VDAC regulation by the Bcl-2 family of proteins (original) (raw)
Introduction
In several phylogenically divergent metazoans including nematodes, fruit flies and mammals, there are structural and functional homologues that play a key role in apoptosis, such as death proteases (caspases), the Bcl-2 family of proteins, and caspase activators, indicating that the basic mechanism of apoptosis has been well conserved throughout evolution. The apoptotic signal transduction mechanism appears to consist of individual and common pathways. The common pathway is driven by caspases,1 which cleave a set of cellular proteins including inhibitor of caspase-activated DNase (ICAD),2,3 Acinus,4 and many others1 to cause apoptotic changes in cells, and uniquely constitute an irreversible step in the signal transduction of apoptosis. The activation of caspases is regulated by several sets of genes, among which the best characterized is the bcl-2 family.5,6 A major role of the Bcl-2 family of proteins in regulating caspase activation is to alter mitochondrial membrane permeability, thus controlling the release of caspase-activating cytochrome c. Through different modification of various members, the Bcl-2 family constitutes a convergence point via which a number of apoptotic signals eventually act on the mitochondria.
This article reviews our recent work together with that of others concerning the modulation of mitochondrial membrane permeability by the Bcl-2 family, and discusses how cell viability or death is decided.
The Bcl-2 family of proteins
Bcl-27,8,9 was initially shown to inhibit cell death induced by deprivation of IL-310 and was subsequently found to also inhibit cell death induced by various stimuli, including a chemotherapy agent and heat shock.11 In recent years, it has been well established that Bcl-2 and its relative Bcl-xL can block most forms of apoptotic cell death as well as certain forms of necrotic cell death by preventing mitochondrial changes.6,12 Based on their structural and functional characteristics, the Bcl-2 family of proteins has been divided into three categories.5,6 (1) The anti-apoptotic members include Bcl-2, Bcl-xL, Bcl-w, Mcl-1, A1 (Bfl-1) and Boo. These proteins mostly share sequence homology within four regions, BH (Bcl-2 homology)1 through BH4, although some members lack an obvious BH4 domain but still carry a conserved α-helical structure.13 (2) The pro-apoptotic members include Bax, Bak, Mtd (Bok), and Diva. These proteins share sequence homology of BH1, BH2, and BH3, but not BH4, although a significant BH4 homology has been found in some members. (3) The ‘BH3-only proteins’ are also pro-apoptotic, and they include Bik, Bad, Bid, Bim, Hrk (DP5), Blk, Bnip3, and Bnip3L. These proteins only share sequence homology of BH3 region.
One of the unique features of the Bcl-2 family of proteins is heterodimerization between anti-apoptotic and pro-apoptotic family members, which is considered to inhibit the activity of their partner proteins.14,15 This heterodimerization process is mediated by the insertion of a BH3 region of a pro-apoptotic protein into a hydrophobic cleft composed of BH1, BH2 and BH3 on an anti-apoptotic protein.16 In addition to BH1 and BH2, the BH4 domain is required for anti-apoptotic activity.17,18,19,20,21,22 In contrast, BH3 is essential and sufficient for pro-apoptotic activity.23
One of the known activities of Bcl-2 family members is formation of ion channels in synthetic lipid membranes, with Bcl-2, Bcl-xL, Bax, and Bid all having the ability to form such ion channels.24,25,26,27,28 However, it still remains to be determined whether these proteins actually form ion channels in vivo and whether apoptosis is regulated via the creation of ion channels.
Sites of Bcl-2 family activity: mitochondria versus apoptosome
Mitochondria play an essential role in many forms of apoptosis in mammals29 by releasing apoptogenic factors such as cytochrome c30 from the intermembrane space into the cytoplasm.31,32 Mitochondria are also reported to release other apoptogenic factors such as AIF33 and some of the caspases. Cytochrome c release is inhibited by anti-apoptotic Bcl-2 family members, while it is directly enhanced by the pro-apoptotic members.5,6 Many of the pro-apoptotic family members (such as Bax, Bid, Bim and Bad) are localized in the cytoplasm of living cells.5,6 Apoptotic stimuli cause various modifications of these proteins, such as proteolytic cleavage and dephosphorylation, probably leading to conformational changes, after which translocation to the mitochondria occurs.6 In addition to directly preventing an apoptotic increase of mitochondrial membrane permeability, Bcl-2 and Bcl-xL inhibit apoptosis by indirectly preventing the translocation of Bax to the mitochondria through a cytoplasmic factor.34,35
Bcl-xL, but probably not Bcl-2, also has the ability to prevent caspase activation by sequestering Apaf-1.36,37 Although this is similar to the mechanism through which CED-4 (an Apaf-1 homologue that activates Ced-3 caspase) is regulated by CED-9 (a Bcl-2 homologue) via formation of a protein complex called an apoptosome in C. elegans,38 whether Bcl-2 family proteins actually sequester Apaf-1 to prevent caspase activation in mammalian cells has recently been questioned, based upon the failure to detect a physiologically relevant level of stable interaction between Apaf-1 and Bcl-2/Bcl-xL.39,40 Thus, at least in mammals, modulation of mitochondrial membrane permeability seems to be one of the major mechanisms by which Bcl-2 family proteins regulate apoptosis.
Bcl-2/Bcl-xL and Bax/Bak act independently
In addition to the regulation of apoptosis by heterodimerization of anti-apoptotic and pro-apoptotic members of the Bcl-2 family, some of these proteins have been suggested to regulate apoptosis independently of each other, based on observations in transgenic and knockout mice,41 although the data have never been conclusive because of the presence of other family members. The independent action of Bcl-2/BclxL and Bax/Bak was more clearly shown by recent studies of ours and others on yeast cells, which lack Bcl-2 family proteins.42,43,44,45 It was found that Bax/Bak can induce cytochrome c release, which is inhibited by Bcl-2/Bcl-xL, in yeast mitochondria42,44 as well as in yeast cells,43,45 and that Bcl-xL prevents cytochrome c release from isolated mitochondria induced by several chemicals such as ATP and ethanol that are known to induce a ‘permeability transition (PT)’-like phenomenon, including Δψ loss and swelling of isolated mitochondria.43 Thus, Bcl-2 family members, particularly Bcl-2/Bcl-xL and Bax/Bak, seem to regulate apoptotic mitochondrial changes in two ways, i.e., through heterodimerization and through various independent functions.
Regulation of cytochrome c release by the Bcl-2 family
Regarding the translocation of mitochondrial cytochrome c to the cytoplasm during apoptosis, three models have been proposed.46 One possibility is that cytochrome c release is mediated by physical rupture of the outer mitochondrial membrane, resulting from mitochondrial swelling47 or membrane instability induced by molecules such as Bax,48 although previous studies have suggested that mitochondrial swelling and membrane rupture rarely accompany apoptosis. The other models involve release of cytochrome c via specific channels such as the VDAC-Bax channel (Figure 1) or a channel constituted by Bax alone (or possibly another pro-apoptotic family member alone).
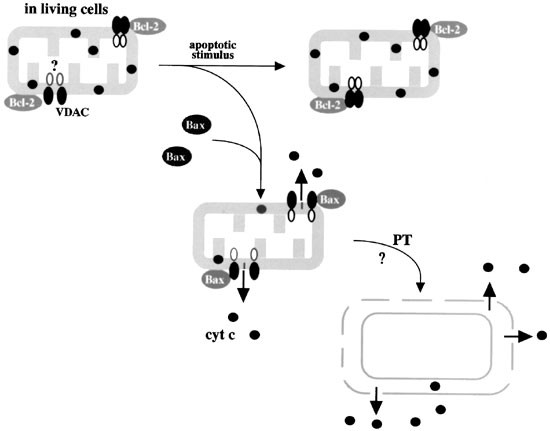
Figure 1
VDAC-dependent mechanism of cytochrome c release. Bax/Bak, once activated interacts with and opens the VDAC through which cytochrome c is released. Opening of the VDAC probably affects a channel(s) on the inner membrane and thus induces loss of membrane potential. Anti-apoptotic Bcl-2/Bcl-xL has the ability to close the VDAC. It may be possible that Bcl-2/Bcl-xL is loosely associated with VDAC in living cells while the channel remains functional, and tightly binds to and closes the VDAC to prevent cytochrome c release in response to an apoptotic stimulus. Opening of the VDAC results in cytochrome c release and, at least in vitro, induction of the PT could eventually occur, leading to further cytochrome c release
Mechanism of cytochrome c release through mitochondrial membrane channels
Permeability transition and Bax/Bak
A sytem with isolated mitochondria and recombinant Bcl-2 family proteins has proven very useful for studying the mechanisms by which Bcl-2 family proteins regulate apoptotic mitochondrial changes, and has been employed quite extensively. Although there is some controversy about the findings, we and others have shown that addition of recombinant Bax or Bak to isolated mitochondria induces cytochrome c release and Δψ loss (as well as other permeability transition (PT)49,50-associated changes such as swelling).51,52,53,54,55 Note that Bax/Bak-induced mitochondrial swelling is much less severe compared with Ca2+-induced swelling51 (our unpublished observations). We have also shown that, unlike Bax/Bak, some of the BH3-only proteins such as Bid and Bik induce cytochrome c release but not the PT (Δψ loss).55 Importantly, these observations on isolated mitochondria have been confirmed in cells that were transfected with Bax- or Bid-expressing plasmids. It was found that Bax-induced apoptotic cell death was accompanied by Δψ loss,55,56,57 whereas Bid-induced apoptotic death was not.55 Expression of BH3-only proteins could also eventually lead to Δψ loss, which was probably a consequence of cell death. All of the mitochondrial changes induced by pro-apoptotic Bcl-2 members are known to be inhibited by Bcl-2 and Bcl-xL in isolated mitochondria as well as in mammalian cells. Consistent with induction of the PT by Bax/Bak, but not by Bid/Bik, Bax/Bak-mediated cytochrome c release and Δψ loss are inhibited by PT inhibitors, including cyclosporin A (a cyclophilin D inhibitor),51,52,54,55 bonkrekic acid (an ANT inhibitor), and calcium depletion,52,55,58 whereas Bid/Bik-induced cytochrome c release is not sensitive to PT inhibitors.55 More importantly, it should be noted that Bax-induced apoptosis is inhibited by cyclosporine A, but not FK506 (another immunophilin that does not react with mitochondrial cyclophilin D)57 (our unpublished results). It should also be noted that several modes of apoptosis are inhibited by cyclosporine A and bonkrekic acid.53 Although PT inhibitors do not block all kinds of apoptotic cell death, this might depend on which pro-apoptotic member of the Bcl-2 family is dominant in different modes of apoptosis, as discussed below. The PT is regulated by the PT pore, which is an oligo-protein channel that consists of the VDAC on the outer membrane, adenine nucleotide translocator (ANT) on the inner membrane, matrix protein cyclophilin D, and probably other proteins,59 although the molecular nature of this pore is still elusive. Our group and Kroemer's have independently shown that the PT pore is a target for the Bcl-2 family of proteins since Bcl-2/Bcl-xL and Bax/Bak interact with the VDAC42,43 and ANT.42,60
Bax/Bak induces cytochrome c release through the VDAC
We have shown that VDAC activity is directly modulated by the Bcl-2 family of proteins:42 Bcl-xL closes the VDAC on liposomes, whereas Bax/Bak opens this channel so that cytochrome c can pass through it (Figure 1). Similarly, although Bax does not affect ANT, it was found to enhance ANT channel activity in the presence of an ANT inhibitor, atractyloside, whereas ANT activity is inhibited by Bcl-2.61
An essential role of the VDAC in Bax/Bak-mediated cytochrome c was suggested by the observation that Bax/Bak induces cytochrome c release from the mitochondria of wild-type yeast cells, but not from the mitochondria of VDAC-1-deficient yeast cells using isolated mitochondria and cells,42,43 although there was a controversial report that Bax induces cytochrome c release, as spectrophotometrically assessed, in yeast cells lacking VDAC-1.44 Yeast cells carry another VDAC, called VDAC-2, but there is no evidence that VDAC2 forms a channel. Supporting our model involving the VDAC in cytochrome c release, we have more recently shown that Bax-induced cytochrome c release and Δψ loss are prevented in isolated mitochondria by two kinds of anti-VDAC antibodies, both of which inhibit VDAC activity, and also that microinjection of either of these antibodies into cells prevents both Bax-induced cytochrome c release and cell death.62 We also found that Bid-induced cytochrome c release and cell death were not inhibited by the same antibodies, providing stronger evidence for the essential role of VDAC in Bax-induced apoptotic mitochondrial changes and cell death.62 Although it may be argued that Bax kills yeast cells lacking VDAC-1, it should be noted that Bax kills yeast cells by a mechanism which is independent on cytochrome c release.45,63
How does Bax/Bak make the VDAC permeable to cytochrome c? An electrophysiological study of the VDAC-Bax channel has revealed that it is nearly continuously in an open state, unlike the rapidly opening closing VDAC, and its pore is four times as large as that of the VDAC.64 It was also shown that a single VDAC-Bax channel formed in a planar lipid bilayer is permeable to cytochrome c.64 Although VDAC-Bax channels in liposomes are permeable to cytochrome c (14 kDa), but not to GST-GFP with a molecular weight of approximately 50 kDa,42 the VDAC-Bax channels formed in the mitochondrial membrane in a physiological context might be permeable to larger molecules known to be released from the mitochondria during apoptosis.65 Bax/Bak might induce conformational changes of the VDAC to form larger pores or might be involved in creating a larger pore together with the VDAC. If Bax/Bak has the same role in inducing cytochrome c release and Δψ loss in yeast mitochondria as in mammalian mitochondria,42,43,52 since yeast mitochondria lacking the Bcl-2 family of proteins still show inhibition by Bcl-2/Bcl-xL of the release of cytochrome c in response to chemicals such as ATP and ethanol,43 the possibility is greater that Bax/Bak induces conformational changes of the VDAC rather than forming a hybrid VADC-Bax channel. However, it is also conceivable that yeast mitochondria might possess a protein that does not belong to the Bcl-2 family but functions similarly to Bax/Bak in forming a hybrid channel.
The stoichiometry of the VDAC-Bax channel, e.g., how many Bax/Bak and VDAC molecules are required to form it, is still to be determined. Mutation studies have indicated that the BH3 domain of Bax/Bak, which is required for inducing cell death, is also indispensable for opening of the VDAC by Bax/Bak, but not for binding to the channel.42
VDAC and anti-apoptotic Bcl-2/Bcl-xL
Recombinant Bcl-xL inhibits VDAC activity and competes with Bax/Bak on liposomes.42 A picture of how the VDAC is inhibited by Bcl-xL was revealed by an electrophysiological study, which indicated that Bcl-xL almost completely closes the channel.64 Given that the VDAC functions as a gate for various materials moving in and out of the mitochondria, it may be argued that closure of this channel by Bcl-2/Bcl-xL in living cells could cause respiratory failure, but this never actually occurs. It might be that only a fraction of the total VDAC, e.g., the VDAC associated with the PT pore, is directly targeted by Bcl-2/Bcl-xL. Alternatively, since the interaction of Bcl-xL with the VDAC is considerably enhanced by apoptotic stimuli (our unpublished observations), it might be that Bcl-2/Bcl-xL only closes the channel in the presence of apoptotic signals, as seen in an in vitro study. Thus, the functional interaction between Bcl-2/Bcl-xL and the VDAC might be suppressed in healthy living cells. The BH1 domain of Bcl-xL is required for binding to the VDAC,42 while the BH4 domain of Bcl-2/Bcl-xL is necessary and sufficient for Bcl-2/Bcl-xL to close the VDAC in liposome and electrophysiological studies.22 Even after opening the VDAC by Bax, BH4 oligopeptides are able to compete with Bax and close it again.22 Consistent with the notion that BH4 is essential and sufficient for inhibiting the VDAC, BH4 oligopeptides are able to prevent Ca2+-induced apoptotic changes (cytochrome c release and Δψ loss) in isolated mitochondria.22 Interestingly, Ca2+ induces cytochrome c release and Δψ loss in a PT inhibitor-sensitive manner as does Bax/Bak.55 Furthermore, BH4 oligopeptides, when fused with the protein transduction domain of HIV Tat protein (allowing the peptides to cross the plasma membrane), inhibit apoptotic cell death induced by the chemotherapy agent etoposide.22 Since the BH4 domain is separated by the regions of Bcl-2/Bcl-xL (α-helices 5 and 6) required for formation of an ion channel,66,67 this finding may imply that ion channel formation by Bcl-2/Bcl-xL is not required for anti-apoptotic activity per se.22
ANT
Concerning the possible involvement of ANT in Bax/Bak-induced cytochrome c release,60 probably via the PT, our studies on yeast mutants have revealed that none of the three ANTs are essential for Bax/Bak-mediated cytochrome c release from isolated mitochondria,43 apparently ruling out a role of ANTs in Bax/Bak-mediated cytochrome c release, although the possibility was not excluded that an ANT-like channel might compensate for the ANT functions.
Δψ loss and the PT pore
Can the controversy concerning whether or not Bax/Bak induces Δψ loss (the PT) be resolved? It arises at least partly from differences in the experimental conditions that have been used. Since Δψ loss (the PT) is Ca2+-dependent, Ca2+ depletion prevents it and this might explain the occurrence of Bax/Bak-induced cytochrome c release without Δψ loss, because Ca2+ chelator was added to the mitochondrial buffers used in some studies.68,69 Since the presence of Ca2+ outside the mitochondria is more physiological and since cells transfected by Bax-expressing DNA show Δψ loss,55,56,57 the Δψ loss induced in isolated mitochondria by Bax/Bak seems more likely to represent the physiological role of Bax. In the absence of Ca2+, Bax/Bak opens the VDAC and allows the efflux of cytochrome c, whereas two modes of cytochrome c release via the VDAC and through PT-dependent rupture of the outer membrane, can occur in the presence of Ca2+ (Figure 1). Another possible explanation for the controversy about Bax-induced Δψ loss came from the study of Pastorino et al.,54 showing that different amounts of Bax can have different effects, even though Bax activates the same PT mechanism, i.e., a small amount of Bax induces a transient PT with cytochrome c release and Δψ loss that can be reversed subsequently, whereas a larger amount of Bax induces a complete PT with irreversible Δψ loss. The PT-dependent process, however, need to be assessed carefully because the interaction of Bax/Bak with VDAC in cells might not trigger all of the changes seen during the PT in studies on isolated mitochondria. For example, mitochondrial swelling might be an extreme reaction to the in vitro PT that may not occur in actual cells. Furthermore, how extensive the PT actually is during apoptosis might vary between cell types.
How does Bax/Bak induce Δψ loss? In addition to being essential for cytochrome c release, the VDAC has been shown to be required for apoptotic Δψ loss in yeast mitochondria,42 whereas ANTs are dispensable.43 Studies using the neutralizing anti-VDAC antibodies have revealed that the VDAC is essential for Bax/Bak-induced Δψ loss in isolated mammalian mitochondria.62 These results suggest that the interaction of Bax/Bak with the VDAC may trigger Δψ loss. Since the VDAC is a component of the PT pore, it probably communicates with other channels on the inner membrane, and opening of the VDAC sufficiently for cytochrome c release might trigger the opening of inner membrane channels, resulting in Δψ loss. Bax/Bak directly interacts with ANT42,60 and also possibly with several ANT-like proteins in the mitochondria, such as the Pi/OH− antiporter and uncoupling proteins, and these channels might be directly involved in Δψ loss. It may be that Bax/Bak initially interacts with the VDAC and then translocates to the inner membrane.60 It also remains possible that Bax might induce Δψ loss via a totally different mechanism, which might be indirectly regulated by the PT pore.
Since different components of the PT pore complex could interact with each other, involvement of this pore in apoptotic mitochondrial changes could also explain how the ANT inhibitor bonkrekic acid (which is assumed to close the ANT channel) and cyclosporine A (which targets mitochondrial cyclophilin D) can prevent VDAC opening and thereby inhibit cytochrome c release. Although Bax induces cytochrome c release from isolated mitochondria in both the presence and absence of Ca2+, only Bax-mediated cytochrome c release occurring in the presence of Ca2+ is blocked by PT inhibitors. Since the VDAC possesses a Ca2+-binding module, Ca2+ might modify the structure of the channel directly or via other Ca2+ sensor(s) so that the VDAC comes to interact with other parts of the PT pore complex, and thus, Bax-mediated VDAC opening could be regulated by ANT and cyclophilin D in a Ca2+-dependent manner.
Bax/Bak versus Bid/Bik
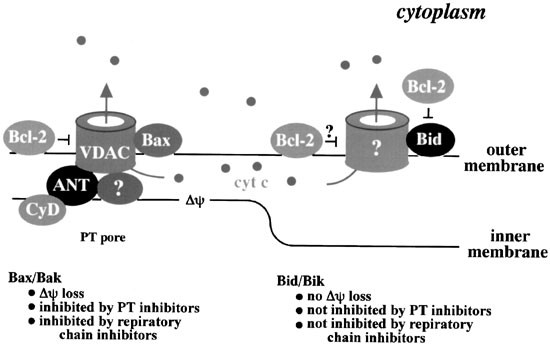
Unlike Bax and Bak, BH3-only proteins such as Bid and Bik neither bind to the VDAC nor affect VDAC activity in liposome studies,55 suggesting that the BH3-only proteins function via a totally different mechanism, via the inhibition of anti-apoptotic Bcl-2 family members or via the activation of pro-apoptotic Bcl-2 family members like Bax and Bak. A third possibility might be supported by the finding that Bid induces conformational changes of Bax and Bak.70,71 However, support for the first possibility is provided by the finding that Bax/Bak-induced cytochrome c release is substantially different from Bid (or Bik)-mediated cytochrome c release under the same experimental conditions55 (also see Figure 2). One of the notable differences is the concomitant occurrence of Δψ loss with Bax/Bak-mediated, but not Bid/Bik-mediated, cytochrome c release in isolated mitochondria as well as mammalian cells.55 Consistent with the idea that Δψ loss is one of the characteristics of the PT, Bax/Bak-mediated (but not Bid/Bik-mediated) cytochrome c release from isolated mitochondria can be inhibited by PT inhibitors.51,52,53,55 Furthermore, Bax/Bak-mediated cytochrome c release is inhibited by respiratory chain inhibitors such as antimycin, protonophore, KCN, and oligomycin, whereas Bid/Bik-induced cytochrome c release is insensitive to these drugs,55 although the detailed mechanism through which Bax/Bak-induced cytochrome c release is blocked by respiratory chain inhibitors remains to be determined. Based on these observations, it has been proposed that Bid/Bik probably targets a distinct molecule on the mitochondrial membrane to induce cytochrome c release.55 Although there is considerable variation in the primary structures of BH3-only proteins, there tertiary structure might be sufficiently similar to target the same molecule on the mitochondria, given that the 3D structure of Bid is extremely similar to that of Bcl-xL although primary sequence homology only exists within the BH3 domain.72,73 Since Bid/Bik-induced cytochrome c release is prevented by Bcl-2/Bcl-xL, these anti-apoptotic Bcl-2 family members might form heterodimers with Bid/Bik to inhibit their activity or directly close the Bid/Bik target channel in a similar manner as occurs with the VDAC (Figure 2).
Figure 2
Functional interaction of Bcl-2/Bcl-xL and Bax/Bak, but not Bid/Bik, with the VDAC. Bax/Bak opens the VDAC to induce cytochrome c release, while Bcl-2/Bcl-xL closes this channel. Bid/Bid does not interact with the VDAC, but probably has the ability to open an unidentified channel(s) that is involved in cytochrome c release. The VDAC is a component of the PT pore, which would explain the concomitant induction of Δψ loss by Bax/Bak and inhibition of Bax/Bak-induced cytochrome c and Δψ loss by PT inhibitors. Bcl-2/Bcl-xL inhibits Bid/Bik-induced cytochrome c release, probably through heterodimerization with Bid/Bik or by closing an unidentified channel(s)
Given the recent view that different apoptotic signals probably activate different pro-apoptotic Bcl-2 family members,6 induction of Δψ loss by Bax/Bak (but not by Bid/Bik) might also explain the previous controversial observations that Δψ loss is associated with some forms of apoptotic cell death but not others, assuming that different pro-apoptotic Bcl-2 family members may play a major role in certain modes of apoptotic cell death.
VDAC-Bax channel versus Bax channel: Is one favored over the other?
Given that two models exist for apoptotic cytochrome c release involving specific channels,46 i.e., (1) a channel formed by Bax alone and (2) a channel formed from the VDAC plus Bax, should one model be favored over the other in the light of our current knowledge?
- 1
Independent action of Bcl-2/Bcl-xL and Bax/Bak: As discussed above, Bcl-2/Bcl-xL and Bax/Bak can function independently from each other. If Bax (or another pro-apoptotic Bcl-2 family member), itself, forms a large pore responsible for cytochrome c release, how is Bcl-2/Bcl-xL able to prevent this process independently from Bax or without forming heterodimes with Bax? An independent action of anti-apoptotic Bcl-2/Bcl-xL and pro-apoptotic Bax/Bak is more favorable for a model of VDAC- or other types of mitochondrial channel-mediated cytochrome c release. Since yeast mitochondria lack Bcl-2 family proteins, but still have a mechanism for cytochrome c release that is Bcl-2/Bcl-xL-inhibitable43 and VDAC-dependent,42,43 there must be a way to open the VDAC and release cytochrome c independently of any of proteins from the Bcl-2 family. A similar mechanism is probably conserved in mammalian mitochondria, underlying the independent action of Bcl-2/Bcl-xL and Bax/Bak. - 2
If Bax (or another pro-apoptotic Bcl-2 family member) forms a large pore responsible for cytochrome c release, Bax as well as BH3-only proteins would also be expected to induce cytochrome c release from yeast mitochondria. However, only Bax/Bak (and not Bid/Bik) induces cytochrome c release from yeast mitochondria42 (our unpublished observations), making it unlikely that Bid/Bik itself forms a large pore in the mitochondrial membrane that allows cytochrome c release. - 3
Although Bax readily forms ion channels in a synthetic lipid membrane, it does not form its own channel in liposomes incorporating the VDAC,64 which represent the outer mitochondrial membrane better than does a synthetic lipid membrane.
In addition to our observations obtained with VDAC-deficient yeast cells and anti-VDAC antibodies, these findings also favor the VDAC-Bax channel model over the Bax channel model of cytochrome c release. The data also suggest that a target molecule for Bid/Bik probably exists on mammalian mitochondria, which is either missing or less conserved in yeast cells.
Summary
Apoptotic mitochondrial changes, including release of cytochrome c and Δψ loss, are central to signal transduction in the process of apoptosis. We propose that the Bcl-2 family of proteins, consisting of anti-apoptotic and pro-apoptotic members, functions as a channel regulator to control apoptotic mitochondrial changes rather than providing the actual channel involved, and that the VDAC is one of the major channels directly regulated by the Bcl-2 family. We also suggest that some of the BH3-only proteins may target an unidentified channel on the outer mitochondrial membrane and thus promote cytochrome c release.
Abbreviations
VDAC:
voltage-dependent anion channel
Δψ:
mitochondrial membrane potential
ANT:
adenine nucleotide translocator
References
- Thronberry NA and Lazebnik Y . 1998 Caspases: Enemies within. Science 281: 1312–1316
Article Google Scholar - Liu X, Zou H, Slaughter C and Wang X . 1997 DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89: 175–184
Article CAS Google Scholar - Sakahira H, Enari M and Nagata S . 1998 Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 391: 96–99
Article CAS Google Scholar - Sahara S, Aoto M, Eguchi Y, Imamoto N, Yoneda Y and Tsujimoto Y . 1999 Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature 401: 168–173
Article CAS Google Scholar - Adams JM and Cory S . 1998 The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326
Article CAS Google Scholar - Tsujimoto Y . 1998 Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells 3: 697–707
Article CAS Google Scholar - Tsujimoto Y, Cossman J, Jaffe E and Croce CM . 1985 Science 228: 1440–1443
- Bakhshi A, Jensen JP, Goldman P, Wright JJ, McBride OW, Epstein AL and Korsmeyer SJ . 1985 Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosomes 14 and near a transcriptional unit on 81. Cell 41: 899–906
Article CAS Google Scholar - Cleary ML and Sklar J . 1985 Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc. Natl. Acad. Sci. USA 82: 7439–7443
Article CAS Google Scholar - Vaux DL, Cory S and Adams JM . 1988 bcl-2 gene promotes haematopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335: 440–442
Article CAS Google Scholar - Tsujimoto Y . 1989 Stress-resistance conferred by high level of bcl-2α protein in human B lymphoblastoid cell. Oncogene 4: 1331–1336.
CAS PubMed Google Scholar - Shimizu S, Eguchi Y, Kamiike W, Waguri S, Uchiyama Y, Matsuda H and Tsujimoto Y . 1996 Bcl-2 blocks loss of mitochondrial membrane potential while ICE inhibitors act at different step during inhibition of death induced by respiratory chain inhibitors. Oncogene 13: 21–29
CAS PubMed Google Scholar - Zhang H, Cowan-Jacob SW, Simonen M, Greenhalf W, Heim J and Meyhack B . 2000 Structural basis of BFL-1 for its interaction with BAX and its anti-apoptotic action in mammalian and yeast cells. J. Biol. Chem. 275: 11092–11099
Article CAS Google Scholar - Oltvai ZN, Milliman CL and Korsmeyer SJ . 1993 Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74: 609–619
Article CAS Google Scholar - Yang E, Zha J, Jockel J, Boise LH, Thompson CB and Korsmeyer SJ . 1995 Bad, a heterodimeric partner for Bcl-xL and Bcl-2, displaces Bax and promotes cell death. Cell 80: 285–291
Article CAS Google Scholar - Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB and Fesik SW . 1997 Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 275: 983–986
Article CAS Google Scholar - Borner C, Martinou I, Mattmann C, Irmler M, Schaerer E, Martinou JC and Tschopp J . 1994 The protein bcl-2 alpha does not require membrane attachment, but two conserved domains to suppress apoptosis. J. Cell Biol. 126: 1059–1068
Article CAS Google Scholar - Hanada M, Aime-Sempe C, Sato T and Reed JC . 1995 Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J. Biol. Chem. 270: 11962–11969
Article CAS Google Scholar - Hunter JJ, Bond BL and Parslow TG . 1996 Functional dissection of the human Bcl2 protein: sequence requirements for inhibition of apoptosis. Mol. Cell. Biol. 16: 877–883
Article CAS Google Scholar - Lee LC, Hunter JJ, Mujeeb A, Turck C and Parslow TG . 1996 Evidence for alpha-helical conformation of an essential N-terminal region in the human Bcl2 protein. J. Biol. Chem. 271: 23284–23288
Article CAS Google Scholar - Huang DC, Adams JM and Cory S . 1998 The conserved N-terminal BH4 domain of Bcl-2 homologues is essential for inhibition of apoptosis and interaction with CED-4. EMBO J. 17: 1029–1039
Article CAS Google Scholar - Shimizu S, Konishi A, Kodama T and Tsujimoto Y . 2000 BH4 domain of anti-apoptotic Bcl-2 family members closes VDAC, and inhibits apoptotic mitochondrial changes and cell death. Proc. Natl. Acad. Sci. USA 97: 3100–3105
Article CAS Google Scholar - Chittenden T, Flemington C, Houghton AB, Ebb RG, Gallo GJ, Elangovan B, Chinnadurai G and Lutz RJ . 1995 A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 14: 5589–5596
Article CAS Google Scholar - Minn AJ, Velez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M and Thompson CB . 1997 Bcl-xL forms as ion channel in synthetic lipid membranes. Nature 385: 353–357
Article CAS Google Scholar - Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G and Korsmeyer SJ . 1997 Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc. Natl. Acad. Sci. USA 94: 11357–11362
Article CAS Google Scholar - Schendel SL, Xie Z, Montal MO, Matsuyama S, Montal M and Reed JC . 1997 Channel formation by antiapoptotic protein Bcl-2. Proc. Natl. Acad. Sci. USA 94: 5113–5118
Article CAS Google Scholar - Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R and Martinou JC . 1997 Inhibition of Bax channel-forming activity by Bcl-2. Science 277: 370–372
Article CAS Google Scholar - Schendel SL, Azimov R, Pawlowski K, Godzik A, Kagan BL and Reed JC . 1999 Ion channel activity of the BH3 only Bcl-2 family member, BID. J. Biol. Chem. 274: 21932–21936
Article CAS Google Scholar - Green DR and Reed JC . 1998 Mitochondria and apoptosis. Science 281: 1309–1312
CAS Google Scholar - Liu X, Kim CN, Yang J, Jemmerson R and Wang X . 1996 Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 86: 147–157
Article CAS Google Scholar - Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP and Wang X . 1997 Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 275: 1129–1132
Article CAS Google Scholar - Kluck RM, Bossy-Wetzel E, Green DR and Newmeyer DD . 1997 The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 275: 1132–1136
Article CAS Google Scholar - Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM and Kroemer G . 1999 Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397: 441–446
Article CAS Google Scholar - Gross A, Jockel J, Wei MC and Korsmeyer SJ . 1998 Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 17: 3878–3885
Article CAS Google Scholar - Nomura M, Shimizu S, Ito T, Narita M, Matsuda H and Tsujimoto Y . 1999 Apoptotic cytosol facilitates Bax translocation to mitochondria that involves cytosolic factor regulated by Bcl-2. Cancer Res. 59: 5542–5548
CAS PubMed Google Scholar - Hu Y, Benedict MA, Wu D, Inohara N and Nunez G . 1998 Bcl-xL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc. Natl. Acad. Sci. USA 95: 4386–4391
Article CAS Google Scholar - Pan G, O'Rourke K and Dixit VM . 1998 Caspase-9, Bcl-xL, and Apaf-1 form a ternary complex. J. Biol. Chem. 273: 5841–5845
Article CAS Google Scholar - Golstein P . 1997 Controlling cell death. Science 275: 1081–1082
Article CAS Google Scholar - Moriishi K, Huang DC, Cory S and Adams JM . 1999 Bcl-2 family members do not inhibit apoptosis by binding the caspase activator Apaf-1. Proc. Natl. Acad. Sci. USA 96: 9683–9688
Article CAS Google Scholar - Hausmann G, O'Reilly LA, van Driel R, Beaumont J, Strasser A, Adams JM and Huang DC . 2000 Pro-apoptotic apoptosis protease-activating factor 1 (Alpha-1) has a cytoplasmic localization distinct from Bcl-2 or Bcl-xL. J. Cell Biol. 149: 623–634
Article CAS Google Scholar - Knudson CM and Korsmeyer SJ . 1997 Bcl-2 and Bax function independently to regulate cell death. Nature Genet. 16: 358–363
Article CAS Google Scholar - Shimizu S, Narita M and Tsujimoto Y . 1999 Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399: 483–487
Article CAS Google Scholar - Shimizu S, Shinohara Y and Tsujimoto Y . 2000 Bax and Bcl-xL independently regulate apoptotic changes of yeast mitochondria that require VDAC but not adenine nucleotide translocator. Oncogene in press
- Priault M, Chaudhuri B, Clow A, Camougrand N and Manon S . 1999 Investigation of bax-induced release of cytochrome c from yeast mitochondria permeability of mitochondrial membranes, role of VDAC and ATP requirement. Eur. J. Biochem. 260: 684–691
Article CAS Google Scholar - Roucou X, Prescott M, Devenish RJ and Nagley P . 2000 A cytochrome c-GFP fusion is not released from mitochondria into the cytoplasm upon expression of Bax in yeast cells. FEBS Lett. 471: 235–239
Article CAS Google Scholar - Tsujimoto Y and Shimizu S . 2000 Bcl-2 family: Life-or-death switch. FEBS Lett. 466: 6–10
Article CAS Google Scholar - Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT and Thompson CB . 1997 Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 91: 627–637
Article CAS Google Scholar - Basanez G, Nechushtan A, Drozhinin O, Chanturiya A, Choe E, Tutt S, Wood KA, Hsu Y, Zimmerberg J and Youle RJ . 1999 Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl. Acad. Sci. USA 96: 5492–5497
Article CAS Google Scholar - Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Macho A, Haeffner A, Hirsch F, Geuskens M and Kroemer G . 1996 Mitochondrial permeability transition is a central coordinating event of apoptosis. J. Exp. Med. 184: 1155–1160
Article CAS Google Scholar - Zoratti M and Szabo I . 1995 The mitochondrial permeability transition. Biochim. Biophys. Acta. 1241: 139–176
Article Google Scholar - Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D and Reed JC . 1998 Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 95: 4997–5002
Article CAS Google Scholar - Narita M, Shimizu S, Ito T, Chittenden T, Lutz RJ, Matsuda H and Tsujimoto Y . 1998 Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc. Natl. Acad. Sci. USA 95: 14681–14686
Article CAS Google Scholar - Kroemer G, Dallaporta B and Resche-Rigon M . 1998 The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol. 60: 619–642
Article CAS Google Scholar - Pastorino JG, Tafani M, Rothman RJ, Marcineviciute A, Hoek JB and Farber JL . 1999 Functional consequences of the sustained or transient activation by Bax of the mitochondrial permeability transition pore. J. Biol. Chem. 274: 31734–31739
Article CAS Google Scholar - Shimizu S and Tsujimoto Y . 1999 Pro-apoptotic BH3-only Bcl-2 family members induce cytochrome c release, but not mitochondrial membrane potential loss, and do not directly modulate VDAC activity. Proc. Natl. Acad. Sci. USA 97: 577–582
Article Google Scholar - Xiang J, Chao DT and Korsmeyer SJ . 1996 BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc. Natl. Acad. Sci. USA 93: 14559–14563
Article CAS Google Scholar - Pastorino JG, Chen ST, Tafani M, Snyder JW and Farber JL . 1998 The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J. Biol. Chem. 273: 7770–7775
Article CAS Google Scholar - Zamzami N, Susin SA, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M and Kroemer G . 1996 Mitochondrial control of nuclear apoptosis. J. Exp. Med. 183: 1533–1544
Article CAS Google Scholar - Zamzami N, Brenner C, Marzo I, Susin SA and Kroemer G . 1998 Subcellular and submitochondrial mode of action of Bcl-2-like oncoproteins. Oncogene 16: 2265–2282
Article CAS Google Scholar - Marzo I, Brenner C, Zamzami N, Jurgensmeier JM, Susin SA, Vieira HL, Prevost MC, Xie Z, Matsuyama S, Reed JC and Kroemer G . 1998 Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 281: 2027–2031
Article CAS Google Scholar - Brenner C, Cadiou H, Vieira HL, Zamzami N, Marzo I, Xie Z, Leber B, Andrews D, Duclohier H, Reed JC and Kroemer G . 2000 Bcl-2 and Bax regulate the channel activity of the mitochondrial adenine nucleotide translocator. Oncogene 19: 329–336
Article CAS Google Scholar - Shimizu S, Matsuoka Y, Shinohara Y, Yoneda Y and Tsujimoto Y. . Essential role of voltage-dependent anion channel in various forms of apoptosis in mammalian cells. J. Cell. Biol. in press
- Gross A, Pilcher K, Blachly-Dyson E, Basso E, Jockel J, Bassik MC, Korsmeyer SJ and Forte M . 2000 Biochemical and genetic analysis of the mitochondrial response of yeast to BAX and BCL-X. Mol. Cell. Biol. 20: 3125–3136
Article CAS Google Scholar - Shimizu S, Ide T, Yanagida T and Tsujimoto Y . 2000 Electrophysiological study of a novel large pore formed by Bax and VDAC, which is permeable to cytochrome c. J. Biol. Chem. 275: 12321–12325
Article CAS Google Scholar - Patterson SD, Spahr CS, Daugas E, Susin SA, Irinopoulou T, Koehler C and Kroemer G . 2000 Mass spectrometric identification of proteins released from mitochondria undergoing permeability transition. Cell Death Differ. 7: 137–144
Article CAS Google Scholar - Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, Ng SL and Fesik SW . 1996 X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 381: 335–341
Article CAS Google Scholar - Schendel SL, Montal M and Reed JC . 1998 Bcl-2 family proteins as ion-channel. Cell Death Differ. 5: 372–380
Article CAS Google Scholar - Kluck RM, Esposti MD, Perkins G, Renken C, Kuwana T, Bossy-Wetzel E, Goldberg M, Allen T, Barber MJ, Green DR and Newmeyer DD . 1999 The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. J. Cell. Biol. 147: 809–822
Article CAS Google Scholar - Eskes R, Antonsson B, Osen-Sand A, Montessuit S, Richter C, Sadoul R, Mazzei G, Nichols A and Martinou JC . 1998 Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J. Cell. Biol. 143: 217–224
Article CAS Google Scholar - Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B and Martinou JC . 1999 Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell. Biol. 144: 891–901
Article CAS Google Scholar - Eskes R, Desagher S, Antonsson B and Martinou JC . 2000 Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20: 929–935
Article CAS Google Scholar - Chou JJ, Li H, Salvesen GS, Yuan J and Wagner G . 1999 Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell 96: 6156–6124
Article Google Scholar - McDonnell JM, Fushman D, Milliman CL, Korsmeyer SJ and Cowburn D . 1999 Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell 96: 625–634
Article CAS Google Scholar
Acknowledgements
We wish to thank all the members of my laboratory at Osaka University Graduate School of Medicine. The work performed at this laboratory was supported in part by a grant for Scientific Research on Priority Areas, by a Center of Excellence Research grant, and by grants for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
Author information
Authors and Affiliations
- Department of Medical Genetics, Biomedical Research Center, Osaka University Graduate School of Medicine, CREST of Japan Science and Technology Corp. (JST), Suita, Osaka, Japan
Y Tsujimoto & S Shimizu
Authors
- Y Tsujimoto
- S Shimizu
Corresponding author
Correspondence toY Tsujimoto.
Additional information
Edited by G Kroemer
Rights and permissions
About this article
Cite this article
Tsujimoto, Y., Shimizu, S. VDAC regulation by the Bcl-2 family of proteins.Cell Death Differ 7, 1174–1181 (2000). https://doi.org/10.1038/sj.cdd.4400780
- Received: 01 June 2000
- Accepted: 14 September 2000
- Published: 13 December 2000
- Issue Date: 01 December 2000
- DOI: https://doi.org/10.1038/sj.cdd.4400780