Surface expression of phosphatidylserine on macrophages is required for phagocytosis of apoptotic thymocytes (original) (raw)
Introduction
A crucial step in programmed cell death, or apoptosis, is recognition and phagocytosis of the dying cell by neighboring cells or professional phagocytes.1 Engulfment before cell lysis prevents the inflammation which might ensue from release of the cell's intracellular contents.1,2 In order for apoptotic cells to be distinguished from their healthy neighbors, the apoptotic cell surface must be recognizably different from that of non-apoptotic cells. The expression of the membrane phospholipid, phosphatidylserine (PS), on the cell surface is one signal which identifies cells as apoptotic.3,4 The importance of this surface PS for recognition is demonstrated by the finding that masking PS on the surface of apoptotic cells with the PS-specific binding protein, annexin V, specifically reduces their phagocytosis.5,6
In the specific case of recognition of apoptotic lymphocytes by macrophages, two different PS-dependent recognition systems operate.7 One system is utilized by unactivated macrophages such as mouse bone marrow macrophages, human monocyte-derived macrophages and the murine macrophage cell line J774. Phagocytosis using this system is inhibited by erythrocytes lysed and resealed under conditions where PS becomes exposed on the cell surface.8 Phagocytosis of these PS-presenting (lipid-symmetric) erythrocytes, but not of apoptotic thymocytes, is completely inhibited by PS vesicles.9 Phagocytosis using the other system, utilized by activated macrophages such as mouse peritoneal macrophages and β-glucan-activated mouse bone marrow macrophages, is inhibitable by PS vesicles, but not by lipid-symmetric erythrocytes.8 Phagocytosis by both systems, however, is inhibited by annexin V.6
The two macrophage recognition systems are also distinguished by their different sensitivities to other inhibitors. The synthetic peptide RGDS and antibodies to the vitronectin receptor inhibit phagocytosis of apoptotic lymphocytes by unactivated macrophages,7,8 implicating this integrin in recognition, whereas N-acetylglucosamine (GlcNAc) inhibits phagocytosis by activated macrophages, implicating a lectin-like receptor in recognition.8,10 Phagocytosis by both recognition systems is inhibited by treating macrophages with antibodies to the lipoprotein receptor CD3611 and to the lipopolysaccharide receptor CD14.12,13 However, the ligands which any of the receptors may recognize on the apoptotic cell surface have yet to be identified. In contrast, ICAM-3 on the lymphocyte surface participates in the interaction of apoptotic lymphocytes and unactivated macrophages, although the receptor with which ICAM-3 interacts has not been identified.14
Normal, healthy cells sequester PS to the inner leaflet of the bilayer.4,15 During the course of apoptosis, however, PS equilibrates between the two leaflets16 resulting in exposure of PS on the cell surface.3,4 Before the development of annexin V as a tool to detect PS exposed on the apoptotic cell surface resulting from loss of membrane asymmetry,17 the membrane probe merocyanine 540 (MC540) was utilized for that purpose. MC540 is a fluorescent dye sensitive to lipid packing,18 which binds to cells in which membrane asymmetry has been lost.19,20 Although normal lymphocytes, monocytes and neutrophils do not bind the dye,21 apoptotic lymphocytes are readily distinguished by their increased fluorescence following staining with MC540.3,22,23 Surprisingly, primary macrophages and macrophage cell lines stain with MC540.24 This result suggests that normal, non-apoptotic macrophages do not maintain the same scrupulously asymmetric distribution of phospholipids that other cells do, and thus may express PS on their surface. The studies presented here demonstrate that normal macrophages do indeed express PS on their surface, and that expression of PS on the macrophage surface is specifically involved in the engulfment of PS-exposing target cells.
Results
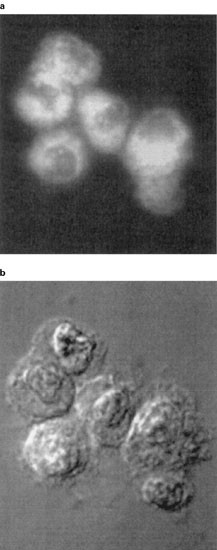
To investigate whether normal, non-apoptotic macrophages express PS on their surface, adherent J774 macrophages were stained with the PS-specific probe, fluorescent annexin V, and examined by fluorescence microscopy. Because of the prodigious rate of membrane internalization and recycling characteristic of macrophages, staining was performed at 4°C to slow these processes. As shown in Figure 1A, macrophages do stain with annexin V. Although plasma membrane staining predominates, nuclei can be discerned in the cells, outlined by annexin V internalized during the staining procedure. This result is consistent with the previous report that the plasma membrane of normal macrophages labeled with MC540 is very rapidly internalized.24 To confirm that any annexin V internalized was bound to the plasma membrane, and not simply taken up directly from solution, macrophages were stained with annexin V in the presence or absence of Ca2+. Because binding of annexin V to PS is Ca2+-dependent, staining in the absence of Ca2+ should reflect fluid-phase internalization. As shown in Figure 2A, annexin V staining of J774 macrophages in the presence of Ca2+ is significantly higher than in its absence, indicating that staining in the presence of Ca2+ is predominantly plasma membrane staining. That such staining is not restricted to cell lines or to just unactivated macrophages is also shown in Figure 2A where activated, elicited peritoneal macrophages are seen to stain with annexin V. The expression of PS on the surface of macrophages raises the question of whether it plays a functional role in recognition and phagocytosis of apoptotic cells.
Figure 1
Detection by microscopy of fluorescent annexin V binding to J774 macrophages. Monolayer cultures of J774 macrophages were stained with fluorescent annexin V, fixed and photographed using fluorescence (A) or Nomarski (B) optics. The data presented are representative of two separate experiments with similar results
Figure 2
Flow cytometric detection of fluorescent annexin V binding to macrophages and DO11.10 target cells. (A) Either J774 or elicited peritoneal macrophages (PM) released from monolayer were stained with fluorescent annexin V in the presence or absence of Ca2+ and analyzed by flow cytometry. (B) DO11.10 cells incubated with or without Ca2+ ionophore in the presence of Ca2+, or induced to undergo apoptosis by dexamethasone, were stained with fluorescent annexin V, and analyzed by flow cytometry. In both (A) and (B), cells stained with propidium iodide were gated out and do not appear in the profiles. The data presented are representative of three separate experiments with similar results
As reported previously, pre-treating apoptotic thymocytes with annexin V significantly inhibits their phagocytosis by macrophages.6 In those studies, however, treated thymocytes were not washed free of excess, soluble annexin V before presentation to macrophages. In light of the fact that macrophages express PS on their surface, it is possible that in those experiments excess annexin V, carried over with the target cells, might be acting at the macrophage surface. This possibility was tested by pretreating target cells with annexin V, washing to remove unbound annexin V and then presenting the treated cells to macrophages. The targets used were DO11.10 cells, an ovalbumin-specific murine T cell hybridoma, which can be induced by a variety of agents to undergo apoptosis and expose PS.16,25 As with all cell populations, induction of apoptosis in DO11.10 cells is not synchronous and at any given timepoint only a small fraction of intact cells expose PS. However, a uniform population of PS-expressing cells can be produced by elevating cytosolic Ca2+ concentrations with the Ca2+ ionophore A23187. This treatment activates a non-specific lipid flipsite, termed the scramblase, also activated by the apoptotic program, that allows rapid diffusion of PS to the cell surface.25 To facilitate initial studies identifying the site at which annexin V works, these ionophore-treated cells were used as targets.
As shown in Figure 2B, virtually all DO11.10 cells treated for 10 min with Ca2+ ionophore expose PS on their surface as detected by staining with fluorescent annexin V; the staining of cells induced with dexamethasone to undergo apoptosis is shown for comparison. As shown previously, cells induced by ionophore to expose PS are phagocytosed in the same PS-dependent fashion as cells in which PS is exposed as part of the apoptotic program.25 Phagocytosis of these cells by J774 macrophages is reduced about fourfold by pretreating them with 10 μM annexin V, and, importantly, the extent of inhibition was not affected by washing the targets to remove excess annexin V before presenting them to macrophages (data not shown), verifying that the PS exposed on target cells is required for their phagocytosis.
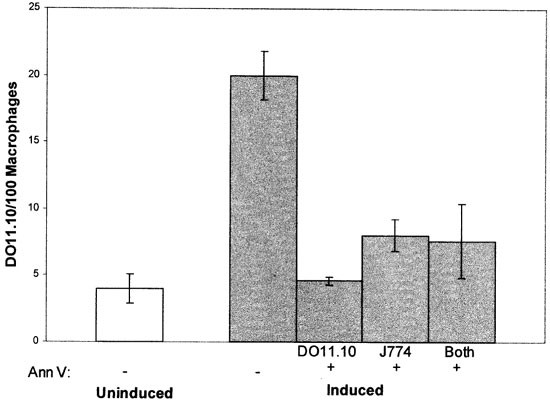
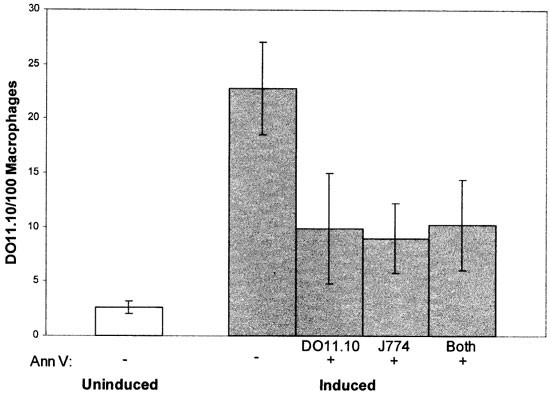
As well as confirming that masking PS on the target cell surface blocks phagocytosis, the above results provide protocols for asking whether the PS exposed at the macrophage surface plays any role in phagocytic clearance. To address this question, targets, macrophages or both were pre-treated with annexin V and washed, then the targets and macrophages combined and phagocytosis measured. As shown in Figure 3, phagocytosis was inhibited to a similar extent (approximately 80%) regardless of whether targets or macrophages were pre-treated with annexin V. Additionally, inhibition produced by treating both target and macrophage with annexin V (without washing) was statistically indistinguishable from treating either just targets or just macrophages alone. These results imply that the PS exposed on the macrophage surface is required for phagocytosis of PS-expressing target cells.
Figure 3
Phagocytosis following pre-treatment of DO11.10 targets, J774 macrophages, or both cell types with annexin V. DO11.10 cells were induced to express PS by incubating with Ca2+ ionophore in the presence of Ca2+. These targets, J774 macrophages, or both cell types were pre-treated with 10 μM annexin V (Ann V) before presenting the targets to macrophages. When only one cell type was pre-treated, cells were washed before targets were presented to macrophages. Phagocytosis of uninduced DO11.10 cells is shown for comparison. The data presented are representative of three separate experiments with similar results
Previous studies demonstrated that the concentration of fluorescent annexin V required for maximal binding to apoptotic cells was similar to the concentration which produced maximal inhibition of phagocytosis,6 as expected if inhibition is the result of high affinity specific binding of annexin V to PS on the target cell surface. If inhibition at the macrophage surface also is the direct result of specific high affinity binding and masking of PS, versus some non-specific effect at the cell surface, the dose-response for annexin V inhibition might be expected to be similar for treatment of either cell type. In fact, inhibition at the target cell surface was near maximal at 0.1 μM annexin V, with only slight increases at higher concentrations; similarly, annexin V pre-treatment of macrophages inhibited phagocytosis with a maximal effect near 0.l μM annexin V (data not shown). This result implies that the effects of annexin V on macrophages result from the same high affinity binding as its effects on targets, identifying PS as the target of its action.
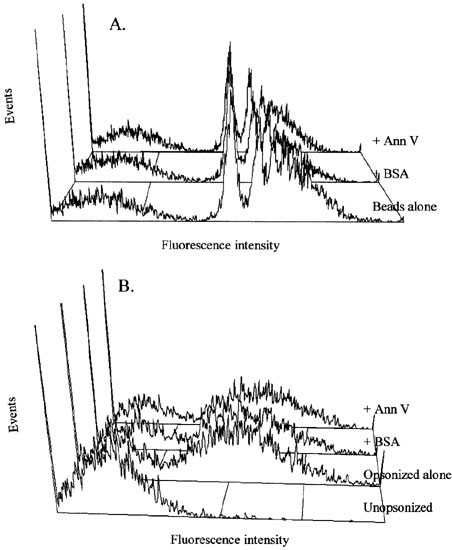
While these experiments rule out the possibility of non-specific binding as the mechanism of annexin V effects on macrophages, they leave open the possibility that this specific binding might non-specifically inhibit phagocytosis, i.e., that annexin V might inhibit phagocytosis in general by interfering with a membrane process (such as membrane bending or fusion) required for phagocytosis. This possibility was tested by asking whether annexin V treatment of macrophages prevented uptake of targets which do not present PS. As shown in Figure 4A, treating macrophages with annexin V did not inhibit the phagocytosis of fluorescent latex beads. Nor, as shown in Figure 4B, was the Fc-mediated phagocytosis of opsonized erythrocytes impaired by treatment with annexin V. Thus, the inhibition by annexin V at the macrophage surface is specific to PS-presenting targets and is not a non-specific consequence of interference with general phagocytic processes of the cell.
Figure 4
Phagocytosis of latex beads and Fc-mediated phagocytosis by macrophages pre-treated with annexin V. (A) Fluorescent latex beads were added to adherent J774 macrophages that were pre-treated with BSA or annexin V (Ann V). After 30 min, cells were released from culture dishes and analyzed by flow cytometry. The broad peak of low fluorescence represents cells which have not phagocytosed beads. The sharp positive peaks represent macrophages that have phagocytosed increasing numbers of beads. The data presented are representative of three separate experiments with similar results. (B) Fluorescently-labeled erythrocytes opsonized with anti-glycophorin antibody were added to J774 macrophages that were pre-treated with BSA or annexin V (Ann V). After 30 min, uningested erythrocytes were lysed with NH4Cl, and the macrophages were analyzed by flow cytometry. Phagocytosis of un-opsonized erythrocytes is shown for comparison. The data presented are representative of four separate experiments with similar results
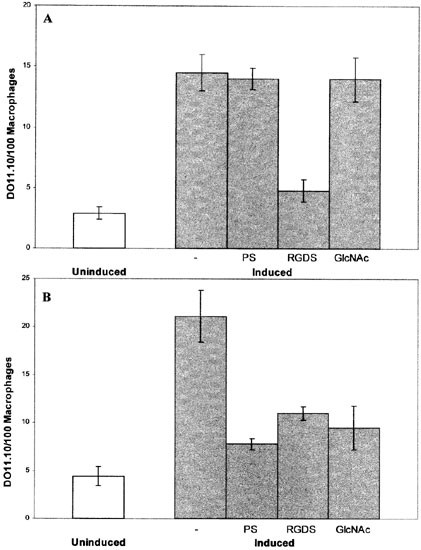
As indicated in the Introduction, and shown in Figure 5A, J774 macrophages behave as unactivated macrophages in that phagocytosis of PS-presenting cells is inhibited by RGDS and not by PS vesicles or GlcNAc which inhibit phagocytosis by activated macrophages.8 When primary unactivated macrophages ingest β-glucan particles, they acquire the activated macrophage recognition system.26 To determine whether the same was true for the J774 cell line, cultures were incubated with β-glucan for ⩾3 days, and then phagocytosis was measured in the presence of a variety of inhibitors. As shown in Figure 5B, after β-glucan treatment, phagocytosis by J774 macrophages became sensitive to the presence of either PS vesicles or GlcNAc. In contrast to the case of primary macrophages activated by β-glucan, however, phagocytosis by activated J774 cells remained sensitive to RGDS, a point considered further in the Discussion. To determine whether activated J774 macrophages, like their unactivated counterparts, require PS on their surface for phagocytosis, the experiment described for Figure 3 was repeated with β-glucan-activated J774 macrophages. As shown in Figure 6, the results were similar to those obtained with unactivated cells: phagocytosis of PS-presenting DO11.10 cells was inhibited to a similar extent regardless of whether target, phagocyte, or both, were pre-treated with annexin V. Thus, the dependence of phagocytosis on PS expressed on the macrophage surface is not confined to unactivated macrophages.
Figure 5
Phagocytosis of DO11.10 cells by unactivated or β-glucan-activated J774 macrophages in the presence of various inhibitors. DO11.10 cells induced to express PS by incubating with Ca2+ ionophore in the presence of Ca2+ were suspended with PS vesicles (PS), RGDS, or GlcNAc and presented to unactivated (A) or β-glucan activated (B) J774 macrophages. Phagocytosis of uninduced DO11.10 cells is shown for comparison. The data presented are representative of three separate experiments with similar results
Figure 6
Phagocytosis following pre-treatment of DO11.10 targets, β-glucan-activated J774 macrophages, or both cell types with annexin V. DO11.10 cells were induced to express PS by incubating with Ca2+ ionophore in the presence of Ca2+. These targets, β-glucan-activated J774 macrophages, or both cell types were pre-treated with 10 μM annexin V (Ann V) before presenting the targets to macrophages. When only one cell type was pre-treated, cells were washed before targets were presented to macrophages. Phagocytosis of uninduced DO11.10 cells is shown for comparison. The data presented are representative of two separate experiments with similar results
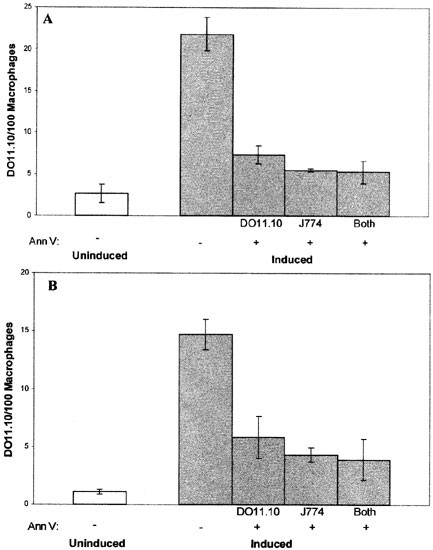
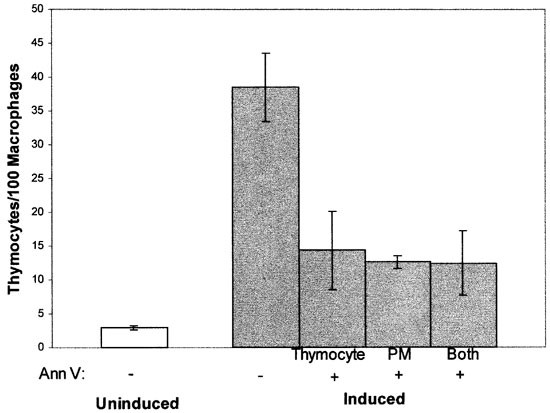
In these model experiments, both targets and phagocytes were cell lines, and the exposure of PS on target cells was artificially induced. Even though the phagocytosis of these cells requires the same integrin and/or lectin receptors, as well as PS, that are required for phagocytosis of true apoptotic cells, experiments were carried out with authentic apoptotic cells to ensure that the results were not peculiar to the model systems employed. DO11.10 cells were induced with dexamethasone to undergo apoptosis and the resulting apoptotic cell populations (see Figure 2B) were presented to unactivated and activated J774 macrophages. As shown in Figure 7, the phagocytosis of these apoptotic target cells in both cases was inhibited by annexin V treatment of the macrophages to a level comparable to that observed by masking PS on the surface of the ionophore-treated target cells. Similar experiments were also performed using both primary apoptotic target cells and primary macrophages. Elicited peritoneal macrophages, which express an activated macrophage inhibition phenotype, and which are shown in Figure 2A to expose PS on their surface, were presented with primary murine thymocytes induced by dexamethasone to undergo apoptosis. As shown in Figure 8, the results using primary cells accurately recapitulate the sensitivity to annexin V seen using cell lines. Together, these results indicate that the sensitivity of the phagocytosis of target cells to annexin V treatment of the phagocyte is a general property of PS-exposing targets and professional phagocytes.
Figure 7
Phagocytosis of apoptotic DO11.10 cells by J774 macrophages following pre-treatment with annexin V. DO11.10 cells were induced by dexamethasone to undergo apoptosis. These targets, unactivated (A) or β-glucan-activated (B) J774 macrophages, or both cell types were pre-treated with 10 μM annexin V (Ann V). When only one cell type was pre-treated, cells were washed before targets were presented to macrophages. Phagocytosis of uninduced DO11.10 cells is shown for comparison. The data presented are representative of two separate experiments with similar results
Figure 8
Phagocytosis of apoptotic thymocytes by elicited peritoneal macrophages following pre-treatment with annexin V. Primary thymocytes were induced by dexamethasone to undergo apoptosis. These targets, elicited peritoneal macrophages (PM), or both cell types were pre-treated with 10 μM annexin V (Ann V). When only one cell type was pre-treated, cells were washed before targets were presented to macrophages. Phagocytosis of uninduced thymocytes is shown for comparison. The data presented are representative of two separate experiments with similar results
Discussion
Expression of PS on the macrophage surface is at odds with the generalization that normal, non-apoptotic cells restrict PS to the inner leaflet of the plasma membrane. However, organism-wide surveys of cells which bind annexin V in vivo have already identified exceptions to this general rule,4,27,28 including fusing myoblasts and megakaryocytes. Regions of annexin V binding were also observed on potential phagocytes in brain, clustered near apoptotic targets, and were interpreted as possible sites of membrane exchange. The results presented here suggest alternative possibilities. The staining of macrophages with annexin V and MC540 suggests that PS exposure is constitutive in these cells, and that membrane exchange is thus not required to explain the annexin V binding observed in vivo. However, the exposure of PS on the macrophages examined here seems to occur over the entire cell surface, suggesting that any concentration of PS in the region near a potential target, as seen in the in vivo studies, may be an induced redistribution.
Previous studies established that phagocytosis of apoptotic thymocytes by macrophages is sensitive to inhibition by annexin V;6 the studies presented here examine more carefully the site at which annexin V acts. Given the well-known exposure of PS on apoptotic cells at an early stage in the apoptotic program, there is little drama in the confirmation that binding of annexin V to the PS exposed at the apoptotic target cell surface inhibits phagocytosis. Much more surprising is the discovery that pre-treating macrophages with annexin V similarly inhibits phagocytosis. This finding indicates that the PS exposed on the macrophage surface, identified by fluorescent annexin V staining, plays a functional role in phagocytosis. Because the pre-treatment with annexin V preceded the addition of target cells, the constitutive expression of PS on the macrophage cell surface is sufficient for its functional activity. Further, the absence of an inhibitory effect of annexin V on phagocytosis of latex beads or opsonized cells implies that the PS exposed on the macrophage surface is not required for general engulfment processes, such as membrane bending and membrane fusion at the external leaflet, but rather is specific to PS-expressing targets. Since treating both targets and macrophages with annexin V was no more effective than treating either alone, the PS exposed on each of the target cell and macrophage surfaces must be two elements of the same mechanism. However, why the mechanism requires PS on both target and macrophage, or even whether PS plays the same role at both cell surfaces, is unclear.
The simplest mechanism to explain the dual role of PS is that it serves as a ligand for a divalent molecule that bridges the target and macrophage surfaces. Ca2+ ions might be imagined to play such a role,29,30 except that inhibition of phagocytosis by PS is stereospecific,3,7 which is not consistent with this simplest model. Bridging between the target and macrophage surface might also occur via a multivalent PS-binding protein. Although attractive, there is no evidence for the existence of such a protein and the fact that phagocytosis occurs within 30 min after washed targets and macrophages are combined, in the absence of serum, argues against a soluble protein mediating this effect. PS stereospecificity has been taken to mean that a receptor on macrophages recognizes PS on the target cell surface. However, it is not clear whether stereospecificity applies at the macrophage surface, the target cell surface or both. Therefore, it remains possible that a receptor on lymphocytes recognizes PS on the macrophage surface. Although both target and macrophage may possess PS receptors, both of which are required for phagocytosis, it is just as possible that PS on one of the cell surfaces does not act as a ligand, but rather serves some other purpose, such as a co-factor required for the operation of the recognition machinery.
One macrophage membrane protein implicated in the phagocytosis of apoptotic cells is ABC1, a member of the large family of ATP-binding cassette (ABC) ATPases which mediate or regulate the movement of a wide variety of substrates across cell membranes.31,32 Treating mouse macrophages with antibodies to ABC1 blocks their ability to phagocytose apoptotic lymphocytes.33 In C. elegans, the ced-7 gene has been identified as a homolog of ABC1 whose expression is required in both target and engulfing cell for removal of apoptotic cells,34 suggesting that CED-7-dependent transport of the same molecule is required in both cell types for phagocytosis. Recently, inhibitors of ABC1 were shown to block phagocytosis when applied to either macrophage or apoptotic target, and also block the induced exposure of PS in either cell type.35 The apparent requirement for exposure of PS on both macrophage and target for phagocytosis argues that the engulfment role of CED-7 in C. elegans or the corresponding ABC-1 protein in mammals stems from its involvement in the pathway leading to the exposure of PS on both the target and macrophage surface.35 Externalization of PS in apoptotic lymphocytes results from activation of a non-specific lipid flipsite termed the scramblase.16,36 A Ca2+-dependent lipid scrambling activity has been reconstituted in artificial lipid vesicles with proteins isolated from erythrocytes37 and platelets,38 and the protein from erythrocytes responsible for this activity cloned.39 However, the activity of the reconstituted protein is very low and other proteins may also be involved in PS externalization.39,40 Although it appears that CED-7/ABC-1 is involved in PS externalization,27 ATP does not seem to be required for the dissipation of membrane phospholipid asymmetry,41 leaving open the question of what that involvement might be.
In the studies presented here, several experiments used target cells treated with a Ca2+ ionophore to induce PS exposure, making possible a comparison between these cells and cells on which PS is exposed as a part of the apoptotic program. First, inhibition of uptake when macrophages were treated with annexin V was equally effective for apoptotic and ionophore-treated targets, implying that loss of asymmetry in the target cell is sufficient to engage the PS-dependent mechanism on the macrophage surface. Second, no marked difference was seen in the inhibitor sensitivity of the uptake of ionophore-treated versus apoptotic targets; RGDS inhibited the phagocytosis of ionophore-treated targets by unactivated macrophages and GlcNAc inhibited the phagocytosis of ionophore-treated targets by activated macrophages. These agents are generally considered to block recognition of apoptotic target cell ligands by an integrin on unactivated macrophages or by a lectin-like receptor on activated macrophages. The fact that these agents block uptake of ionophore-treated targets argues that loss of transbilayer asymmetry and/or exposure of PS on the cell surface is sufficient to generate those ligands and that those ligands develop over a very short period of time. Changes in the fluidity, ordering, and lipid packing of the outer leaflet of the plasma membrane and an increase in surface hydrophobicity accompany loss of asymmetry in erythrocytes42,43,44,45 and could be involved in the generation of ligands on the lymphocyte surface. Taken together, these results suggest that ionophore-treated thymocytes are a remarkably good model for studying the mechanisms of recognition and phagocytosis of apoptotic cells by macrophages.
To date, all macrophages studied have utilized either the recognition system inhibitable by PS vesicles and GlcNAc or the recognition system inhibitable by lipid-symmetric erythrocytes and RGDS, but not both.7,8 In particular, this either/or rule applies to mouse bone marrow macrophages activated by β-glucan, which convert from one system to the other, acquiring sensitivity to PS vesicles and GlcNAc, but becoming refractile to inhibition by RGDS, even though the vitronectin receptor is still expressed on the activated macrophage surface.26 This finding implies that the vitronectin receptor is functionally neutralized on activated macrophages. The experiments presented here show that J774 macrophages activated by β-glucan can acquire sensitivity to PS vesicles and GlcNAc, and lose their sensitivity to lipid-symmetric erythrocytes (not shown), while retaining their sensitivity to RGDS. This result suggests that the either/or rule does not result from a requirement for neutralization of the vitronectin receptor in order for the PS vesicle/GlcNAc-sensitive mechanism to become operational. The nature of the switch between these two PS-dependent recognition mechanisms, and their relationship to PS exposure on the macrophage surface, remain to be clarified.
Materials and Methods
Materials
Dexamethasone, propidium iodide, paraphenylenediamine mounting media, anti-glycophorin A, B (clone E3), bovine serum albumin (BSA), bovine brain PS, Ca2+ ionophore A23187, PKH26 labeling kit, and β-glucan from barley were purchased from Sigma Chemical Co. Diff-Quik staining reagents were purchased from Baxter. 5-carboxyfluorescein succinimidyl ester (5-FAM) was purchased from Molecular Probes. J774A.1 macrophages, DO11.10 hybridoma cells, and E. coli TG1 containing a plasmid encoding human placental annexin V (clone pRK6) were purchased from American Type Culture Collection. Fluoresceinated latex beads (1 μ in diameter) were purchased from Polyscience.
Animals
Male CBA/J mice, 4–8 weeks of age, were maintained on food and water ad libitum in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Macrophages
Macrophages were elicited in the peritoneal cavity of 6–8 week old mice by intraperitoneal injection of 1 ml of 3% Brewer's thioglycollate media. Cells were harvested 5 days later by peritoneal lavage using 10 ml of ice-cold PBS (7.4 mM Na2HPO4, 2.6 mM NaH2PO4, 137 mM NaCl, 10 mM KCl) containing 10 U/ml of heparin. Collected cells were washed in PBS and suspended in RPMI 1640 medium containing 10% fetal bovine serum (FBS). Approximately 3×105 cells were pipetted onto 18 mm bicarbonate-treated glass coverslips kept in 30 mm petri dishes. After 2 h at 37°C, nonadherent cells were removed by aspiration, and the medium replaced with fresh RPMI 1640 medium containing 10% FBS. These macrophage cultures were used for phagocytosis assays within 24 h of plating. J774A.1 macrophages were grown in RPMI 1640 medium supplemented with 10% FBS at 37°C in 5% CO2. Cultures of 3×105 J774 macrophages per bicarbonate-treated coverslip or tissue culture well (24 well plate) were prepared within 24 h prior to phagocytosis assays. J774 macrophages activated by incubating in RPMI 1640 medium plus 10% FBS and 100 μg/mL of β-glucan for 3–5 days were used exactly as unactivated J774 macrophages for phagocytosis assays.
Thymocytes
Thymuses were removed from 4–6 week old mice and dissociated in PBS. After collecting cells by centrifugation and resuspending in 17 mM Tris, 140 mM NH4Cl, pH 7.2, to lyse erythrocytes, thymocytes were washed and resuspended at 107 cells/ml in RPMI 1640 medium containing 10% FBS. DO11.10 cells were grown in RPMI 1640 medium supplemented with 1 mM sodium pyruvate and 10% FBS at 37°C in 5% CO2. Apoptosis was induced in thymocytes (106 cells/mL) or log phase DO11.10 (106 cells/mL) by addition of 10−6 M or 5×10−6 M dexamethasone, respectively, and incubation at 37°C in 5% CO2 for 6 h. Apoptosis was monitored by fluorescent annexin V staining. To induce PS expression with ionophore, log phase DO11.10 cells in RPMI 1640 medium were treated for 10 min at 37°C with either 1 mM CaCl2 plus 10 μg/ml of the Ca2+ ionophore A23187 or 1 mM CaCl2 alone as a control. After the appropriate treatment, cells were collected by centrifugation, washed, and resuspended in annexin V buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2).
Phagocytosis assays
106 thymocytes or DO11.10 cells in 150 μl of annexin V buffer were overlayed onto coverslip cultures of 3×105 macrophages prepared as described above. When appropriate, either the target cells or macrophages were first incubated in 150 μL of various concentrations of annexin V in annexin V buffer for 15 min at room temperature and then washed twice with annexin V buffer before targets in 150 μL of annexin V buffer were overlayed onto macrophages. When both targets and macrophages were pre-treated with annexin V, neither was washed prior to overlaying. In some experiments target cells resuspended in annexin V buffer were mixed in a total volume of 150 μL with either 7.5 nM PS vesicles prepared as previously described,8 1 mM RGDS, or 20 mM GlcNAc, immediately before being overlayed onto macrophages. After 30 min at 37°C in 5% CO2, coverslips were washed vigorously in PBS and fixed in 1.8% formaldehyde for 15 min before staining with Diff-Quik. Cells were counted as phagocytosed as previously described in detail.8 Results are presented as the mean±standard deviation of triplicate coverslips. Each experiment was performed at least twice with similar results. For phagocytosis of fluorescent latex beads, beads were washed twice in RPMI 1640 medium and diluted 10 000 fold in annexin V buffer, then 100 μl was added to 3×105 macrophages per well of a 24-well tissue culture plate that either had or had not been pre-treated for 10 min with 200 μL of either 1 μM annexin V or BSA at room temperature. After 30 min at 37°C in 5% CO2, macrophages were washed and released from the dishes with 0.5 mM EDTA in PBS and analyzed by flow cytometry. For Fc-mediated phagocytosis, erythrocytes from fresh venous blood obtained from volunteers according to institutional guidelines were first labeled with the fluorescent dye PKH26 according to the manufacturer's instructions. Briefly, 50 μL of packed erythrocytes were resuspended in 500 μL of diluent C. PKH26 dye was added to 2 μM and incubated for 4 min at room temperature with constant shaking followed by the addition of 500 μL of human serum for 1 min to terminate labeling. Cells were washed and then treated with a 1 : 400 dilution of anti-glycophorin monoclonal antibody in PBS for 15 min at room temperature. Opsonized cells were washed and resuspended at 107 cells/mL in annexin V buffer. One hundred and fifty μL of this cell suspension was added to 3×105 macrophages per well of a 24-well tissue culture plate that were pre-treated with 200 μL of either 1 μM annexin V or BSA for 15 min at room temperature. After 30 min at 37°C in 5% CO2, the medium was removed by aspiration and cultures were treated with 17 mM Tris, 140 mM NH4Cl, pH 7.2, to lyse uningested cells. Macrophages were removed from the wells with 0.5 mM EDTA in PBS and analyzed by flow cytometry.
Preparation of fluorescent annexin V
Recombinant human placental annexin V was expressed in E. coli and purified as described previously.6,46 Purified annexin V at 15 mg/ml was dialyzed overnight into 1.0 M sodium bicarbonate buffer, pH 8.5. One mg of a freshly prepared stock solution of 5-FAM dissolved in dimethylformamide at 1 mg/ml was added to 10 mg of purified annexin V. Following incubation for 1 h at room temperature with shaking, unconjugated dye was removed by dialysis overnight into fresh sodium bicarbonate buffer.
Fluorescent annexin V staining
106 thymocytes or DO11.10 cells were incubated with 1 μg of fluorescent annexin V for 15 min at room temperature in 100 μL of annexin V buffer and then brought to 500 μL with annexin V buffer for flow cytometry. For flow cytometric analysis of macrophages, 106 cells removed from monolayer by scraping were stained as described above except that incubation was performed on ice and annexin V dilution buffer was ice-cold. For fluorescent microscopy, 3×105 macrophages in monolayer culture on bicarbonate-treated glass coverslips were incubated with 1 μg of fluorescent annexin V for 15 min at 4°C in 150 μL of annexin V buffer. After staining, the cultures were washed three times with ice-cold annexin V buffer, fixed in 2% formaldehyde for 15 min and mounted in 90% glycerol containing 0.1 mg/mL of paraphenylenediamine, to inhibit fading, in PBS.47
Microscopy
Annexin V-stained cells were examined using a Zeiss Axioplan fluorescence microscope. Images were captured with a Spot 2 camera (Diagnostic Instruments) and recorded using Adobe Photoshop.
Flow cytometry
A minimum of 10 000 cells/sample was analyzed using an EPICS-XL-MCL flow cytometer (Coulter Electronics, Hialeah, FL, USA) fitted with a single 15 mW argon ion laser providing excitation at 488 nm. Annexin V staining was monitored through a 525 nm bandpass filter; propidium iodide was added at 10 μg/mL immediately prior to analysis and cells stained by the dye were gated out of profiles. Macrophages phagocytosing PKH26-labeled opsonized erythrocytes or fluorescent latex beads were monitored through a 575 nm bandpass filter.
Abbreviations
PS:
phosphatidylserine
PBS:
phosphate-buffered saline
FBS:
fetal bovine serum
RPMI:
Roswell Park Memorial Institute
GlcNAc:
N-acetylglucosamine
MC540:
merocyanine 540
References
- Wyllie AH, Kerr JFR and Currie AR . (1980) Cell death: the significance of apoptosis. Int. Rev. Cytol. 68: 251–306
Article CAS PubMed Google Scholar - Ellis RE, Yuan J and Horvitz HR . (1991) Mechanisms and functions of cell death. Ann. Rev. Cell Biol. 7: 663–698
Article CAS PubMed Google Scholar - Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton JL and Henson PM . (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148: 2207–2216
CAS PubMed Google Scholar - Van den Eijnde SM, Boshart L, Reutelingsperger CPM, De Zeeuw CI and Vermeij-keers C . (1997) Phosphatidylserine plasma membrane asymmetry: a pancellular phenomenon which alters during apoptosis. Cell Death Differ. 4: 311–316
Article CAS PubMed Google Scholar - Bennett MR, Gibson DF, Schwartz SM and Tait TF . (1995) Binding and phagocytosis of apoptotic vascular smooth muscle cells is mediated in part by exposure of phosphatidylserine. Circ. Res. 77: 1136–1142
Article CAS PubMed Google Scholar - Krahling S, Callahan MK, Williamson P and Schlegel RA . (1999) Exposure of phosphatidylserine is a general feature in the phagocytosis of apoptotic lymphocytes by macrophages. Cell Death Differ. 6: 183–189
Article CAS PubMed Google Scholar - Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA and Henson PM . (1992) Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J. Immunol. 149: 4029–4035
CAS PubMed Google Scholar - Pradhan D, Krahling S, Williamson P and Schlegel RA . (1997) Multiple systems for recognition of apoptotic lymphocytes by macrophages. Mol. Biol. Cell 8: 767–778
Article CAS PubMed PubMed Central Google Scholar - Pradhan D, Williamson P and Schlegel RA . (1994) Phosphatidylserine vesicles inhibit phagocytosis of erythrocytes with a symmetric transbilayer distribution of phospholipids. Mol. Memb. Biol. 11: 181–188
Article CAS Google Scholar - Duvall E, Wyllie AH and Morris RG . (1985) Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunol. 56: 351–358
CAS Google Scholar - Fadok VA, Warner ML, Bratton DL and Henson PM . (1998) CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor. J. Immunol. 161: 6250–6257
CAS PubMed Google Scholar - Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL and Gregory CD . (1998) Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature 392: 505–509
Article CAS PubMed Google Scholar - Schlegel RA, Krahling S, Callahan MK and Williamson P . (1999) CD14 is a component of multiple recognition systems used by macrophages to phagocytose apoptotic lymphocytes. Cell Death Differ. 6: 583–592
Article CAS PubMed Google Scholar - Moffat OD, Devitt A, Bell ED, Simmons DL and Gregory CD . (1999) Macrophage recognition of ICAM-3 on apoptotic leukocytes. J. Immunol. 162: 6800–6810
Google Scholar - Williamson P and Schlegel RA . (1994) Back and forth: the regulation and function of transbilayer phospholipid movement in eukaryotic cells (Review). Mol. Memb. Biol. 11: 199–216
Article CAS Google Scholar - Verhoven B, Schlegel RA and Williamson P . (1995) Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J. Exp. Med. 82: 1597–1601
Article Google Scholar - Koopman G, Reutelingsperger CPM, Kuijten GAM, Keehnen RMJ, Pals ST and van Oers HJ . (1994) Annexin V for flow cytometric detection of phosphatidylserine on B cells undergoing apoptosis. Blood 84: 1415–1420
CAS PubMed Google Scholar - Williamson P, Mattocks K and Schlegel RA . (1983) Merocyanine 540: A fluorescent probe sensitive to lipid packing. Biochim. Biophys. Acta 732: 387–393
Article CAS PubMed Google Scholar - Williamson P, Bateman J, Kozarsky K, Mattocks K, Hermanowitz N, Choe HR and Schlegel RA . (1982) Involvement of spectrin in the maintenance of erythrocyte membrane phase state. Cell 30: 725–733
Article CAS PubMed Google Scholar - McEvoy L, Williamson P and Schlegel RA . (1986) Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc. Natl. Acad. Sci. USA 83: 3311–3315
Article CAS PubMed PubMed Central Google Scholar - McEvoy L, Schlegel RA, Williamson P and Del Buono BJ . (1988) Merocyanine 540 as a flow cytometric probe of membrane lipid organization in leukocytes. J. Leuk. Biol. 44: 337–344
Article CAS Google Scholar - Schlegel RA, Stevens M, Lumley-Sapanski K and Williamson P . (1993) Altered lipid packing identifies apoptotic thymocytes. Immunol. Lett. 36: 283–288
Article CAS PubMed Google Scholar - Mower DA, Peckham DW, Illera VA, Fishbaug JK, Stunz LI and Ashman RF . (1994) Decreased membrane phospholipid packing and decreased cell size precede DNA cleavage in mature mouse B cell apoptosis. J. Immunol. 152: 4832–4842
CAS PubMed Google Scholar - Schlegel RA and Williamson P . (1988) Role of phospholipid asymmetry in cellular membrane fusion. In Molecular Mechanisms of Membrane Fusion, Ohki S, Doyle D, Flanagan TD, Hui SE and Mayhew E, eds (New York, Plenum Press) pp. 289–301
Chapter Google Scholar - Verhoven B, Krahling S, Schlegel RA and Williamson P . (1999) Regulation of phosphatidylserine exposure and phagocytosis of apoptotic T lymphocytes. Cell Death Differ. 6: 262–270
Article CAS PubMed Google Scholar - Fadok VA, Laszlo DJ, Noble PW, Weinstein L, Riches DWH and Henson PM . (1993) Particle digestibility is required for induction of the phosphatidylserine recognition mechanism used by murine macrophages to phagocytose apoptotic cells. J. Immunol. 151: 4274–4285
CAS PubMed Google Scholar - Van den Eijnde SM, Luijsterburg AJM, Boshart L, Vermeij-Keers C, Reutelingsperger CPM and De Zeeuw CI . (1997) In situ detection of apoptosis during embryogenesis with annexin V: from whole mount to ultrastructure. Cytometry 29: 313–320
Article CAS PubMed Google Scholar - Van den Eijnde SM, Lips J, Boshart L, Vermeij-Keers C, Reutelingsperger CPM and De Zeeuw CI . (1999) Spatiotemporal distribution of dying neurons during early mouse development. Eur. J. Neurosci. 11: 712–724
Article CAS PubMed Google Scholar - Papahadjopoulos D, Poste G, Schaeffer BE and Vail WJ . (1974) Membrane fusion and molecular segregation in phospholipid vesicles. Biochim. Biophys. Acta 352: 10–28
Article CAS PubMed Google Scholar - Papahadjopoulos D, Vail WJ, Panghorn WA and Post G . (1976) Studies on membrane fusion. II. Induction of fusion in pure phospholipid membranes by calcium and other divalent metals. Biochim. Biophys. Acta 448: 265–283
Article CAS PubMed Google Scholar - Doige CA and Ames CF-L . (1993) ATP-dependent transport systems in bacteria and humans: relevance to cystic fibrosis and multidrug resistance. Ann. Rev. Microbiol. 47: 291–319
Article CAS Google Scholar - Higgins CF . (1992) ABC transporters: from microorganisms to man. Ann. Rev. Cell Biol. 8: 67–113
Article CAS PubMed Google Scholar - Luciani M and Chimini G . (1996) The ATP binding cassette transporter ABC1, is required for the engulfment of corpses generated by apoptotic cell death. EMBO J. 15: 226–235
Article CAS PubMed PubMed Central Google Scholar - Wu Y and Horvitz HR . (1998) The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell 93: 951–960
Article CAS PubMed Google Scholar - Marguet D, Luciani M-F, Moynault A, Williamson P and Chimini G . (1999) Engulfment of apoptotic cells involves the redistribution of membrane phosphatidylserine on phagocyte and prey. Nature Cell Biol. 1: 454–456
Article CAS PubMed Google Scholar - Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA and Henson PM . (1997) Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J. Biol. Chem. 272: 26159–26165
Article CAS PubMed Google Scholar - Basse F, Stout JG, Sims PJ and Wiedmer T . (1996) Isolation of an erythrocyte membrane protein that mediates Ca2+-dependent transbilayer movement of phospholipid. J. Biol. Chem. 271: 17205–17210
Article CAS PubMed Google Scholar - Comfurius P, Williamson P, Smeets EF, Schlegel RA, Bevers EM and Zwaal RFA . (1996) Reconstitution of phospholipid scramblase activity from human blood platelets. Biochemistry 35: 7631–7634
Article CAS PubMed Google Scholar - Zhou Q, Ahzo J, Stout JG, Luhm RA, Wiedmer T and Sims PJ . (1997) Molecular cloning of human plasma membrane phospholipid scramblase. J. Biol. Chem. 272: 18240–18244
Article CAS PubMed Google Scholar - Stout JG, Basse F, Luhm RA, Weiss HJ, Wiedmer T and Simms PJ . (1997) Scott Syndrome erythrocytes contain a membrane protein capable of mediating Ca2+-dependent transbilayer migration of membrane phospholipids. J. Clin. Invest. 99: 2232–2238
Article CAS PubMed PubMed Central Google Scholar - Verhoven B, Schlegel RA and Williamson P . (1992) Rapid loss and restoration of lipid asymmetry by different pathways in resealed erythrocyte ghosts. Biochim. Biophys. Acta 1104: 15–23
Article CAS PubMed Google Scholar - Tanaka KI and Ohnishi SI . (1976) Heterogeneity in the fluidity of intact erythrocyte membranes and its homogenization upon hemolysis. Biochim. Biophys Acta 426: 218–231
Article CAS PubMed Google Scholar - Seigneuret M, Zachowski A, Hermann A and Devaux PF . (1984) Asymmetric lipid fluidity in human erythrocyte membranes: new spin label evidence. Biochemistry 23: 4271–4275
Article CAS PubMed Google Scholar - Williamson P, Algarin L, Bateman J, Choe H-R and Schlegel RA . (1985) Phospholipid asymmetry in human erythrocyte ghosts. J. Cell. Physiol. 123: 209–214
Article CAS PubMed Google Scholar - Morot G, Cribier S, Devaux PF, Geldwerth D, Davoust J, Bureau JF, Fellmann P, Herve P and Frilley B . (1986) Asymmetric lateral mobility of phospholipids in the human erythrocyte membrane. Proc. Natl. Acad. Sci. USA 83: 6863–6867
Article Google Scholar - Burger A, Berendes R, Voges D, Huber R and Demange P . (1993) A rapid and efficient purification method for recombinant annexin V for biophysical studies. FEBS Lett. 329: 25–28
Article CAS PubMed Google Scholar - Johnson DG and Nogueira Araujo GM . (1981) A simple method of reducing the fading of immunofluorescence during microscopy. J. Immunol. Methods 43: 349–350
Article CAS PubMed Google Scholar
Acknowledgements
Supported by the United States National Science Foundation. We thank Elaine Kunze for help with flow cytometry, Davis Ng and Eric Spear for help with immunofluorescence microscopy, and Michael Earley for valuable discussions.
Author information
Authors and Affiliations
- Department of Biochemistry and Molecular Biology, Penn State University, University Park, 16802, PA, USA
M K Callahan & R A Schlegel - Department of Biology, Amherst College, Amherst, 01002, MA, USA
P Williamson
Authors
- M K Callahan
You can also search for this author inPubMed Google Scholar - P Williamson
You can also search for this author inPubMed Google Scholar - R A Schlegel
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toR A Schlegel.
Additional information
Edited by BA Osborne
Rights and permissions
About this article
Cite this article
Callahan, M., Williamson, P. & Schlegel, R. Surface expression of phosphatidylserine on macrophages is required for phagocytosis of apoptotic thymocytes.Cell Death Differ 7, 645–653 (2000). https://doi.org/10.1038/sj.cdd.4400690
- Received: 15 October 1999
- Revised: 12 February 2000
- Accepted: 06 March 2000
- Published: 27 June 2000
- Issue Date: 01 July 2000
- DOI: https://doi.org/10.1038/sj.cdd.4400690