Sphingosine generation, cytochrome c release, and activation of caspase-7 in doxorubicin-induced apoptosis of MCF7 breast adenocarcinoma cells (original) (raw)
Introduction
The anthracycline antibiotics constitute an important group of drugs that have been used to successfully produce regression in disseminated neoplasias, including acute lymphoblastic leukemia, acute myeloblastic leukemia, Wilm's tumor, soft tissue and bone sarcomas, breast carcinoma, ovarian carcinoma, and many others. The molecular mechanisms responsible for the cytotoxic effects of anthracyclines in malignant cells are not fully understood but are mainly attributed to their ability to intercalate into DNA and cause localized uncoiling of the double helix or by stabilization of the complex between DNA and topoisomerase II.1 In addition, anthracyclines can mediate oxygen free radical generation originating from quinone-generated redox activity that can also damage DNA.1
Ceramide, a sphingolipid produced by the hydrolysis of membrane-associated sphingomyelin, has previously been implicated as a gauge of apoptosis (reviewed in 2). Diverse apoptotic stimuli, including the anthracycline drug daunorubicin,3,4,5,6,7,8,9 increase ceramide levels, which in turn leads to activation of downstream signals important for the apoptotic process. Daunorubicin triggers apoptosis and ceramide generation by activating a neutral sphingomyelinase in the leukemic cell lines U937 and HL-60,3 whereas other studies suggest that it stimulates ceramide elevation in lymphocytic P388 and U937 cells by activation of ceramide synthase in the de novo pathway, rather than by activation of sphingomyelinase.4 Although Bcl-2 overexpression inhibits ceramide-mediated apoptosis, it does not intervene in ceramide generation induced by daunorubicin, suggesting that Bcl-2 suppresses apoptosis downstream of ceramide generation.5 In agreement, doxorubicin, another member of the anthracycline family, induces apoptosis in cardiac myocytes and activates the sphingomyelin-ceramide pathway.10,11
Sphingosine, formed from ceramide by ceramidase, has also been implicated in cell growth arrest and apoptosis. Indeed, sphingosine is rapidly produced during TNFα-mediated apoptosis in human neutrophils12 and in cardiac myocytes,13 and is capable of inducing apoptosis when added exogenously to many cell types.12,13,14,15,16,17,18 More recently, we found that sphingosine is also transiently produced during the early phase of Fas- and ceramide-induced apoptosis in Jurkat T cells, acting in a mitochondria-dependent manner.19
Here, we report that treatment of human breast cancer MCF7 cells with doxorubicin induces transient generation of sphingosine, preceding cytochrome c release and activation of the executioner caspase-7. In addition, exogenous sphingosine recapitulates doxorubicin-induced apoptosis, as well as cytochrome c redistribution and activation of caspase-7 in a Bcl-xL-sensitive fashion. Our findings suggest the potential involvement of sphingosine, in addition to ceramide, in mitochondria-dependent apoptosis triggered by doxorubicin in human breast cancer MCF7 cells.
Results
In agreement with previous studies,20 we found that treatment of MCF7 cells with doxorubicin caused extensive cell death (Figure 1A). As sphingosine has been implicated in cytokine-induced apoptosis,12,13,19 it was of interest to determine whether treatment with doxorubicin could also induce apoptosis in a manner dependent on sphingosine generation. After treatment with 1 μg/ml doxorubicin, a therapeutic concentration, sphingosine levels significantly increased by 16 h, peaked at 24 h, and declined thereafter (Figure 1B). Interestingly, sphingosine levels also somewhat increased in untreated cells, probably as a result of changing to fresh serum-free media, in agreement with previous studies demonstrating that sphingolipid synthesis was stimulated by replacement of the media.21,22 In line with previous studies in other cell types,12,13,15,16,17,18,19,23,24 addition of exogenous sphingosine also induced apoptosis in MCF7/Fas cells in a time- (Figure 1A) and concentration-dependent manner (data not shown).
Figure 1
The effect of doxorubicin on cell death and sphingosine levels in MCF7 cells. (A) MCF7/Fas cells were treated with 1 μg/ml of doxorubicin (filled squares) or 10 μM sphingosine (open squares) for the indicated times. The percentage of cell death was assessed by the MTT assay. The values are means of triplicate determinations±S.D. Similar results were obtained in three additional experiments. (B) Sphingosine levels were measured in MCF7/Fas cells cultured in serum-free media without (untreated) or with 1 μg/ml doxorubicin for the indicated times. Results are means±S.D. of three independent experiments performed in triplicate
To examine the possibility that sphingosine-induced cell death might be due to its conversion to ceramide by the action of ceramide synthase, we investigated the effect of the mycotoxin fumonisin B1, a competitive inhibitor of ceramide synthase.25 Treatment of MCF7/Fas cells with 25 μM fumonisin B1 had no effect on cell death induced by doxorubicin (Figure 2A) nor did it affect the extent of cell death induced by sphingosine (Figure 2B). However, surprisingly, a higher concentration of fumonisin B1 (100 μM) inhibited cell death in MCF7/Fas cells treated with doxorubicin to a lesser extent than that induced by sphingosine (Figure 2C). These data suggest that sphingosine- and doxorubicin-induced cell death in MCF7/Fas cells is partially dependent on ceramide synthase activity.
Figure 2
Effects of fumonisin B1 on cell death. (A) MCF7/Fas cells were pre-incubated without (filled squares) or with (open squares) 25 μM fumonisin B1 for 30 min in serum-free medium and then 1 μg/ml doxorubicin was added for the indicated times. (B) MCF7/Fas cells were pre-incubated without (filled squares) or with (open squares) 25 μM fumonisin B1 for 30 min in serum-free medium and then incubated in the presence of 10 μM sphingosine for the indicated times. (C) MCF7/Fas cells were incubated in the presence of 1 μg/ml doxorubicin or 10 μM sphingosine without or with 100 μM fumonisin B1 for 72 h. The percentage of cell death was assessed by the MTT assay. The values are means±S.D. of triplicate determinations
The generation of sphingosine during cell death induced by doxorubicin suggests that the increase in sphingosine might result from degradation of ceramide. This is a likely possibility, as sphingosine is not synthesized de novo and can only be produced by metabolism of ceramide.25 To gain further insight into the relative function of endogenous sphingosine, we used the ceramidase inhibitor, dMAPP.26 Treatment of MCF7/Fas cells with 5 μM dMAPP not only attenuated cell death induced by doxorubicin (Figure 3A), but also surprisingly, that induced by exogenous sphingosine to an even greater extent (Figure 3B).
Figure 3
Effects of dMAPP on cell death. MCF7/Fas cells were incubated without (filled squares) or with (open squares) 5 μM dMAPP for 30 min and then 1 μg/ml doxorubicin (A) or 10 μM sphingosine (B) were added for the indicated times. The percentage of cell death was assessed by the MTT assay. The values are means±S.D. of triplicate determinations
Because overexpression of Bcl-2 or Bcl-xL protects against diverse apoptotic stimuli, including ceramide-induced apoptosis,5,19,27,28,29,30 it was important to determine whether the effects of sphingosine were also blocked by these proteins. MCF7/Fas cells stably overexpressing Bcl-xL were treated with doxorubicin or sphingosine and cell death was measured. In agreement with the anti-apoptotic effects of Bcl-xL family members in other cell types, including human small cell lung cancer cells,3 neuroblastoma cells,32 myeloid leukemia HL-60 cells33,34 and U937 cells,35 overexpression of Bcl-xL inhibited doxorubicin-induced cell death and completely protected against sphingosine-induced cell death (Figure 4A). However, Bcl-xL expression did not have any significant effect on the increase in sphingosine induced by doxorubicin (Figure 4B). These results suggest that the sphingosine increase is not merely a consequence of cell death as it is also observed in the Bcl-xL overexpressing cells which do not die and imply that sphingosine may act upstream of Bcl-xL.
Figure 4
Bcl-xL overexpression blocks doxorubicin-induced cell death but not sphingosine generation. (A) MCF7/Fas and MCF7/Fas/Bcl-xL cells were treated with 1 μg/ml doxorubicin or 10 μM sphingosine and cell death was assessed by the MTT assay. The values are means±S.D. of triplicate determination. This experiment was repeated three times with similar results. (B) Sphingosine levels were determined in MCF7/Fas and MCF7/Fas/Bcl-xL cells treated with 1 μg/ml doxorubicin for the indicated times. Results are means±S.D. of three independent experiments performed in triplicate
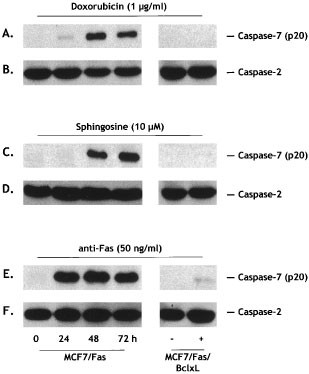
Because our previous findings suggested that sphingosine may function proximal to executioner caspases in Jurkat T lymphoma cells,19 we next investigated whether doxorubicin and sphingosine activated executioner caspases in MCF7/Fas cells. Based on their substrate specificities, caspase-3, -2, and -7, believed to be the final executioner caspases, belong to the group II subfamily that preferentially cleave the peptide sequence DExD found in numerous death substrates.36 Proteolytic activation of these caspases was examined by Western blotting analysis. Only caspase-7 was activated by both doxorubicin (Figure 5A) and sphingosine (Figure 5C) in MCF7/Fas cells. Surprisingly, although caspase-2 is clearly expressed in MCF7/Fas cells, it was not cleaved after treatment with doxorubicin (Figure 5B) or sphingosine (Figure 5D). Similarly, extracts from MCF7/Fas cells treated with Fas mAb, a strong inducer of apoptosis in this cell line,37,38,39,40 did not activate caspase-2 (Figure 5F), whereas caspase-7 was processed to the active fragment (Figure 5E). As the MCF7 cell line is devoid of caspase-3 owing to the functional deletion of the CASP-3 gene,41 caspase-7 appears to be the only executioner caspase activated in these cells. Despite activation of caspase-7, Ac-DEVD-AMC-cleaving activity was not detectably increased after treatment of MCF7/Fas cells with sphingosine or doxorubicin (data not shown), likely a result of the deficiency of caspase-3 activity.
Figure 5
Activation of caspase-7 and caspase-2 in response to doxorubicin, sphingosine, and anti-Fas mAb. MCF7/Fas and MCF7/Fas/Bcl-xL cells were incubated in serum-free conditions without (−) or with (+) 1 μg/ml doxorubicin (A, B), 10 μM sphingosine (C, D), or 50 ng/ml anti-Fas mAb (E, F) and extracts prepared at the indicated times or 72 h for MCF7/Fas/Bcl-xL cells. Proteins were resolved by 15% SDS–PAGE, blotted, and probed with anti-caspase-7 antibody (A,C,E), or with anti-caspase-2 antibody (B,D,F). Migrations indicated: active subunit p20 of caspase-7; full-length caspase-2
Having established that doxorubicin- and sphingosine-induced cell death was prevented by Bcl-xL overexpression, we also examined caspase activation in MCF7/Fas/Bcl-xL cells. As shown in Figure 5A,C,E, activation of caspase-7 in these cells was markedly inhibited by overexpression of Bcl-xL.
After initiation of the apoptotic program, release of cytochrome c from mitochondria plays an important role in activation of downstream caspases. Cytochrome c binds to Apaf-1, a mammalian homologue of the Caenorhabditis elegans death-promoting CED-4 protein, inducing it to associate with caspase-9, thereby triggering its autoactivation into a mature form, which in turn can then directly cleave caspase-3 or -7 (reviewed in42,43,44). Because expression of Bcl-xL has been shown to interfere with cytochrome c release,44 we next determined whether doxorubicin and sphingosine could also trigger mitochondrial cytochrome c release. As shown in Figure 6A, treatment with doxorubicin resulted in a significant increase in cytosolic cytochrome c levels by 24 h. This is in agreement with a previous study establishing cytochrome c release upon doxorubicin treatment in neuroblastoma cells.32 Similarly, treatment with exogenous sphingosine also caused accumulation of cytochrome c in the cytosol (Figure 6C). Cytochrome c release induced by doxorubicin or sphingosine was prevented by Bcl-xL overexpression (Figure 6A,C). The absence of detectable cytochrome oxidase in cytosolic extracts confirmed that our preparations were free of mitochondrial contamination (Figure 6B,D).
Figure 6
Cytosolic accumulation of cytochrome c induced by doxorubicin or sphingosine is inhibited by Bcl-xL overexpression. MCF7/Fas and MCF7/Fas/Bcl-xL cells treated without (−) or with (+) 1 μg/ml doxorubicin (A, B) or 10 μM sphingosine (C, D) were harvested after 48 h, and cytosolic and mitochondrial proteins were separated by 15% SDS–PAGE and analyzed by immunoblotting with anti-cytochrome c (A, C) or anti-cytochrome oxidase (subunit II; B, D). Cytochrome oxidase serves as a marker for mitochondrial contamination of cytosolic extracts. A mitochondrial extract from nontreated cells (mit. Fr. Con) was used as a positive control for cytochrome c and cytochrome oxidase (subunit II)
As doxorubicin and sphingosine were capable of inducing caspase-7 activation as well as cytochrome c translocation to the cytosol, it was important to determine whether cytochrome c mediated activation of caspase-7 in caspase-3 null MCF7 cells. When cytochrome c was added in vitro to cell-free MCF7/L/neo extracts in the presence of 1 mM dATP, cleavage of caspase-7 increased with time of incubation (data not shown) and cytochrome c concentration (Figure 7D). However, as recently reported,45 DEVDase activity was only slightly increased under these conditions (Figure 7A), presumably owing to the absence of caspase-3 in these cells (Figure 7C). Interestingly, but in line with our results showing that caspase-2 was not processed in apoptotic MCF7/Fas cells (Figure 5B,D,F), no activation of caspase-2 was detected in this in vitro system (Figure 7E). These results demonstrate that cytochrome _c_-dependent caspase-7 activation can take place in caspase-3 deficient MCF7 cell lines. In a comparative in vitro study performed with MCF7/L cells stably expressing caspase-3 (denoted MCF7/L/casp-3), processing of caspase-3 (Figure 7C) was accompanied by increased cleavage of caspase-7 (Figure 7D). Furthermore, increased processing of caspase-2 (Figure 7E) demonstrates that caspase-3 activation is required for proteolysis of caspase-2. Likewise, MCF7/L/casp-3 extracts have several fold higher DEVDase activity than MCF7/L/neo extracts, probably as a result of the combined activity of caspase-3 and caspase-7. In contrast to cytosolic extracts prepared from MCF7 cells and in agreement with previous results, complete processing of caspase-9,46-3,46,47-7,46,47 and -246 was readily observed in cytosolic extracts prepared from Jurkat cells incubated with a concentration of cytochrome c as low as 10 μg/ml within 30 min (Figure 8A–D). Moreover, cytochrome _c_-triggered caspase activation resulted in rapid cleavage of PARP (Figure 8E), confirming that this in vitro system recapitulates the apoptotic cascade from initiation of executioner caspases activation to cleavage of death substrates. Taken together, these assays demonstrate that caspase-7 does not require the presence of caspase-3 to be activated in MCF7 cells, although overexpression of caspase-3 does enhance its processing.
Figure 7
Cytochrome _c_-initiated activation of caspases in a cell-free system from MCF7/L/neo and MCF7/L/casp3 cells. (A) DEVDase activity was measured with the fluorogenic substrate Ac-DEVD-AMC in cytosolic extracts from MCF7/L/neo cells treated without or with the indicated concentrations of horse heart cytochrome c for 30 min (open squares) or 90 min (filled squares) at 37°C. Results are means±S.D. of at least three independent experiments performed in triplicate. (B) DEVDase activity was measured in cytosolic extracts from MCF7/L/casp3 cells treated without or with the indicated concentrations of horse heart cytochrome c for 30 min (open squares) or 90 min (filled squares) at 37°C. Results are means±S.D. of at least three independent experiments. Cytosolic extracts from MCF7/L/neo cells treated without or with the indicated concentrations of horse heart cytochrome c for 90 min at 37°C were separated by SDS–PAGE and analyzed by immunoblotting with anti-caspase-3 (C), anti-caspase-7 (D), or anti-caspase-2 (E). Migrations indicated: full-length caspase-3, cleavage intermediate p20; active subunit p17; active subunit p20 of caspase-7; full-length caspase-2
Figure 8
Cytochrome _c_-initiated activation of casp-9, -3, -7,and -2, and cleavage of PARP in Jurkat cell extracts. Cytosolic extracts from Jurkat T cells treated without or with the indicated concentrations of horse heart cytochrome c for 30 min at 37°C were separated by SDS–PAGE and analyzed by immunoblotting with anti-caspase-9 (A), anti-caspase-3 (B), anti-caspase-7 (C), anti-caspase-2 (D), anti-PARP (E). Migrations indicated: full-length caspase-9, cleavage intermediate p35; full-length caspase-3, cleavage intermediate p20, active subunit p17; active subunit p20 of caspase-7; full-length caspase-2. full-length PARP, active form p89
The Fas receptor/ligand system has been proposed to play a role in doxorubicin-mediated apoptosis.48,49,50 Levels of FasL protein were examined by Western blotting to determine whether its expression was increased by doxorubicin or sphingosine treatment. There were no detectable changes in FasL protein expression discernible after doxorubicin (Figure 9C) or sphingosine treatment (Figure 9E). To further examine the possible requirement for Fas signaling in cell death induced by doxorubicin in MCF7/Fas cells, we determined whether caspase-8 could be activated. Caspase-8 cleavage often represents the first detectable event in Fas death receptor-mediated apoptosis.51 Notwithstanding, in type II cells, such as Jurkat T cells, caspase-8 can be activated in a post-mitochondrial manner, and sphingosine treatment can lead to caspase-8 processing in this cell type.19 As shown in Figure 7B, caspase-8 was only activated in anti-Fas mAb-treated, but not in doxorubicin- (Figure 9D) or sphingosine-treated (Figure 9F) MCF7/Fas cells. In agreement with previous studies,37,40,52 Bcl-xL, which strongly inhibits apoptosis, had no effect on the processing of caspase-8 induced by Fas ligation (Figure 9B). Together, these findings negate a role for the Fas ligand/receptor signaling in doxorubicin-induced cell death in MCF7 carcinoma cells.
Figure 9
Caspase-8 activation and FasL expression in response to doxorubicin, sphingosine, or anti-Fas mAb. MCF7/Fas and MCF7/Fas/Bcl-xL cells were incubated in serum-free conditions without (−) or with (+) 50 ng/ml anti-Fas mAb (A, B), 1 μg/ml doxorubicin (C, D), or 10 μM sphingosine (E, F) and extracts prepared at the indicated times or 72 h for MCF7/Fas/Bcl-xL cells. Proteins were resolved by 15% SDS–PAGE, blotted, and probed with anti-FasL antibody (A, C, E), or with anti-caspase-8 (B, D, F). Migrations indicated: full-length caspase-8; FasL
Discussion
Accumulating evidence indicates that chemotherapeutic agents such as anthracyclines kill neoplastic cells by the induction of apoptosis. Although the pathways involved in cell death induced by these agents are not fully understood, abundant reports have suggested that the sphingolipid ceramide play an important role in apoptosis initiated by anthracyclines.3,4,5,6,7,8,9,10,11
Accumulation of glucosylceramides, simple glycosylated forms of ceramide, is a feature of some multidrug-resistant cancer cells and tumors from patients who are less responsive to chemotherapy, demonstrating a correlation between cellular drug resistance and alterations in ceramide metabolism.53 Reduction of ceramide levels by transfection with glucosylceramide synthase, the enzyme that converts ceramide to glucosylceramide, confers doxorubicin resistance in MCF7 cells.54 Conversely, transfection of antisense glucosylceramide synthase to limit cellular ceramide glycosylation, overcomes doxorubicin resistance.55 Hence, tumor cells may have reduced sensitivity to chemotherapy because of an inability to produce sufficient apoptotic signal via sphingomyelin hydrolysis to ceramide.56 Indeed, sphingomyelin co-administration potentiates chemotherapy of human cancer xenografts.57
In contrast to doxorubicin, which induces apoptosis only in human epidermoid carcinoma KB-3-1 cells and not in a multidrug-resistant subclone that expresses P-glycoprotein; the ceramide breakdown product, sphingosine, induces apoptosis in both cell types.14 Furthermore, neither the protein kinase C (PKC) inhibitor H7 nor staurosporine induced apoptosis in these cell lines, suggesting that PKC-independent signaling is involved in apoptosis induced by sphingosine.14 An analog of sphingosine, L-threo-dihydrosphingosine (known as safingol), enhanced doxorubicin accumulation and sensitivity of MCF7 drug resistant cells. However, this effect correlated with inhibition of PKC rather than interference with P-glycoprotein drug binding.58 Because sphingosine and its analogs induce apoptosis regardless of P-glycoprotein expression, they may provide a new strategy for the treatment of anticancer drug-resistant cancers. Indeed, in pilot clinical phase I trials with safingol, which potentiates the effect of doxorubicin in tumor-bearing animals, it was found that safingol can be given safely with doxorubicin at a dose that is potentially pharmacologically active without dose-limiting toxicity.59
The results presented here demonstrate that transient generation of endogenous sphingosine also occurs in MCF7 breast cancer cells during apoptosis induced by the anthracycline doxorubicin, preceding release of cytochrome c from the mitochondria and activation of the executioner caspase-7. Similar to doxorubicin, exogenous sphingosine induced apoptosis in a caspase-7-dependent manner. Consistent with other studies,31,32,33,34,35 overexpression of Bcl-xL protected MCF7 cells from doxorubicin-induced apoptosis and rendered them resistant to sphingosine-induced apoptosis, even though Bcl-xL overexpression did not inhibit sphingosine generation after doxorubicin treatment. Despite the lack of caspase-3, MCF7 cells underwent apoptosis suggesting that other executioner caspases, such as caspase-7 or -2 may be activated. In this study, we established that caspase-7 is indeed activated during apoptosis of MCF7 cells. Furthermore, even in a cell-free caspase activation assay, caspase-2, which shares the same substrate specificity, was not detectably activated, in agreement with previous studies showing that caspase-2 was not processed in MCF7 cells following TNFα or staurosporine treatment.60 However, introduction of CASP-3 cDNA into MCF7 cells restored caspase-2 cleavage. To date, caspase-3-dependent caspase-2 activation has only been reported in Jurkat T cell extracts immunodepleted of caspase-3.46 Thus our results support the notion that activation of caspase-2 but not -7 depend on the presence of caspase-3.
Our observations establish that cytochrome _c_-inducible caspase-7 activation takes place in caspase-3 null MCF7 cells during apoptosis and raises the possibility that caspase-7 may be able to compensate for the lack of caspase-3, as overexpression of caspase-3 in MCF7 cells did not greatly enhance their apoptotic susceptibility (data not shown). Our results also demonstrate that activation of Fas/FasL signaling is not involved in apoptosis triggered by doxorubicin in MCF7 breast carcinoma cells. In addition, caspase-8, the first caspase to be processed in death receptor-mediated apoptosis,51 was only proteolyzed when cells were treated with anti-Fas mAb, whereas caspase-8 was not activated during doxorubicin-induced apoptosis.
Interestingly, we have found that the involvement of sphingolipid metabolites in cell death machinery triggered by doxorubicin may be more complex than previously thought. Accumulation of ceramide in anthracycline-mediated cell death has been shown to be due to activation of sphingomyelinase3 or ceramide synthase.4 In agreement, our results suggest that doxorubicin might in fact activate both pathways to mediate cell death, de novo sphingolipid synthesis4 and a sphingolipid degradation pathway mediated by a sphingomyelinase3 and a ceramidase. Unexpectedly, we found that cell death induced by exogenous sphingosine was inhibited by ceramide synthase or ceramidase inhibitors. These findings support the notion that exogenously added sphingosine can first be converted to ceramide, which can then be hydrolyzed to sphingosine by the action of a ceramidase, presumably in another compartment.
In sum, our results suggest that sphingosine, the metabolite of ceramide, is also involved in the doxorubicin-mediated, Bcl-xL inhibitable pathway of apoptosis in human breast cancer cells.
Materials and Methods
Cell culture and reagents
The MCF7/L cell variant was obtained from the Cell Culture Core Resource (Lombardi Cancer Center, Washington, DC, USA), and was maintained in RPMI 1640 containing 10% fetal bovine serum, 1% L-glutamine and 1% penicillin/streptomycin. MCF7 cells stably transfected to express the Fas antigen (denoted MCF7/Fas) or both Fas and Bcl-xL (denoted MCF7/Fas/Bcl-xL) were a kind gift of Dr. Vishva Dixit (Genentech Inc., San Francisco, CA, USA), and were grown in the same media supplemented with 200 μg/ml G418 and 150 μg/ml hygromycin. The MCF7/L/neo and MCF7/L/casp-3 cell lines were obtained by transfecting MCF7/L cells with either empty pcDNA3 or pcDNA3 engineered to express caspase-3, respectively (the CASP-3 expression vector was a kind gift of Dr. Donald Nicholson, Merck-Frosst Centre for Therapeutic Research, Pointe Claire-Dorval, Québec). These cells were propagated in the same media containing 1 mg/ml G418.
Cells were subcultured twice weekly and 48 h prior to the initiation of an experiment. For induction of apoptosis, cells at ⩽60–75% confluence were treated in serum-free media with either doxorubicin (Sigma), anti-Fas antibody (Upstate Biotechnology, Lake Placid, NY, USA) or sphingosine (Biomol, Plymouth Meeting, PA, USA). Jurkat T cells were cultured in RPMI 1640 containing 10% FBS.
Ac-DEVD-AMC was from Bachem (King of Prussia, PA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), horse heart cytochrome c and phosphocreatine were from Sigma. ATP, dATP and rabbit muscle creatine kinase were from Roche Diagnostics. [γ-32P]ATP (3000 Ci/mmol) was from NEN Life Science. FB1 and dMAPP were from Biomol (Plymouth Meeting, PA, USA). Other reagents and solvents were analytical grade.
Mass measurement of sphingosine
Sphingosine was measured after phosphorylation to sphingosine-1-phosphate, using recombinant sphingosine kinase as described by Olivera et al.61 For each experiment, known amounts of sphingosine were used to generate a standard curve. Total phospholipids mass in the lipid extracts was quantified as previously described62 using a colorimetric phosphate assay.63
Cell death assay
The tetrazolium based MTT assay was used to determine cell death as previously described.64 Briefly, approximately 5000 cells per well were plated in 96-well tissue culture plates as described above, and 24 h later, the medium was replaced with 100 μl serum-free medium containing the indicated amounts of doxorubicin, anti-Fas antibody, or sphingosine. After various times of incubation at 37°C, 25 μl of MTT solution (5 mg/ml) was added and the plates were incubated for approximately 4 h. After solubilization for 16 h with 100 μl of lysis buffer (20% SDS in 50% _N,N_-dimethylformamide), formazan was quantified with a microplate reader at a wavelength of 570 nm.
Preparation of mitochondria and Western blot analysis of cytochrome c
Mitochondrial preparations were carried out as previously described.65 In brief, cells were collected by centrifugation at 600×g for 5 min at 4°C. After washing once with ice-cold PBS, mitochondrial and cytosolic fractions were prepared by resuspending cell pellets in 5 vol of ice-cold buffer A (20 mM HEPES-KOH pH 7.5, 0.1% BSA, 1 mM sodium EDTA, 1 mM DTT, 0.1 mM PMSF, 20 μg/ml leupeptin, 10 μg/ml aprotinin and 10 μg/ml pepstatin A] containing 250 mM sucrose. After swelling on ice for 15 min, cells were homogenized with 15 to 20 strokes of a number 22 Kontes Dounce homogenizer with a B pestle (Kontes Glass Company, Vineland, NJ, USA), and the homogenates centrifuged at 750×g for 5 min at 4°C. Supernatants were then centrifuged at 10 000×g for 15 min at 4°C, and the resulting mitochondria pellets resuspended in cold buffer A. For Western blot analysis, equal amounts of mitochondrial and cytosolic proteins were separated on 15% SDS–PAGE and then transblotted to nitrocellulose. Anti-cytochrome c mAb (PharMingen) and anti-cytochrome oxidase subunit II mAb (Molecular Probes, Eugene, OR, USA) were used as primary antibodies.
Western blot analysis
Cell lysate preparation and Western blotting were carried out as previously described.65,66 Rabbit anti-caspase-3 (gift of Dr. Donald Nicholson, Merck-Frosst Centre for Therapeutic Research, Pointe Claire-Dorval, Québec), mouse anti-caspase-8 (PharMingen, San Diego, CA, USA), rabbit anti-caspase-7, rabbit anti-caspase-2 (Santa Cruz Biotechnology), rabbit anti-caspase-9 (Research Diagnostic, Flanders, NJ, USA or Oncogene Research), anti-PARP (PharMingen), and anti-FasL (Transduction Laboratories, Lexington, KY, USA) were used as primary antibodies. Proteins were visualized by ECL using anti-rabbit or anti-mouse HRP-conjugated IgG (Bio-Rad).
Fluorogenic DEVD cleavage enzyme assays
Enzyme reactions were performed in 96-well plates with 20 μg of cytosolic proteins and a final concentration of 20 μM Ac-DEVD-AMC substrate as previously described.65,66
In vitro assay for cytochrome **c**-dependent activation of caspases
Cytosolic fractions (40 μl, 400 μg proteins), prepared as described above, were incubated at 37°C for various times in 96-well plates in a final volume of 200 μl in buffer B (10 mM HEPES pH 7.4, 2 mM MgCl2, 5 mM sodium EGTA, 5 mM DTT, 1 mM ATP, 1 mM PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml pepstatin A, 10 mM phosphocreatine, 150 μg/ml creatine kinase) supplemented without or with 1 mM dATP and horse heart cytochrome c. Proteins were then separated by SDS–PAGE, blotted, and probed with anti-caspase and anti-PARP antibodies.
Abbreviations
Ac-DEVD-AMC:
acetyl-Asp-Glu-Val-Asp-aminomethylcoumarin
dMAPP:
D-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol
FB1:
fumonisin B1
PARP:
poly(ADP-ribose) polymerase
References
- Gewirtz DA . 1999 A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 57: 727–741
Article CAS PubMed Google Scholar - Hannun YA and Luberto C . 2000 Ceramide in the eukaryotic stress response. Trends Cell Biol. 10: 73–80
Article CAS PubMed Google Scholar - Jaffrézou J, Levade T, Bettaieb A, Andrieu N, Bezombes C, Maestre N, Vermeersch S, Rousse A and Laurent G . 1996 Daunorubicin-induced apoptosis: triggering of ceramide generation through sphingomyelin hydrolysis. EMBO J. 15: 2417–2424
Article PubMed PubMed Central Google Scholar - Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z and Kolesnick R . 1995 Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell 82: 405–414
Article CAS PubMed Google Scholar - Allouche M, Bettaieb A, Vindis C, Rousse A, Grignon C and Laurent G . 1997 Influence of Bcl-2 overexpression on the ceramide pathway in daunorubicin-induced apoptosis of leukemic cells. Oncogene 14: 1837–1845
Article CAS PubMed Google Scholar - Mansat V, Laurent G, Levade T, Bettaieb A and Jaffrézou JP . 1997 The protein kinase C activators phorbol esters and phosphatidylserine inhibit neutral sphingomyelinase activation, ceramide generation, and apoptosis triggered by daunorubicin. Cancer Res. 57: 5300–5304
CAS PubMed Google Scholar - Mansat V, Bettaieb A, Levade T, Laurent G and Jaffrézou JP . 1997 Serine protease inhibitors block neutral sphingomyelinase activation, ceramide generation, and apoptosis triggered by daunorubicin. FASEB J. 11: 695–702
Article CAS PubMed Google Scholar - Come MG, Battaieb A, Skladanowski A, Larsen AK and Laurent G . 1999 Alteration of the daunorubicin-triggered sphingomyelin-ceramide pathway and apoptosis in MDR cells: influence of drug transport abnormalities. Int. J. Cancer 81: 580–587
Article CAS PubMed Google Scholar - Bettaieb A, Plo I, Mas VMD, Quillet-Mary A, Levade T, Laurent G and Jaffrézou JP . 1999 Daunorubicin- and mitoxantrone-triggered phosphatidylcholine hydrolysis: implication in drug-induced ceramide generation and apoptosis. Mol. Pharmacol. 55: 118–125
Article CAS PubMed Google Scholar - Andrieu-Abadie N, Jaffrézou JP, Hatem S, Laurent G, Levade T and Mercadier JJ . 1999 L-carnitine prevents doxorubicin-induced apoptosis of cardiac myocytes: role of inhibition of ceramide generation. FASEB J. 13: 1501–1510
Article CAS PubMed Google Scholar - Delpy E, Hatem SN, Andrieu N, de Vaumas C, Henaff M, Rucker-Martin C, Jaffrézou JP, Laurent G, Levade T and Mercadier JJ . 1999 Doxorubicin induces slow ceramide accumulation and late apoptosis in cultured adult rat ventricular myocytes. Cardiovasc. Res. 43: 398–407
Article CAS PubMed Google Scholar - Ohta H, Yatomi Y, Sweeney EA, Hakomori S and Igarashi Y . 1994 A possible role of sphingosine in induction of apoptosis by tumor necrosis factor-alpha in human neutrophils. FEBS Lett. 355: 267–270
Article CAS PubMed Google Scholar - Krown KA, Page MT, Nguyen C, Zechner D, Gutierrez V, Comstock KL, Glembotski CC, Quintana PJ and Sabbadini RA . 1996 Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J. Clin. Invest. 98: 2854–2865
Article CAS PubMed PubMed Central Google Scholar - Shirahama T, Sweeney EA, Sakakura C, Singhal AK, Nishiyama K, Akiyama S, Hakomori S and Igarashi Y . 1997 In vitro and in vivo induction of apoptosis by sphingosine and N,N-dimethylsphingosine in human epidermoid carcinoma KB-3-1 and its multidrug-resistant cells. Clin. Cancer Res. 3: 257–264
CAS PubMed Google Scholar - Sweeney EA, Sakahura C, Shirahama T, Masamune A, Ohta H, Hakomori S and Igarashi Y . 1996 Sphingosine and its methylated derivative N,N-dimethylsphingosine (DMS) induce apoptosis in a variety of human cancer cell lines. Int. J. Cancer 66: 358–366
Article CAS PubMed Google Scholar - Sweeney EA, Inokuchi J and Igarashi Y . 1998 Inhibition of sphingolipid induced apoptosis by caspase inhibitors indicates that sphingosine acts in an earlier part of the apoptotic pathway than ceramide. FEBS Lett. 425: 61–65
Article CAS PubMed Google Scholar - Hung WC, Chang HC and Chuang LY . 1999 Activation of caspase-3-like proteases in apoptosis induced by sphingosine and other long-chain bases in Hep3B hepatoma cells. Biochem. J. 338: 161–166
Article CAS PubMed PubMed Central Google Scholar - Jarvis WD, Fornari FA, Traylor RS, Martin HA, Kramer LB, Erukulla RK, Bittman R and Grant S . 1996 Induction of apoptosis and potentiation of ceramide-mediated cytotoxicity by sphingoid bases in human myeloid leukemia cells. J. Biol. Chem. 271: 8275–8284
Article CAS PubMed Google Scholar - Cuvillier O, Edsall L and Spiegel S . 2000 Involvement of sphingosine in mitochondria-dependent Fas-induced apoptosis of type II Jurkat T cells. J. Biol. Chem. 275: 15691–15700
Article CAS PubMed Google Scholar - Ravid A, Rocker D, Machlenkin A, Rotem C, Hochman A, Kessler-Icekson G, Liberman UA and Koren R . 1999 1,25-Dihydroxyvitamin D3 enhances the susceptibility of breast cancer cells to doxorubicin-induced oxidative damage. Cancer Res. 59: 862–867
CAS PubMed Google Scholar - Smith ER, Jones PL, Boss JM and Merrill Jr AH . 1997 Changing J774A.1 cells to new medium perturbs multiple signaling pathways, including the modulation of protein kinase C by endogenous sphingoid bases. J. Biol. Chem. 272: 5640–5646
Article CAS PubMed Google Scholar - Smith ER and Merrill Jr AH . 1995 Differential roles of de novo sphingolipid biosynthesis and turnover in the ‘burst’ of free sphingosine and sphinganine, and their 1-phosphates and N-acyl-derivatives, that occurs upon changing the medium of cells in culture. J. Biol. Chem. 270: 18749–18758
Article CAS PubMed Google Scholar - Ohta H, Sweeney EA, Masamune A, Yatomi Y, Hakomori S and Igarashi Y . 1995 Induction of apoptosis by sphingosine in human leukemic HL-60 cells: a possible endogenous modulator of apoptotic DNA fragmentation occurring during phorbol ester-induced differentiation. Cancer Res. 55: 691–697
CAS PubMed Google Scholar - Shirahama T, Sakakura C, Sweeney EA, Ozawa M, Takemoto M, Nishiyama K, Ohi Y and Igarashi Y . 1997 Sphingosine induces apoptosis in androgen-independent human prostatic carcinoma DU-145 cells by suppression of bcl-X(L) gene expression. FEBS Lett. 407: 97–100
Article CAS PubMed Google Scholar - Merrill Jr AH, van Echten G, Wang E and Sandhoff K . 1993 Fumonisin B1 inhibits sphingosine (sphinganine) N-acyltransferase and de novo sphingolipid biosynthesis in cultured neurons in situ. J. Biol. Chem. 268: 27299–27306
CAS Google Scholar - Bielawska A, Greenberg MS, Perry D, Jayadev S, Shayman JA, McKay C and Hannun YA . 1996 (1S,2R)-D-erythro-2-(N-Myristoylamino)-1-phenyl-1-propanol as an inhibitor of ceramidase. J. Biol. Chem. 271: 12646–12654
Article CAS PubMed Google Scholar - Zhang J, Alter N, Reed JC, Borner C, Obeid LM and Hannun YA . 1996 Bcl-2 interrupts the ceramide-mediated pathway of cell death. Proc. Natl. Acad. Sci. USA 93: 5325–5328
Article CAS PubMed PubMed Central Google Scholar - Geley S, Hartmann BL and Kofler R . 1997 Ceramides induce a form of apoptosis in human acute lymphoblastic leukemia cells that is inhibited by Bcl-2, but not by CrmA. FEBS Lett. 400: 15–18
Article CAS PubMed Google Scholar - Smyth MJ, Perry DK, Zhang J, Poirier GG, Hannun YA and Obeid LM . 1996 prICE: a downstream target for ceramide-induced apoptosis and for the inhibitory action of Bcl-2. Biochem. J. 316: 25–28
Article CAS PubMed PubMed Central Google Scholar - Dbaibo GS, Perry DK, Gamard CJ, Platt R, Poirier GG, Obeid LM and Hannun YA . 1997 Cytokine response modifier A (CrmA) inhibits ceramide formation in response to tumor necrosis factor (TNF)-α: CrmA and Bcl-2 target distinct components in the apoptotic pathway. J. Exp. Med. 185: 481–490
Article CAS PubMed PubMed Central Google Scholar - Ohmori T, Podack ER, Nishio K, Takahashi M, Miyahara Y, Takeda Y, Kubota N, Funayama Y, Ogasawara H, Ohira T et al.1993 Apoptosis of lung cancer cells caused by some anti-cancer agents (MMC, CPT-11, ADM) is inhibited by bcl-2. Biochem. Biophys. Res. Commun. 192: 30–36
Article CAS PubMed Google Scholar - Fulda S, Susin SA, Kroemer G and Debatin KM . 1998 Molecular ordering of apoptosis induced by anticancer drugs in neuroblastoma cells. Cancer Res. 58: 4453–4460
CAS PubMed Google Scholar - Ruvolo PP, Deng X, Carr BK and May WS . 1998 A functional role for mitochondrial protein kinase Calpha in Bcl2 phosphorylation and suppression of apoptosis. J. Biol. Chem. 273: 25436–25442
Article CAS PubMed Google Scholar - Naumovski L, Martinovsky G, Wong C, Chang M, Ravendranath Y, Weinstein H and Dahl G . 1998 BCL-2 expression does not not correlate with patient outcome in pediatric acute myelogenous leukemia. Leuk. Res. 22: 81–87
Article CAS PubMed Google Scholar - Katoh O, Takahashi T, Oguri T, Kuramoto K, Mihara K, Kobayashi M, Hirata S and Watanabe H . 1998 Vascular endothelial growth factor inhibits apoptotic death in hematopoietic cells after exposure to chemotherapeutic drugs by inducing MCL1 acting as an antiapoptotic factor. Cancer Res. 58: 5565–5569
CAS PubMed Google Scholar - Nicholson DW . 1999 Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 6: 1028–1042
Article CAS PubMed Google Scholar - Srinivasan A, Li F, Wong A, Kodandapani L, Smidt Jr R, Krebs JF, Fritz LC, Wu JC and Tomaselli KJ . 1998 Bcl-xL functions downstream of caspase-8 to inhibit Fas- and Tumor Necrosis Factor Receptor 1-induced apoptosis of MCF7 breast carcinoma Cells. J. Biol. Chem. 273: 4523–4529
Article CAS PubMed Google Scholar - Jäättelä M, Benedict M, Tewari M, Shayman JA and Dixit VM . 1995 Bcl-x and Bcl-2 inhibit TNF and Fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene 10: 2297–2305
PubMed Google Scholar - Li F, Srinivasan A, Wang Y, Armstrong RC, Tomaselli KJ and Fritz LC . 1997 Cell-specific induction of apoptosis by microinjection of cytochrome c. J. Biol. Chem. 272: 30299–30305
Article CAS PubMed Google Scholar - Medema JP, Scaffidi C, Krammer PH and Peter ME . 1998 Bcl-xL acts downstream of caspase-8 activation by the CD95 death-inducing signaling complex. J. Biol. Chem. 273: 3388–3393
Article CAS PubMed Google Scholar - Janicke RU, Sprengart ML, Wati MR and Porter AG . 1998 Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 273: 9357–9360
Article CAS PubMed Google Scholar - Cecconi F . 1999 Apafl and the apoptotic machinery. Cell Death Differ. 6: 1087–1098
Article CAS PubMed Google Scholar - Slee EA, Adrain C and Martin SJ . 1999 Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ. 6: 1067–1074
Article CAS PubMed Google Scholar - Budihardjo I, Oliver H, Lutter M, Luo X and Wang X . 1999 Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15: 269–290
Article CAS PubMed Google Scholar - Wolf BB, Schuler M, Echeverri F and Green DR . 1999 Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J. Biol. Chem. 274: 30651–30656
Article CAS PubMed Google Scholar - Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR and Martin SJ . 1999 Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9- dependent manner. J. Cell. Biol. 144: 281–292
Article CAS PubMed PubMed Central Google Scholar - Hampton MB, Zhivotovsky B, Slater AF, Burgess DH and Orrenius S . 1998 Importance of the redox state of cytochrome c during caspase activation in cytosolic extracts. Biochem. J. 329: 95–99
Article CAS PubMed PubMed Central Google Scholar - Herr I, Wilhelm D, Bohler T, Angel P and Debatin K . 1997 Activation of CD95 (APO-1/Fas) signaling by ceramide mediates cancer therapy-induced apoptosis. EMBO J. 16: 6200–6208
Article CAS PubMed PubMed Central Google Scholar - Friesen C, Fulda S and Debatin KM . 1997 Deficient activation of the CD95 (APO-1/Fas) system in drug-resistant cells. Leukemia 11: 1833–1841
Article CAS PubMed Google Scholar - Fulda S, Scaffidi C, Pietsch T, Krammer PH, Peter ME and Debatin K-M . 1998 Activation of the CD-95 (APO-1/Fas) pathway in drug- and γ-irradiation-induced apoptosis of brain tumor cells. Cell Death Differ. 5: 884–893
Article CAS PubMed Google Scholar - Peter ME and Krammer PH . 1998 Mechanisms of CD95 (APO-1/Fas)-mediated apoptosis. Curr. Opin. Immunol. 10: 545–551
Article CAS PubMed Google Scholar - Li H, Zhu H, Xu CJ and Yuan J . 1998 Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501
Article CAS PubMed Google Scholar - Lavie Y, Cao H, Bursten SL, Giuliano AE and Cabot MC . 1996 Accumulation of glucosylceramides in multidrug-resistant cancer cells. J. Biol. Chem. 271: 19530–19536
Article CAS PubMed Google Scholar - Liu YY, Han TY, Giuliano AE and Cabot MC . 1999 Expression of glucosylceramide synthase, converting ceramide to glucosylceramide, confers adriamycin resistance in human breast cancer cells. J. Biol. Chem. 274: 1140–1146
Article CAS PubMed Google Scholar - Liu YY, Han TY, Giuliano AE, Hansen N and Cabot MC . 2000 Uncoupling ceramide glycosylation by transfection of glucosylceramide synthase antisenser reverses adriamycin resistance. J. Biol. Chem. 275: 7138–7143
Article CAS PubMed Google Scholar - Bezombes C, Maestre N, Laurent G, Levade T, Bettaieb A and Jaffrézou JP . 1998 Restoration of TNF-alpha-induced ceramide generation and apoptosis in resistant human leukemia KG1a cells by the P-glycoprotein blocker PSC833. FASEB J. 12: 101–109
Article CAS PubMed Google Scholar - Modrak DE, Lew W, Goldenberg DM and Blumenthal R . 2000 Sphingomyelin potentiates chemotherapy of human cancer xenografts. Biochem. Biophys. Res. Commun. 268: 603–606
Article CAS PubMed Google Scholar - Sachs CW, Safa AR, Harrison SD and Fine RL . 1995 Partial inhibition of multidrug resistance by safingol is independent of modulation of P-glycoprotein substrate activities and correlated with inhibition of protein kinase C. J. Biol. Chem. 270: 26639–26648
Article CAS PubMed Google Scholar - Schwartz GK, Ward D, Saltz L, Casper ES, Spiess T, Mullen E, Woodworth J, Venuti R, Zervos P, Storniolo AM and Kelsen DP . 1997 A pilot clinical/pharmacological study of the protein kinase C-specific inhibitor safingol alone and in combination with doxorubicin. Clin. Cancer Res. 3: 537–543
CAS PubMed Google Scholar - Janicke RU, Ng P, Sprengart ML and Porter AG . 1998 Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J. Biol. Chem. 273: 15540–15545
Article CAS PubMed Google Scholar - Olivera A, Rosenthal J and Spiegel S . 1994 Sphingosine kinase from Swiss 3T3 fibroblasts: a convenient assay for the measurement of intracellular levels of free sphingoid bases. Anal. Biochem. 223: 306–312
Article CAS PubMed Google Scholar - Edsall LC, Pirianov GG and Spiegel S . 1997 Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J. Neurosci. 17: 6952–6960
Article CAS PubMed PubMed Central Google Scholar - van Veldhoven PP and Mannaerts GP . 1987 Inorganic and organic phosphate measurements in the nanomolar range. Anal. Biochem. 161: 45–48
Article CAS PubMed Google Scholar - Cuvillier O, Pirianov G, Kleuser B, Vanek PJ, Coso OA, Gutkind JS and Spiegel S . 1996 Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381: 800–803
Article CAS PubMed Google Scholar - Cuvillier O, Mayhew E, Janoff AS and Spiegel S . 1999 Induction of a cytochrome c-mediated apoptosis in liposome-associated ether lipid ET-18-O-CH3 . Blood 94: 3583–3592
CAS PubMed Google Scholar - Cuvillier O, Rosenthal DS, Smulson ME and Spiegel S . 1998 Sphingosine 1-phosphate inhibits activation of caspases that cleave poly(ADP-ribose) polymerase and lamins during Fas- and ceramide-mediated apoptosis in Jurkat T lymphocytes. J. Biol. Chem. 273: 2910–2916
Article CAS PubMed Google Scholar
Acknowledgements
This work was supported by a grant from the National Institutes of Health (CA61774) to S Spiegel. Financial support from Inserm (to O Cuvillier and T Levade) and the Association pour la Recherche sur le Cancer (to O Cuvillier) is gratefully acknowledged.
Author information
Authors and Affiliations
- Department of Biochemistry and Molecular Biology, Georgetown University Medical Center, 3900 Reservoir Road NW, DC 20007, Washington, USA
O Cuvillier, V E Nava, S K Murthy & S Spiegel - Unité Inserm 466, CHU Rangueil, 1 avenue Jean Poulhès, Toulouse, 31403, France
O Cuvillier & T Levade - Laboratory of Cellular and Molecular Regulation, NIMH, Bethesda, 20892, MD, USA
L C Edsall & S Milstien
Authors
- O Cuvillier
You can also search for this author inPubMed Google Scholar - V E Nava
You can also search for this author inPubMed Google Scholar - S K Murthy
You can also search for this author inPubMed Google Scholar - L C Edsall
You can also search for this author inPubMed Google Scholar - T Levade
You can also search for this author inPubMed Google Scholar - S Milstien
You can also search for this author inPubMed Google Scholar - S Spiegel
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toS Spiegel.
Additional information
Edited by C Thiele
Rights and permissions
About this article
Cite this article
Cuvillier, O., Nava, V., Murthy, S. et al. Sphingosine generation, cytochrome c release, and activation of caspase-7 in doxorubicin-induced apoptosis of MCF7 breast adenocarcinoma cells.Cell Death Differ 8, 162–171 (2001). https://doi.org/10.1038/sj.cdd.4400793
- Received: 09 May 2000
- Revised: 02 August 2000
- Accepted: 20 September 2000
- Published: 17 April 2001
- Issue Date: 01 February 2001
- DOI: https://doi.org/10.1038/sj.cdd.4400793