We assessed differences in survival rates between the two clones by comparing the original mixture and cell populations recovered from the lungs. Mice were killed at 15 min, 2, 4, 8 and 16 h, and recovered cells were expanded in culture. 435 cells were selected using G418 medium. DNA from 5×106 cells was obtained through phenol-chloroform extraction and 20 μg were electrophoresed on 0.8% agarose gel and then transferred to nylon membrane. Blots were hybridized with the same radiolabeled 0.8-Kb fragment of Bcl-xL in order to analyze the Bcl-xL cDNA in resistant clones.
Abbreviations
HG:
histological grade
i.m.f.p.:
intra-mammary fat pad
TNF-α:
tumor necrosis factor alpha
TGF-β:
transforming growth factor beta
IGF-1:
insulin-like growth factor-1
FCS:
fetal calf serum
LM:
laminin
FN:
fibronectin
CL IV:
collagen IV
G418:
geneticin
i.v.:
intravenous
References
- Nikiforov MA, Kwek SSS, Mehta R, Artwohl JE, Lowe SW, Gupta TD, Deichman GI and Gudkov AV . (1997) Suppression of apoptosis by bcl-2 does not prevent p53-mediated control of experimental metastasis and anchorage dependence. Oncogene 15: 3007–3012
Article CAS Google Scholar - Holmgren L, O'Reilly MS and Folkman J . (1995) Dormancy of micrometastatses: Balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nature Med. 1: 149–153
Article CAS Google Scholar - Cherbonnel-Lasserre C, Gauny S and Kronenberg A . (1996) Suppression of apoptosis by Bcl-2 or Bcl-xL promotes susceptibility to mutagenesis. Oncogene 13: 1489–1497
CAS PubMed Google Scholar - Rudin CM and Thompson CB . (1997) Apoptosis and disease: regulation and clinical relevance of programmed cell death. Ann. Rev. Med. 48: 267–281
Article CAS Google Scholar - Kroemer G . (1997) The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nature Med. 3: 614–620
Article CAS Google Scholar - Newton K and Strasser A . (1998) The Bcl-2 family and cell death regulation. Curr. Opin. Genet. Dev. 8: 68–75
Article CAS Google Scholar - Chao DT and Korsmeyer SJ . (1998) Bcl-2 family: regulators of cell death. Annu. Rev. Immunol. 16: 395–419
Article CAS Google Scholar - Boise LH, González-García M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nuñez G and Thompson CB . (1993) bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74: 597–608
Article CAS Google Scholar - Kim CN, Wang X, Huang Y, Ibrado AM, Liu L, Fang G and Bhalla K . (1997) Overexpression of Bcl-xL inhibits Ara-C-induced mitochondrial loss of cytochrome c and other peturbations that activate the molecular cascade of apoptosis. Cancer Res. 57: 3115–3120
CAS PubMed Google Scholar - Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT and Thompson CB . (1997) Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 91: 627–637
Article CAS Google Scholar - Hu Y, Benedict MA, Wu D, Inohara N and Núñez G . (1998) Bcl-xL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc. Natl. Acad. Sci. USA 95: 4386–4391
Article CAS Google Scholar - Schott AF, Apel IJ, Nuñez G and Clarke MF . (1995) Bcl-xL protects cancer cells from p53-mediated apoptosis. Oncogene 11: 1389–1394
CAS PubMed Google Scholar - Olopade OI, Adeyanju MO, Safa AR, Hagos F, Mick R, Thompson CB and Recant WM . (1997) Overexpression of Bcl-x protein in primary breast cancer is associated with high tumor grade and nodal metastases. Cancer J. Sci. Am. 4: 230–237
Google Scholar - Sierra A, Castellsagué X, Escobedo A, Lloveras B, Moreno A and Fabra A . (1998) Expression of death-related genes and their relationship with loss of apoptosis in T1 ductal breast carcinomas. Int. J. Cancer 79: 103–110
Article CAS Google Scholar - Sierra A, Castellsagué X, Tórtola S, Escobedo A, Lloveras B, Peinado MA, Moreno A and Fabra A . (1996) Apoptosis loss and Bcl-2 expression: key determinants of lymph node metastases in T1 breast cancer. Clin. Cancer Res. 2: 1887–1894
CAS PubMed Google Scholar - Sierra A, Castellsagué X, Escobedo A, Lloveras B, García-Ramirez M, Moreno A and Fabra A . (2000) Bcl-2 with loss of apoptosis allows an accumulation of genetic alterations: a pathway to metastatic progression in human breast cancer. Int. J. Cancer 89: (in press)
- Shtivelman E . (1997) A link between metastasis and resistance to apoptosis of variant small cell lung carcinoma. Oncogene 14: 2167–2173
Article CAS Google Scholar - Takaoka A, Adachi M, Okuda H, Sato S, Yawata A, Hinoda Y, Takayama S, Reed JC and Imai K . (1997) Anti-cell death activity promotes pulmonary metastasis of melanoma cells. Oncogene 14: 2971–2977
Article CAS Google Scholar - Glinsky GV and Glinksy VV . (1996) Apoptosis and metastasis: a superior resistance of metastatic cancer cells to programmed cell death. Cancer Lett. 101: 43–51
Article CAS Google Scholar - Sierra A, Price JE, García-Ramirez M, Mendez O, López L and Fabra A . (1997) Astrocyte-derived cytokines contribute to the metastatic brain specificity of breast cancer cells. Lab. Invest. 77: 357–368
CAS PubMed Google Scholar - Arteaga CL, Dugger TC and Hurd SD . (1996) The multifunctional role of transforming growth factor (TGF)-βs on mammary epithelial cell biology. Breast Cancer Res. Treat. 38: 49–56
Article CAS Google Scholar - Reisss M and Barcelos-Hoff MH . (1997) Transforming growth factor-β in breast cancer: a working hypthesis. Breast Cancer Res. Treat. 45: 81–95
Article Google Scholar - Jäättelä M, Benedict M, Tewari M, Shayman JA and Dixit VM . (1995) Bcl-x and Bcl-2 inhibit TNF and Fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene 10: 2297–2305
PubMed Google Scholar - Srinivasan A, Li F, Wong A, Kodandapani L, Smidt Jr R, Krebs JF, Fritz LC, Wu JC and Tomaselli KJ . (1998) Bcl-xL functions downstream of caspase-8 to inhibit Fas- and tumor necrosis factor receptor 1-induced apoptosis of MCF7 breast carcinoma cells. J. Biol. Chem. 20: 4523–4529
Article Google Scholar - Rodeck U, Jost M, DuHadaway J, Kari C, Jensen PJ, Risse B and Ewert DL . (1997) Regulation of Bcl-xL expression in human keratinocytes by cell-substratum adhesion and the epidermal growth factor receptor. Proc. Natl. Acad. Sci. USA 94: 5067–5072
Article CAS Google Scholar - Yawata A, Adachi M, Okuda H, Naishiro Y, Takamura T, Hareyama M, Takayama S, Reed JC and Imai K . (1998) Prolonged cell survival enhances periotoneal dissemination of gastric cancer cells. Oncogene 16: 2681–2686
Article CAS Google Scholar - Del Bufalo D, Biroccio A, Leonetti C and Zupi G . (1997) Bcl-2 overexpression enhances the metastatic potential of a human breast cancer line. FASEB J. 11: 947–953
Article CAS Google Scholar - Vakkala M, Lähteenmäki K, Raunio H, Pääkkö P and Soini Y . (1999) Apoptosis during breast carcinoma progression. Clin. Cancer Res. 5: 319–324
CAS PubMed Google Scholar - Zhang RD, Fidler IJ and Price JE . (1991) Relative malignant potential of human breast carcinoma cell lines established from pleural effusions and a brain metastasis. Invas. Metast. 11: 204–215
CAS Google Scholar - Chomczynski P and Sacchi N . (1987) Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-cloroform extraction. Anal. Biochem. 162: 156–159
Article CAS Google Scholar
Acknowledgements
We are grateful to the ‘Fundación Científica de la Asociación Española Contra el Cáncer’ for supporting Yolanda Fernández as a Doctoral Fellow. We thank Dra. Angels Torregrosa for their skilful advice in the pathological selection of specimens. We also wish to thank Robin Rycroft of the Language Advisory Service at the University of Barcelona. This study was supported by grants MARATO de TV3 95/56, from the ‘Plan Nacional de Investigación Científica y Desarrollo Technológico’ CICYT, SAF 97/0210 and from ‘Ministerio de Sanidad y Consumo’ FIS 99/0770.
Author information
Authors and Affiliations
- Department of Cancer and Metastasis, Institut de Recerca Oncológica, Hospital Duran i Reynals, Ciutat Sanitaria i Universitaria de Bellvitge, Autovia de Castelldefels, Km 7.2, Barcelona, Spain
Y Fernández, L España, S Mañas, A Fabra & A Sierra
Authors
- Y Fernández
- L España
- S Mañas
- A Fabra
- A Sierra
Corresponding author
Correspondence toA Sierra.
Additional information
Edited by JC Reed
Rights and permissions
About this article
Cite this article
Fernández, Y., España, L., Mañas, S. et al. Bcl-xL promotes metastasis of breast cancer cells by induction of cytokines resistance.Cell Death Differ 7, 350–359 (2000). https://doi.org/10.1038/sj.cdd.4400662
- Received: 01 May 1999
- Revised: 01 November 1999
- Accepted: 12 January 2000
- Published: 11 April 2000
- Issue Date: April 2000
- DOI: https://doi.org/10.1038/sj.cdd.4400662