The role of nitric oxide in cancer (original) (raw)
Over the past decade or so, it has become evident that the free radical gas nitric oxide (NO) acts as a novel transcellular messenger molecule in many key physiological and pathological processes1. NO plays a central role in the cardiovascular system as the endothelium - derived relaxing factor2, 3, 4, 5. Within the central nervous system, NO is a crucial component of the signal transduction pathways used for memory formation, sensory processing, and the regulation of cerebral blood flow6. Interestingly, as early as 1982, NO was implicated in the immuno-defence network, as marked increase in urinary NO3− excretion was observed in human subjects with diarrhoea and fever7, 8. Further work showed that the blood levels and urinary excretion of NO3− increased after exposure to Escherichia coli lipopolysaccharide (LPS) in LPS-sensitive mice and that activated mouse peritoneal macrophages showed increased NO2− and NO3− production in vitro9. The mammalian immuno-defense network is involved in tumour suppression, and macrophages are an important part of this process because of their ability to destroy selectively a broad range of tumour types upon specific activation. The role of NO in macrophage cytotoxicity was first described by Hibbs and colleagues in 198710, and since that time numerous studies have shown that cytokine activated rodent macrophages can generate large concentrations of NO by up-regulation of expression of the inducible nitric oxide synthase gene (iNOS)11. The NO generated by this process is capable of killing a range of tumour cells of differing origin and grade11, 12, 13, 14, 15. Various direct and indirect mechanisms have been proposed for the anti-tumour properties of NO. Mechanisms include direct damage of DNA, inhibition of DNA synthesis and inhibition of the rate-limiting enzyme ribonucleotide reductase. Reduced activity of cis-aconitase and loss of a large fraction of the iron pool, have also been suggested as possible mechanisms. Importantly, NO-generation can efect mitochondrial physiology leading to reduction of O2 consumption and damage to complexes I and II in the mitochondrial electron transport chain, reversible inhibition of complex IV activity and induction of apoptosis10, 11, 12, 13, 14, 15.
Importantly, various studies have shown that all three isoforms of NOS, (iNOS, eNOS and nNOS), have been detected in tumour cells from a wide range of isolates16, 17, 18. NOS activity has been observed in human tumour cell lines and cells from tumour biopsies. However, the precise function(s) of NO in tumour biology remains unclear, and several lines of research have indicated that NO may have dual effects in cancer. In this review, we will present some recent evidence on both the pro- and anti-tumour activities of NO and discuss the implications of these data on the use of NO as a therapeutic agent for the treatment of cancer.
Nitric oxide generation and its biological targets
NO is a diatomic free radical molecule, and is a gas at room temperature. Within mammalian cells a family of NOS enzymes has been shown to be able to generate NO, and all family members require a panel of substrates and co-factors to be fully functional. For example, the NO-generating reaction requires L-arginine, NADPH and oxygen as substrates, and tetrahydrobiopterin (BH4), thiol, flavin adenine dinucleotide (FAD), and flavin mononucleotide (FMN) as cofactors. In addition to NO, the NOS-catalysed reaction produces citrulline and NADP as co-products.
Three different isoforms of the NOS family have been identified; endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS). The gene symbol nomenclatures are: NOS1 for nNOS, NOS2 for iNOS and NOS3 for eNOS6. The nNOS and eNOS isoforms are constitutively expressed in a variety of cell types including the endothelium, platelets, and neurons. Typically, the constitutive NOS isoforms can be activated as a result of calmodulin (CaM) binding following a rise in intracellular calcium. They may also be activated and/or inhibited by phosphorylation via various protein kinases. Unlike nNOS and eNOS, iNOS displays a high affinity for CaM, which is tightly bound within physiological concentrations of calcium. As a consequence of this, the activation of iNOS is not calcium-dependent. However, the expression of iNOS can be transcriptionally regulated by factors such as cytokines (e.g. interferon-γ (IFN-γ), interleukin-1β (IL-1β) and tumour necrosis factor-α (TNF-α), bacterial endotoxin (LPS) and oxidative stress (e.g. under conditions encountered during hypoxia).
A good starting point to assess the function of NO in mammalian physiology is to examine its chemical properties. NO is a gas at room and body temperature, making it highly diffusible within the vasculature. As NO is a free radical, it is a highly reactive molecule within biological systems, reacting with other free radicals, molecular oxygen and heavy metals. It has been suggested that the biological effects of NO can be mediated by the products of different NO metabolites. For example, NO can react rapidly in the intracellular environment to form nitrite and nitrate, S-nitroso-thiols or peroxynitrite. These metabolites may play a key role in mediating many of the key genotoxic effects, (such as DNA damage), that are associated with the generation of NO. Importantly, NO has been shown to bind rapidly, and with high affinity, to ferrous iron (Fe2+). As a consequence of this, NO can bind easily to free iron, iron within iron-sulphur centres, and iron within haemoproteins (especially when the haem contains a free ligand position). Many of the biological processes described for NO (involving smooth muscle relaxation, neurotransmission, and inhibition of platelet aggregation and adhesion) results from NO binding to the ferrous haem iron of the enzyme soluble guanylate cyclase (sGC), which in turn results in an increase in cGMP production. Due its association with haem centres it is not surprising that the binding of NO to haemoglobin is regarded as a significant route by which NO can be broken down in the body.
NO can cause DNA damage via the generation of peroxynitrite (ONOO-) and N2O3. Peroxynitrite can oxidise and nitrate DNA and may potentially cause single-strand DNA breaks through attack on the sugar-phosphate backbone. N2O3 can nitrosate amines to form N-nitrosamines, then alkylate DNA. Nitrosation of primary amines, (e.g. in DNA bases) leads to the formation of diazonium ions and subsequent deamination and DNA-crosslinks. The wide range of differing biological effects arising from exposure to NO is very much dependent upon many factors, such as formation and metabolism of NO, the type of NOS enzymes that are present, the interaction between NO utilising processes, and crucially the concentration of NO that is present in the given system.
Nitric oxide synthase expression in tumours
The iNOS isoform has been mostly studied for its role in immuno-mediated processes, for example, iNOS knock-out mice have been generated and shown to have increased susceptibility to infections18. The first NOS isoform implicated in the macrophage-mediated tumour killing process was also iNOS, and as a consequence this isoform has been at the center of attention for study of its expression in cancer. In normal (non-tumourogenic) cells, iNOS has been detected in macrophages and neutrophils, as well as in hepatocytes, cardiac myocytes, chondrocytes and many other cell types. Recently, lack of iNOS in knock-out mice has been found to promote intestinal tumorigenesis in the Apc(Min/+) colon cancer mouse model, thereby substantiating the role of iNOS within host defence mechanisms19.
An initial study on iNOS expression in human breast cancer suggested that iNOS activity was higher in less differentiated tumours in a panel of 15 invasive breast carcinomas17. iNOS expression could be detected predominantly in peritumoural and intratumoural macrophages. NO biosynthesis was significantly greater for grade III tumours as compared with grade II in specimens from 10 breast cancer tissues. Recently, three relatively large scale studies20, 21, 22, suggested that iNOS is not only expressed in stromal cells and macrophages in the tumour, but also in tumour cells themselves (Tab 1). Reveneau et al reported NOS activity in 27 of 40 tumours studied20. Vakkala et al showed that carcinomas with both iNOS positive tumour and stromal cells had a higher apoptotic index and a higher calculated microvessel density index21. Loibl et al further demonstrated that while none of the benign lesions were positive for iNOS, 67% in situ carcinomas and 61% invasive lesions showed iNOS tumour cell staining. eNOS expression was found in 33 invasive lesions (61%)22. Both iNOS expressing lesions and eNOS expressing lesions showed strong co-expression (p=0.0008).
Table 1 Incidence of iNOS expression in benign, in situ and invasive breast lesions
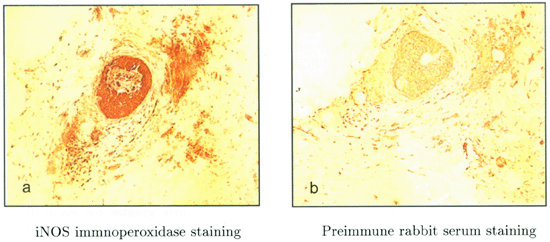
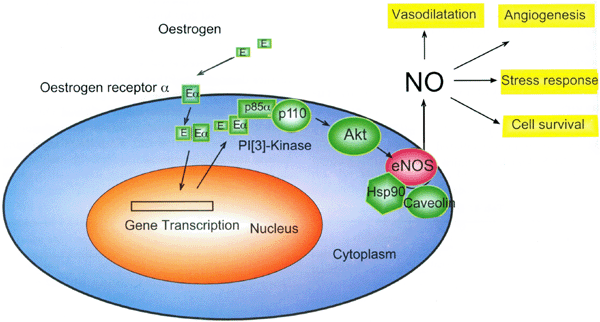
In our recent studies, we have also found strong coexpression of iNOS and eNOS in in situ ductal carcinomas23. Using an antibody raised against a C-terminal peptide of iNOS we demonstrate that there is considerable iNOS expression, not only in the surrounding stroma, but also within the in situ ductal carcinoma itself (Fig 1a) as compared with preimmune rabbit serum control (Fig 1b). Interestingly, in consecutive sections, strong immunopositivity can also be detected with an-eNOS antibody, mainly in the stromal cells, but also in some tumour cells (data not shown). These findings are similar to those reported by Vakkala et al24 who showed that eNOS was expressed in 65% of samples (80 cases in total) with immunopositivity seen both in stromal structures and carcinoma cells24. Interestingly, Martin et al recently reported a significant positive correlation between the percentage of tumour cells with eNOS expression and oestrogen receptors25. We have also observed that some eNOS positive in situ ductal carcinomas show positive staining with antibody to estrogen receptors (Xu et al unpublished data). Recently several studies have shown that the oestrogen/oestrogen receptor complex binds to the p85a regulatory subunit of phosphatidylinositol-3-OH kinase (PI-(3)K), which leads to activation of protein kinase B/Akt 26, 27. It is known that the catalytic activity of eNOS is augmented by phosphorylation of a C-terminal serine residue (Ser-1177 of human eNOS) through the PI-(3)K/Akt pathway28,29. Therefore, it is possible that oestrogen acting on the oestrogen receptor (ERσ) located on the surface of cell membranes, could indirectly activate the release of NO from membrane bound eNOS (Fig 2). It is possible that the NO generated from this activation of eNOS may contribute significantly to tumour cell survival under hypoxia and other stress conditions. It should be noted that there are complicated signalling network(s) in endothelial cells capable of regulating eNOS activity. For example, the Akt-mediated phosphorylation activity can be enhanced by binding to heat shock protein-9030 and inhibited by binding to Caveolin-131 [Fig 2].
Figure 1
(a) Section of human in situ ductal carcinomas (frozen sections) immnuocytochemically stained for human iNOS C-terminal peptide antibody50. (b) is a control which has been stained using preimmune rabbit serum. Solid arrow indicates in situ carcinoma. Dashed arrow indicates surrounding stroma cells. ×100
Figure 2
Schematic presentation of pathway mediating indirect activation of eNOS by oestrogen.
In addition to breast cancer, iNOS has also been shown to be markedly expressed in approximately 60% of human adenomas and in 20-25% of colon carcinomas, while expression was either low or absent in the surrounding normal tissues32, 33. In human ovarian cancer, iNOS activity has been localized in tumour cells and not found in normal tissue16. Other tumours that have demonstrated iNOS gene expression are brain134, head and neck35, esophagus36, lung37, prostate38, bladder39, pancreatic40, and Kaposi's sarcoma41.
In the central nerve system, NO has a variety of biological functions including vasorelaxation and neurotransmision. Interestingly, nNOS has been detected in some oligodendroglioma and neuroblastoma cell lines, althouth further studies are needed to clarified the role of nNOS in tumour pathology34.
NO and tumour cell angiogenesis
While NO had been shown to have anti-tumour properties10, Jenkins et al42[1995] first reported the surprising finding that human carcinoma cells transfected with a murine iNOS cDNA cassette (DLD-1 cells generating 20 pmol min-1 mg-1 NOS activity) showed increased tumour growth, rather than decreased growth. Using a nude mouse/xanograft model it was shown that growth of these NO-generating tumours was accompanied by increased neovascularization. These results were supported by Ambs et al, who used recombinant iNOS expressing Calu-6 and HT-29 human carcinoma cell lines containing mutant p5343 to look at tumour growth. The authors demonstrated that an NO-mediated up-regulation of VEGF corresponded with increased vascularisation in the xenograft tumours. Therefore it is possible that NO generated by NOS (located either within the tumour or in the surrounding stroma) may promote new blood vessel formation by up-regulating VEGF. This neovasculaturization not only enhances the ability of the tumour to grow, but also increases its invasiveness and metastatic ability.
NO, p53, PARP and DNA-PKcs in DNA repair
As NO is a free radical, it is a highly reactive molecule within biological systems, capable of interaction with other free radicals, molecular oxygen and heavy metals. The biological effects of NO can be mediated by the products of different NO metabolites. For example, NO rapidly reacts intracellularly to form nitrite and nitrate, S-nitroso-thiols or peroxynitrate, and these metabolites are believed to play key roles in mediating many of the NO-associated genotoxic effects. These effects include DNA damage, which can be initiated by nitrosative deamination, DNA strand breakage or DNA modification44.
One of the consequences of the NO- mediated DNA damage is to trigger p53 accumulation, which can induce apoptosis. This is a possible process by which NO may induce death of tumour cells. An increase in NOS activity (arising from increased transcriptional activity, or from post-transcriptional/protein regulation activity) in tumour cells can consequently cause the concentration of NO to be elevated such that it triggers p53-mediated growth arrest and apoptosis45,46. Interestingly, it has been demonstrated that accumulation of p53 results ultimately in down-regulation of iNOS expression by inhibition of iNOS promoter activity47. Thus a negative feedback loop is formed between NO-generation and p53 accumulation, that may constitute part of a physiological mechanism, which responds to endogenously produced DNA damage due to NO. Overall, this p53-mediated growth inhibition may be expected to provide a strong selection pressure for mutant p53 expression in tumor cells.
In addition to p53, NO has also been shown to activate poly (ADP-ribose) polymerase (PARP)48 and it has been proposed that this activation is due to DNA damage. This damage may take the form of DNA strand breaks or nitrosative deamination of DNA bases when NO is generated at high concentrations. These high concentrations of NO have been reported for NMDA-mediated neurotoxicity as well as for tumouricidal and bactericidal activation of cells44. Another important DNA repair enzyme, DNA-dependent protein kinase (DNA-PK), is also known to be essential for the maintenance of the structural integrity of the genome. DNA-PK is a serine/threonine protein kinase consisting of a large catalytic subunit (DNA-PKcs) and a regulatory subunit (Ku). Recently, mammalian DNA-PKcs has been shown to be an essential component of the DNA double-strand repair pathway, as well as being crucial for V(D)J recombination, involved in the generation of immunoglobulin and T-cell diversity. Scid mice, which lack DNA-PKcs, show increased susceptibility to ionising radiation in addition to having impaired V(D)J recombination and arrested T- and B-cell development49. Interestingly, although DNA-PK activity cannot be up-regulated by strong doses of radiation, we found that NO can act a signal, increasing the activity of DNA-PK. Importantly, we showed that this increase occurred by transcriptional up-regulation of DNA-PKcs expression and occurred under physiologically relevant ranges of NO concentrations50. Biologically, this NO-mediated increase in enzymatically active DNA-PK not only protected cells from the toxic effects of NO, but also provided cross-protection against clinically important DNA-damaging agents, such as X-ray radiation, adriamycin, bleomycin and cisplatin50.
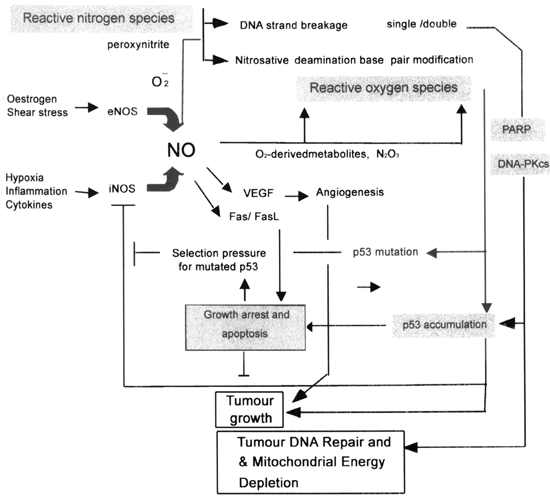
The NO-mediated increase in DNA-PKcs pathway not only plays an important role in tumour DNA repair51, but may also play an important role in other tissue damage processes which involve NO-mediated stress52,53. For example, failing myocardium, (advanced heart failure due to idiopathic dilated cardiomyopathy) undergoes active DNA repair, where DNA-PKcs expression is strongly correlated with iNOS expression (r=0.53, p < 0.01)53. Given the fact that one of the major substrates of DNA-PKcs is p5354 and DNA-PKcs itself is subjected to ADP-ribosylation by PARP, it is possible that NO-mediated DNA damage and repair could play a significant role in tumour development (Fig 3).
Figure 3
A representation of the dual action of nitric oxide on tumour growth
NO as a therapeutic agent for the treatment of cancer
NO-based therapeutics can be traced back for more than a centrury when Willaim Murell proposed the sublingual application of nitroglycerin as a remedy for angina pectoris55. From the time of discovery of the vasodilatory properties of the organic nitrates and nitrites, it took more than hundred years to elucidate their mode of action at the molecular level. For example, it was not until 1987 that NO gas was identified both as the endogenous endothelium-derived relaxing factor3, 4, 5, and as being involved as a primary defence mechanism against tumour cells and intracellular microorganisms10.
Several laboratories have demonstarted that NO-releasing agents can kill tumour cells, and as a consequence there have been attempts deliver NO to cells. While NO-releasing drugs are under developement, an attractive alternative mechanism for delivery would be to transfer NOS- encoding cDNA sequences into cancer cells for gene therapy purposes. Several studies have shown that this approach may work. For example, using a mouse model it was demonstarted that transfection of K-1735 melanoma cells with an iNOS cDNA expression cassette suppressed tumourogenicity and abrogated metastasis14. Transfection of human renal carcinoma cells with a retroviral iNOS cassette showed similar results15. A problem with current approaches however is that constitutive expression of NOS can quickly result in death of the transfectant, shortening the time that NO can be generated, and potentially limiting the utility of the approach. NOS transfectants often have to be cultured under conditions that reduce toxicity (for example in the presence of a NOS inhibitor), and transfection attempts may result in cells that are capable of relatively low levels of NO-generation43. As discussed above, this may result in concentrations of NO that promote tumour growth rather than cell killing. Another significant point is that NOS enzyme activity requires a panel of substrates and co-factors for full activity, and these may be missing from the target cell type. For example synthesis of the important co-factor tetrahydrobiopterin (BH4), requires transcriptional regulation of the rate- limiting enzyme GTP-cyclohydrolase, which may not be induced in all target cells56. Lastly, both retroviral and adenoviral vector maybe hazardous to the host and pose a major health and safety risk57.
A potential strategy to overcome the problems associated with gene therapy is to use a cell-based approach. Cell-based approaches utilise the delivery of recombinant cells (rather than genes) to the target site, with the advantage that the expression of the gene of interest can be optimised prior to delivery. For example, we have recently shown the utility of two novel iNOS-expressing human cell lines that can generate high concentrations of NO following treatment with analogues of either the insect hormone ecdysone or tetracycline50,58. In order to make the NO- generating cells suitable for therapeutic delivery they have been encapsulated within a semipermeable alginate-poly-L-lysine membrane. Encapsulated cells are protected from environmental stresses encountered in the host (such as the host immune response) and can be delivered to tumour site(s) in a nude mouse model58,59. Following delivery, high concentrations of NO and reactive nitrogen species can be generated by administration of the appropriate inducer. This approach has been very successful, and we have used it in a tumour model showing 100% killing of SKOV-3 tumours and 54% killing of DLD-1 tumours58. Importantly, this strategy allowed the mechanism of tumour killing to be determined as it was shown that tumour killing was associated with concomitant up-regulation of the Fas/FasL proteins. Overall we believe that the cell-delivery approach addresses some of the shortcomings of competing strategies and has the potential to inhibit or kill many different types of tumours from various histological origins60.