Cocaine Dependence and D2 Receptor Availability in the Functional Subdivisions of the Striatum: Relationship with Cocaine-Seeking Behavior (original) (raw)
INTRODUCTION
Over the last decade, imaging studies using positron emission tomography (PET) have suggested that alterations in the density of striatal dopamine (DA) D2 receptors might be involved in the initiation or maintenance of cocaine abuse and dependence. First, three studies from one laboratory have observed reduced striatal D2 receptor availability in detoxified cocaine-dependent subjects compared to healthy subjects (Volkow et al, 1990, 1993, 1997). Second, studies from the same laboratory reported that, in healthy subjects, low striatal D2 receptor availability was predictive of a pleasurable experience following administration of the psychostimulant methylphenidate (Volkow et al, 1999, 2002). Third, studies in nonhuman primates demonstrated that low striatal D2 receptor availability was predictive of increased propensity to self-administer cocaine (Morgan et al, 2002). Together, these studies suggest that a low expression of D2 receptors in the striatum might constitute a risk factor for the development of cocaine dependence, and that this neurobiological trait is associated a decreased sensitivity to natural reinforcers (Volkow et al, 2002).
The present study was designed to further evaluate the potential involvement of D2 receptor expression in cocaine dependence. Recently detoxified chronic cocaine-dependent (CCD) subjects and matched healthy control (HC) subjects underwent a PET study with the D2/D3 receptor radiolabeled antagonist [11C]raclopride. PET studies were acquired on the high-resolution camera ECAT EXACT HR+, which permitted the determination of D2 receptor availability, not only in the striatum as a whole, but also in its functional subdivisions (Drevets et al, 2001; Mawlawi et al, 2001; Martinez et al, 2003). Following the scan, CCD subjects underwent cocaine self-administration studies in a laboratory setting. In the first set of laboratory sessions (single doses sessions), subjects rated their subjective response to smoked cocaine. In the second set of laboratory sessions (multiple choice sessions), subjects received a priming dose of cocaine and were then given the choice between smoking more doses of cocaine or receiving an alternative reinforcer (monetary reward). Thus, data from this study enabled us to asses potential relationships between D2 receptor availability, the positive effect of cocaine, and primed drug seeking behavior (a laboratory model of relapse).
Four main hypotheses were tested on this data set. The first hypothesis was that the decreased D2 receptor availability previously observed in CCD subjects at the level of the whole striatum (Volkow et al, 1990, 1993, 1997) would be replicated in this cohort. Studies with laboratory animals have implicated DA transmission in the nucleus accumbens rather than in the corpus striatum in mediating the rewarding effects of psychostimulants (for review see Wise and Romprè, 1989; Di Chiara, 1999). Based on these preclinical data, the second hypothesis was that the decrease in D2 receptor availability associated with cocaine dependence would be more pronounced in the limbic compared to the associative or sensori-motor subdivisions of the striatum. The previous studies which reported an association between low striatal D2 receptor availability and a pleasurable experience following psychostimulant administration were performed in healthy human subjects (Volkow et al, 1999, 2002). However, to our knowledge, this association has not been reported in CCD subjects. Thus, the third hypothesis was that low D2 receptor availability would be predictive of a more pleasurable experience upon smoking cocaine in CCD subjects, and that this relationship would be more pronounced in the limbic striatum. The fourth hypothesis was that low D2 receptor expression in the limbic striatum would be associated with cocaine-seeking behavior following a priming dose of cocaine. Thus, we predicted that low D2 receptor expression in the limbic striatum would be predictive, not only of a positive response to smoked cocaine, but also of the choice for cocaine over the monetary alternative.
MATERIALS AND METHODS
Subjects
The study was approved by the Institutional Review Boards of the Columbia Presbyterian Medical Center and the New York State Psychiatric Institute and all subjects provided written informed consent. A federal certificate of confidentiality was issued by the National Institute of Drug Abuse (NIDA) for this study.
Study criteria for CCD subjects included: (1) males or females between 21 and 45 years old; (2) fulfilling DSM-IV criteria for cocaine abuse or cocaine dependence; (3) weekly use of cocaine in excess of the doses used in this study over the last 6 months; (4) positive urine screen for cocaine; (5) not currently seeking treatment; (6) absence of DSM-IV Axis I disorder other than cocaine abuse or dependence, including abuse or dependence to other drugs and alcohol (nicotine dependence was acceptable); (7) no current (6 months) use of opiates, sedative-hypnotics, and/or cannabis more than twice a week; (8) no current (6 months) use of psychotropic medication such as antipsychotics or antidepressants; (9) no pregnancy; (10) absence of a significant medical condition, including chronic active Hepatitis B or C; (11) no metal implants or paramagnetic objects within the body which may interfere with the MRI scan; (12) no exposure to radiation in the last year; (13) subjects not on parole or probation; (14) no history of violence. Study criteria for control subjects included (1) males or females between 21 and 45 years old; (2) absence of DSM-IV Axis I disorder (nicotine dependence was acceptable); and criteria 7–14 as above.
Screening
CCD and HC subjects were recruited by local newspaper advertisements from the New York City metropolitan area. Following an initial telephone interview, potential participants provided written informed consent and underwent a full screening, which included a psychiatric assessment, physical exam, 12-lead electrocardiogram, and laboratory tests, including urine toxicology and pregnancy test. The psychiatric assessment included: interview with a research psychologist and study psychiatrist, SCID (First et al, 1994, 1995), Drug History Questionnaire, General Health Questionnaire, and Beck Depression Inventory (Beck et al, 1996). The pregnancy test was repeated on the scan day.
Monitored Abstinence Period
After completing of the screening procedures, CCD subjects were admitted to the Irving Center for Clinical Research at the New York Presbyterian Hospital for the duration of the study (19–21 days). Subjects were not permitted to leave the unit unescorted, nor were visitors allowed. During this period, participants were randomly tested to confirm drug abstinence while hospitalized. Subjects were allowed to smoke cigarettes during their admission, except on scanning days. Subjects underwent PET scanning with [11C]raclopride after 2 weeks of monitored abstinence. One to 3 days following the scans, the CCD subjects underwent the first cocaine self-administration sessions (single-sample sessions). On the next 2 days, CCD subjects underwent the multiple choice sessions. While subjects were not seeking treatment as per inclusion criteria, they were offered counseling during the study and referral at the end of the study. Healthy control subjects participated as outpatients and abstained from tobacco smoking on PET scan days. HC subjects did not participate in the cocaine self-administration sessions.
PET Scan Acquisition
[11C]Raclopride was prepared as previously described (Mawlawi et al, 2001). The PET studies were acquired using a bolus plus constant infusion method for delivery of [11C]raclopride, which provides a steady-state concentration of the unmetabolized radiotracer in the plasma and in the brain throughout the time of data acquisition (Mawlawi et al, 2001). This method allows for a direct determination of the equilibrium distribution volume. [11C]Raclopride was delivered in a 60 cc syringe, and a bolus dose of 31 cc was delivered over 3 min using an IMED pump (Gemini PC-1, San Diego, CA). Following the bolus, the pump was reset to deliver the remaining dose at 0.28 cc/min for 80 min. Thus, [11C]raclopride was administered using a bolus to infusion ratio of 105 min (ie 53% of the dose is given in the bolus). We previously demonstrated that, under this administration protocol, activities reach equilibrium at about 40 min, and that an acquisition from 40 to 80 min is adequate and sufficient for reliable determination of activity concentrations in striatal subregions (Mawlawi et al, 2001).
PET imaging was performed with the ECAT EXACT HR+ (Siemens/CTI, Knoxville, TN), which has 63 slices covering an axial field of view of 15.5 cm, an axial sampling of 2.46 mm, and in plane and axial resolution of 4.4 and 4.1 mm full width half-maximum at the center of the field of view in 3D mode. Emission data were collected in the 3D mode as eight frames of 5 min duration obtained from 40 to 80 min. Images were reconstructed with attenuation correction using the data from a 10 min transmission scan and a Shepp 0.5 filter.
Four venous samples (collected at 40, 50, 60, and 70 min) were obtained and analyzed to obtain the plasma concentration of [11C]raclopride as previously described (Mawlawi et al, 2001). Briefly, a 200 μl aliquot of plasma was collected and activity measured in a gamma counter (Wallac 1480 Wizard 3 M Automatic Gamma Counter). The samples were further processed by high-pressure liquid chromatography (HPLC) to measure the fraction of plasma activity representing the parent compound (unmetabolized [11C]raclopride). Plasma-free fraction (_f_1) was measured in triplicate as previously described (Gandelman et al, 1994).
An MRI was acquired on a GE 1.5 T Signa Horizon system. A sagittal scout was initially performed to identify the plane of the anterior and posterior commissures. A transaxial T1 weighted sequence with a 1.5 mm slice thickness was then acquired in a coronal plane orthogonal to the plane of the anterior and posterior commissures. The following parameters were used: three-dimensional SPGR (Spoiled Gradient Recalled Acquisition in the Steady State); TR of 34 ms; TE of 5 ms; flip angle of 45°; slice thickness 1.5 mm and zero gap; 124 slices; FOV 22 × 16 cm; with 256 × 192 matrix, reformatted to 256 × 256, yielding a voxel size of 1.5 mm × 0.9 mm × 0.9 mm.
PET Scan Analysis
Three sets of analysis were performed and are presented. The first analysis was based on a priori defined regions of interest (ROIs). The second analysis was also ROI based, and included partial voluming correction. The third analysis was performed at a voxel-wise level.
Spatial registration
Image analysis was performed in MEDx (Sensor Systems, Inc., Sterling, Virginia) as described previously (Mawlawi et al, 2001). For derivation of registration parameters, PET frames were denoised with a level 2, order 5 Battle–Lemarie wavelet transform (Battle, 1987; Lemarie, 1988; Mallat, 1989). The detail images were then set to zero using a hard threshold, and the resulting image was transformed back into the spatial domain using an inverse wavelet. The first denoised frame of the data set (acquired at 40–45 min) was chosen a priori as the frame of reference and was registered to the MRI using between modality AIR (Woods et al, 1993). Each of the following denoised PET frames were then registered to this frame using within modality AIR (Woods et al, 1992). In seven out of 34 studies, the frame of reference (40–45 min) provided a less than ideal registration to the MRI and the following frame (45–50 min) was successfully registered to the MRI. The transformation matrices determined from the denoised PET frames were then applied to the original (not denoised) PET frames.
Anatomical analysis
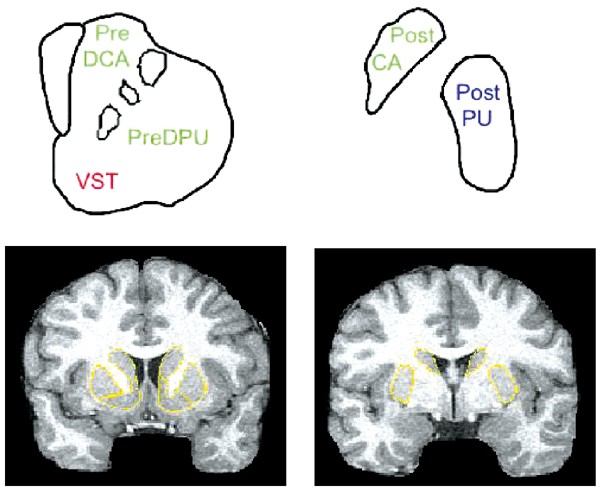
The striatum was divided into five anatomical ROIs and three functional subdivisions (Figure 1), using previously published criteria (Martinez et al, 2003). The ROIs included the ventral striatum (VST), the dorsal caudate rostral to the anterior commissure (precommissural dorsal caudate, preDCA), the dorsal putamen rostral to the anterior commissure (precommissural dorsal putamen, preDPU), the caudate caudal to the anterior commissure (postcommissural caudate, postCA), and the putamen caudal to the anterior commissure (postcommissural putamen, postPU). Activities from left and right regions were averaged. ROIs were classified as belonging to the limbic striatum (LST), associative striatum (AST), or sensorimotor striatum (SMST), based on cortical connectivity (for reviews see Haber and Fudge, 1997; Joel and Weiner, 2000). The LST corresponded to the VST, the AST activity was derived as the spatially weighted average of the activities in the preDCA, preDPU and postCA, and the SMST corresponded to the postPU. See Martinez et al (2003) for discussion of rationale and limitations of this classification scheme. The activity in the striatum as a whole (STR) was derived as the spatially weighted average of the five ROIs. The cerebellum (CER) was used as the reference region. Regions were drawn on the MRI, and applied to the coregistered PET images for activity concentration measurement.
Figure 1
Striatal subregions. The top row is a schematic representation of striatal subregions in the coronal plane. The striatum anterior to the plane of the anterior commissure (AC) is on the left, and includes the VST (ventral striatum), the pre-DCA (precommissural dorsal caudate) and pre-DPU (precommissural dorsal putamen). On the right, the striatum posterior to the AC is shown, which includes the post-CA (postcommissural caudate) and post-PU (postcommissural putamen). Colors indicate functional subdivisions into limbic (red, VST), associative (green, pre-DCA, pre-DPU, and post-CA) and sensorimotor (blue, post-PU) subdivisions. The bottom row illustrates a coronal MRI image anterior (left) and posterior (right) to the AC, with the boundaries of the regions of interest (in yellow). The criteria used to delineate boundaries of VST, pre-DCA, and pre-DPU at the precommissural level are provided in Mawlawi et al (2001).
Derivation of outcome measures
D2 receptor availability was estimated using two outcome measures: [11C]raclopride binding potential (BP) and [11C]raclopride specific to nonspecific equilibrium partition coefficient (V3″). [11C]Raclopride has a similar affinity for D2 and D3 receptor (Sokoloff et al, 1990), and the term D2 receptors is used to denote both D2 and D3 receptors. Both outcome measures were obtained using equilibrium analysis applied to the PET frames obtained from 40 to 80 min. The regional tissue distribution volume (_V_T, ml g−1) was defined as the ratio of the ligand concentration in a region (_C_T, μCi g−1) to the concentration of unmetabolized ligand in venous plasma (_C_P, μCi ml−1) at equilibrium,

The concentration of D2 receptors is negligible in the cerebellum (Hall et al, 1994). Therefore, only free and nonspecifically bound radiotracer were considered to contribute to _V_T in the cerebellum (_V_T CER), and _V_T CER was assumed to be equal to the nondisplaceable distribution volume (_V_2). In the ROIs, _V_T (_V_T ROI) included _V_2 and the specific binding distribution volume, or BP. Assuming that _V_2 in the ROI was equal to _V_T CER, BP was derived as the difference between _V_T ROI and _V_T CER. BP is related to receptor parameters by
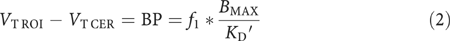
where _f_1 is the plasma-free fraction, _B_max is the concentration of D2 receptors (nM per g of tissue), and _K_D′ is the in vivo equilibrium dissociation constant of the radiotracer (nM per ml of brain water) in the presence of the competitor DA. _K_D′ related to _K_D by _K_D′=_K_D (1+_F_DA/_K_I), where _F_DA is the free concentration of endogenous DA in the vicinity of the receptors, and _K_I is the inhibition constant of DA for the binding of [11C]raclopride (Laruelle et al, 1997). Studies in healthy subjects suggest that about 10% of D2 receptor are occupied by DA in the baseline state in healthy subjects (Abi-Dargham et al, 2000). The proportion of D2 receptors occupied by DA in CCD subjects is unknown.
_V_3″ was calculated as the ratio of BP to _V_T CER. _V_3″ is related to receptor parameters by

where _f_2 is the free fraction in the nonspecific distribution volume of the brain (_f_2=_f_1/_V_2) (Laruelle et al, 1994). The use of BP for the between group comparison assumes that _f_1 is not significantly different between groups, whereas the use of _V_3″ assumes that _f_2 is not significantly different between groups. In this study, both _f_1 and _f_2 were measured to assess the validity of these assumptions.
Partial volume error (PVE) analysis
The measurement of activity in the striatal subregions is affected by the error induced by partial volume effects (PVE). Owing to limitations in resolution, the activity emitted from a given ROI is not fully recovered within that ROI, and activities from adjacent regions contaminate the signal from the ROI. In a previous study in healthy controls, we determined that activity measured in the VST was significantly contaminated by counts spilling over from the adjacent preDCA and preDPU: 70±5% of the specific binding measured in the VST originated from D2 receptors located in the VST, while 12±3 and 18±3% were contributed by D2 receptors in the preDCA and preDPU (Mawlawi et al, 2001). Owing to the importance of the VST measurement in this study, data analysis was repeated after PVE correction, which was performed as previously described (Rousset et al, 1998; Mawlawi et al, 2001). Briefly, the geometric transfer matrix (GTM) was formed by generating binary image sets of the ROI from the MRI, in which the voxels contained within each ROI are set to 1 and all other voxels are set to 0. The regions included the 10 ROIs, and a background region, which included the rest of the brain. The binary images were then realigned to the location of the original PET images in the camera field-of-view, and smoothed using a mathematical model of the point spread function of the PET camera at that location. The true activity in each ROI was calculated from the measured activity and the GTM. PVE correction was performed using a FWHM of 5.1 mm at the center of the field of view. This effective resolution takes into account the resolution of the PET camera, the reconstruction filter, and estimated subject movement (Mawlawi et al, 2001).
Voxel-wise analysis
_V_3″ maps were created for each subject. First, _V_3″(t) images were made by dividing the activity in each MR coregistered frame between 40 and 80 min by the mean cerebellar activity of that frame and subtracting 1. The _V_3″ map was then computed as the mean over frames of _V_3″(t). Each subject's structural MRI image was normalized to the T1 template image in SPM2 (Friston et al, 1995). The same transformation was then applied to the MR coregistered _V_3″ image. Data were smoothed with a 12 mm Gaussian kernel. For SPM analyses, an absolute threshold mask of 0.1 was applied, that is, analysis was restricted to voxels at which all subjects’ _V_3″ exceeded a value of 0.1.
Laboratory Sessions
Following the scan, CCD subjects underwent cocaine self-administration laboratory sessions with doses of 0, 6, and 12 mg smoked cocaine over 3 days. The cocaine base was prepared by the Presbyterian Hospital Manufacturing Pharmacy from cocaine hydrochloride obtained from the National Institute of Drug Abuse (NIDA) as described previously (Foltin et al, 1990). During all sessions, subjects were under continuous EKG and frequent (every 2 min) vital sign monitoring. Subjects were monitored through a one-way mirror and could communicate via an intercom. Participants were presented with cocaine base in a glass stem pipe and a research nurse held a lighter while the subjects inhaled the contents. Subjects were blind to the dose of cocaine. During each session, subjects were asked about their subjective experience of cocaine using the subjective-effects battery described below.
Single-sample sessions
On the first day, subjects had three single-sample sessions, separated in time by at least 2 h. Each session consisted of a single dose of 0, 6, or 12 mg of cocaine, administered in counterbalanced order. During these sessions, the subjective-effects battery was presented to the subjects at baseline, 4, 14, 30, and 60 min following the dose. The computerized subjective effects battery consisted of 26 visual analog scales (VAS) labeled ‘not at all’ at 0 mm and ‘extremely’ at 100 mm. Subjects were asked to indicate with a mark along the 100 mm line (on a computer screen) their response to the following questions: (1) 18 of the VAS start with ‘I feel …’ followed by ‘stimulated’, ‘anxious’, ‘depressed’, ‘sedated’, ‘high’, ‘hungry’, ‘focused’, ‘calm’, ‘able to concentrate’, ‘alert’, ‘tired’, ‘talkative’, ‘self-confident’, ‘social’, ‘irritable’, ‘confused’, ‘a good drug effect’, and ‘a bad drug effect’ (2) Four VAS were used to operationalize drug craving and were labeled ‘I want …’ followed by ‘cocaine’, ‘heroin’, ‘alcohol’, ‘tobacco’ (3). Four VAS were used to rate the dose, three were labeled ‘I liked the choice’ and ‘the choice was …’ followed by ‘high quality’ and ‘potent’ and one scale asked participants to indicate how much they would pay for the dose of cocaine across a range of 0to0 to 0to25.
A previous cluster analysis of these VAS demonstrated five clusters: positive effects (consisting of ‘good drug effect’, ‘high’, and ‘stimulated’), as well as ‘drug quality ratings’, ‘bad drug effect’, ‘mood states’, and ‘on edge/miserable’ (Evans et al, 2002). The positive effects cluster was chosen a priori for correlation with D2 receptor availability, with a post hoc analysis of measures of craving. For each VAS, the area under the curve (AUC) was used as outcome measure for comparison with PET data. The positive effects score was then derived as an average of the AUC for the three VAS within this cluster. Similarly, an average of the AUC was calculated for the drug quality ratings. The VAS for cocaine craving was calculated as the AUC for this scale.
During the single sample sessions blood samples for cocaine were drawn through an intravenous catheter at baseline, 4, 14, 30, 60 min. Plasma cocaine levels were centrifuged and frozen until analyzed. Cocaine plasma concentration was determined using capillary gas chromatograph–mass spectrometry as previously described (Foltin et al, 2003). Cocaine levels (ng/ml) obtained for each dose session were averaged.
Multiple choice sessions
On the second and third laboratory days, subjects underwent three multiple choice sessions, with each the 0, 6, and 12 mg doses, in counterbalanced order, as described previously (Foltin et al, 2003). In these sessions, subjects took an initial response independent or ‘priming’ dose of 0, 6, or 12 mg at _t_=0. Following this dose, subjects were given the choice between this same dose of cocaine or a $5.00 merchandise voucher redeemable at local stores and paid upon discharge. Subjects were presented with this choice 5 times, spaced 14 min apart, and indicated their choice on the computer screen. A progressive ratio was used, such that participants were required to press a space bar on the computer keyboard 200, 600, 1000, 1400, and 1600 times in order to receive their choice. The outcome measure for the choice sessions was the number of times a given dose of cocaine was chosen over voucher (1–5).
Statistical Analysis
Group comparisons were performed with unpaired _t-_test or χ2. Outcomes related to D2 receptor availability ([11C]raclopride BP and _V_3″), which were analyzed by repeated measures ANOVA, with region or functional subdivisions as repeated factor and groups as cofactor. The effects of cocaine were analyzed with repeated measure ANOVA with dose as repeated factor. Voxel-wise analysis was performed with SPM2 (Friston et al, 1995). Relationships between continuous variables were analyzed with the Pearson product moment correlation coefficient. A two-tailed probability value of p<0.05 was chosen as the level of significance.
RESULTS
Group Composition
In total, 19 HC subjects and 20 CCD subjects were enrolled in this study. Two HC subjects were excluded after enrollment: one subject was unable to complete the MRI and another developed an axis I diagnosis after the study. Three CCD subjects were excluded after enrollment: two left the hospital prior to the PET scans for personal reasons and the third was removed by the study physician due to medical illness. Therefore, the final samples included 17 HC and 17 CCD subjects who completed the study. Groups were matched for age, gender, ethnicity, and cigarette smoking (Table 1). Both groups were acquired in parallel, over a 31-month period. CCD subjects reported smoking crack cocaine an average of 4.3±1.7 days per week. They had been using cocaine for 15.5±4.9 years and were spending 264±111264±111 264±111US weekly over the last 6 months.
Table 1 Group Demographic Compositions
Imaging Results
Injected doses
The average decay corrected injected dose was 13.3±3.8 mCi for HC subjects and 12.4±4.3 mCi for CCD subjects (_p_=0.5). The average specific activity was 1473±735 Ci/mmol with an injected mass of 3.6±1.2 μg for HC subjects and 1673±1067 Ci/mmol with an injected mass of 3.1±1.1 μg for CCD subjects (_p_=0.5 for specific activity and _p_=0.2 for mass).
Plasma analysis
The concentration of parent compound ([11C]raclopride) was constant over 40–70 min. The changes in plasma parent concentration over time, during the 40–70 min interval, were calculated as the slope of the regression over time and expressed relative to the average concentration. These changes were not significantly different from zero (one sample _t-_test: HC: 0.6±23%/h, _p_=0.91; CCD: 5±26%/h, _p_=0.44), nor were they different between groups (_p_=0.61). Plasma clearance did not differ between groups (HC: 12.7±3.9 l h−1; CCD: 12.3±2.2 l h−1; _p_=0.7). Likewise, plasma free fraction (_f_1) did not differ between groups (HC: 3.8±0.8%; CCD: 3.4±0.7%; _p_=0.13).
Cerebellum _V_2
The volume of distribution of the cerebellum (_V_2) was 0.40±0.07 ml g−1 in HC subjects and 0.39±0.05 ml g−1 in CCD subjects (_p_=0.6). The free fraction of the cerebellum (_f_2) was 9.8±2.6% in HC subjects and 8.8±1.7% in CCD subjects (_p_=0.2).
ROI volumes
ROIs volumes did not differ between the two groups (Table 2).
Table 2 Region of Interest Volumes (mm3)
D2 receptor availability: non-PVE-corrected data
Representative [11C]raclopride scans in one HC subject and one CCD subject are presented in Figure 2. Regional non-PVE corrected values for BP and _V_3″ are provided in Tables 3 and 4, respectively. Significant group differences in D2 receptor availability were found with both BP and V3″ ([11C]raclopride BP: region factor: p<0.001; group factor: _p_=0.014; group by region interaction: _p_=0.004; [11C]raclopride _V_3″: region factor: p<0.001; group factor: p<0.001; group by region interaction: _p_=0.001). CCD subjects exhibited lower D2 receptor availability compared to HC subjects, and the decreases were of similar magnitude for BP and _V_3″. When the regions were examined individually, a significant difference was found in all regions for BP (Table 3) and _V_3″ (Table 4), with the exception of the postCA, with this region being the source of the significant region by group interaction.
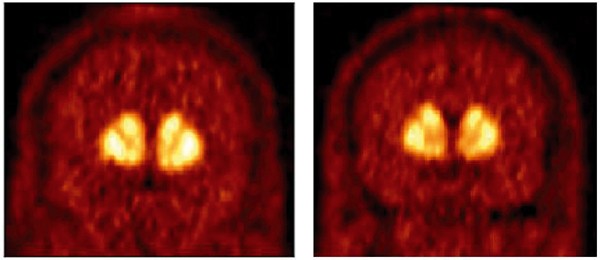
Figure 2
[11C]raclopride distribution in a healthy control subject (left) and a cocaine-dependent subject (right). Both images are the mean of data acquired from 40 to 80 min and the image display was corrected for injected dose. The selected images include the striatum rostral to the anterior commissure. Cocaine-dependent subjects were found to have significantly lower D2 receptor availability compared to healthy controls.
Table 3 [11C]Raclopride Binding Potential (BP, ml g−1)
Table 4 [11C]Raclopride Specific to Nonspecific Partition Coefficient (_V_3″, Unitless)
This analysis was also performed at the level of the subdivisions. Significant group differences in D2 receptor availability were found with both BP and _V_3″ ([11C]raclopride BP: subdivision factor: p<0.001; group factor: _p_=0.013; group by region interaction: _p_=0.042; [11C]raclopride _V_3″: subdivision factor: p<0.001; group factor: p<0.003; group by region interaction: _p_=0.045). When the subdivisions were examined individually, a significant difference was found in all subdivisions for BP (Table 3) and _V_3″ (Table 4). The significance level of the group difference in the SMST was higher than in the AST and LST, a difference being the source of the significant interaction.
D2 receptor availability: PVE-corrected data
PVE corrected values for BP and _V_3″ are provided in Tables 5 and 6. PVE correction resulted in a significant increase in the measured values of BP and _V_3″ for each ROI (RM ANOVA, p<0.05 for all regions). Significant group differences in regional D2 receptor availability were found with both PVE corrected BP and _V_3″ (PVE corrected [11C]raclopride BP: region factor: p<0.001; group factor: _p_=0.010; group by region interaction: _p_=0.06; PVE corrected [11C]raclopride _V_3″: region factor: p<0.001; group factor: _p_=0.002; group by region interaction: _p_=0.06). Thus, even after PVE correction, CCD subjects still exhibited lower D2 receptor availability compared to HC subjects. When ROIs were examined individually, a significant difference was found in all ROIs and subdivisions for PVE corrected BP (Table 5) and _V_3″ (Table 6), except in the postCA. Figure 3 displays the individual values of PVE corrected _V_3″ in LST, AST and SMST in HC and CCD subjects.
Table 5 [11C]Raclopride Binding Potential (BP, ml g−1), Following Partial Volume Effect Correction
Table 6 [11C]Raclopride Specific to Nonspecific Partition Coefficient (V3″, Unitless), Following Partial Volume Effect Correction
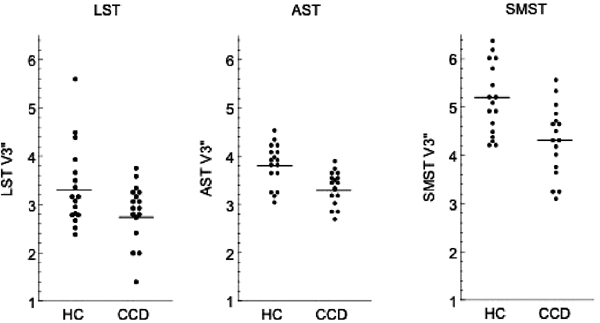
Figure 3
Partial voluming corrected [11C]raclopride _V_3″ in healthy subjects (HC, _n_=17) and cocaine-dependent subjects (CCD, _n_=17) in limbic striatum (LST), associative striatum (AST) and sensorimotor striatum (SMST). In all regions, [11C]raclopride _V_3″ was significantly lower in CCD subjects compared to controls (unpaired _t-_test, p<0.05).
This analysis was also performed at the level of the subdivisions. Significant group differences in PVE-corrected D2 receptor availability were found with both BP and _V_3″ (PVE corrected [11C]raclopride BP: subdivision factor: p<0.001; group factor: _p_=0.009; group by region interaction: _p_=0.084; PVE corrected [11C]raclopride _V_3″: subdivision factor: p<0.001; group factor: _p_=0.002; group by region interaction: _p_=0.14). When the subdivisions were examined individually, a significant difference was found in all subdivisions for BP (Table 5) and _V_3″ (Table 6). Thus, the main difference between PVE and non-PVE corrected analysis of the subregions was that the interaction term became nonsignificant after PVE correction.
D2 receptor availability: voxel-wise analysis
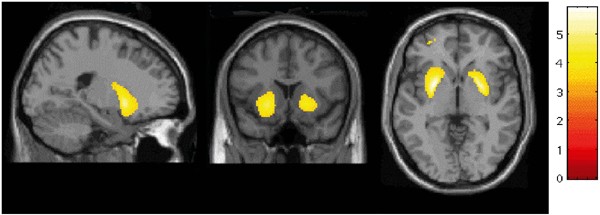
_V_3″ maps for controls and CCD are presented in Figure 4. Results of the voxel-wise group comparison are presented in Figure 5. Two significant clusters appeared corresponding to the left and right sides of the striatum in the contrast for control _V_3″ greater than cocaine user _V_3″. These were significant at the _p_=0.004 and 0.015 levels, respectively, when using the random field family-wise error multiple comparisons correction. The significant regions primarily overlapped putamen (pre- and postcommissural). These were connected to small portions of the precommissural caudate, which, while exceeding the threshold for display, did not reach significance. The display threshold on the image corresponds to an uncorrected _p_-value of 0.001 (_T_=3.37, _n_=34, df=32). A small cluster in the frontal cortex exceeded the display threshold, but did not survive the multiple comparisons procedures (_p_=0.325). No voxels were significant at any uncorrected p level in the contrast for cocaine user _V_3″ greater than control _V_3″.
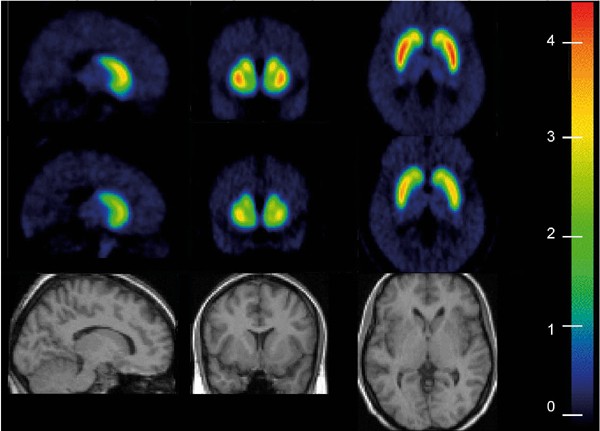
Figure 4
Spatially normalized mean _V_3″ parametric map in healthy controls (_n_=17, top row) and cocaine-dependent subject (_n_=17, middle row). _V_3″ parametric maps were created for each subjects by applying Eq. 3) on each voxel. _V_3″ maps were then spatially normalized to a MRI template. The color scale was calibrated in _V_3″ units, and is identical for both groups. Comparison of the maps illustrates the decreased [11C]raclopride _V_3″ in cocaine-dependent subject compared to controls.
Figure 5
Results of SPM analysis, showing the brain areas were [11C]raclopride _V_3″ was significantly lower in cocaine-dependent subjects compared to controls (display threshold=3.37, the _T_-value associated with uncorrected _p_=0.001).
Laboratory Session Results
Single-sample sessions
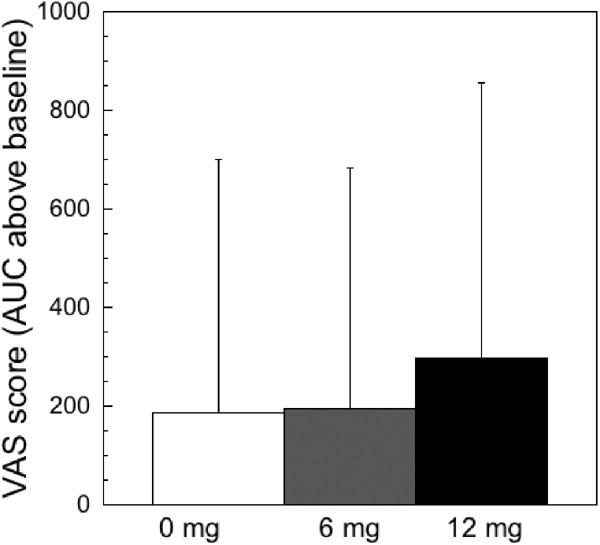
The mean±SD AUC for each VAS of the positive effect cluster and for the positive effects cluster itself are shown in Figure 6 for each dose of cocaine. Ratings of the positive effects of the 6 mg dose did not differ from the 0 mg dose (_p_=0.73), whereas ratings of the 12 mg dose differed significantly from both the 0 and 6 mg doses (p<0.001 for both comparisons). A similar pattern was seen for each of the individual VAS (stimulated, high, and good drug effect): a significant difference was seen between the 12 and 0 mg doses (_p_⩽0.005 for each VAS) and the 12 and 6 mg doses (_p_⩽0.02 for each VAS), with no difference between the scores for the 0 mg and 6 mg doses. Ratings of drug quality followed the same pattern as the positive effects score. The 12 mg dose (378±761) differed from both the 0 mg dose (170±381, _p_=0.01) and the 6 mg dose (219±625, _p_=0.05), whereas the 0 and 6 mg doses did not differ from each other (_p_=0.5). Ratings of craving for cocaine did not differ significantly between the doses: the 0 mg dose was 1609±2271 compared to 1283±2028 for the 6 mg dose and 1526±2016 for the 12 mg dose. Since only the 12 mg dose elicited positive subjective effects different from placebo, the effects of the 12 mg dose were selected for comparison with the scan data.
Figure 6
Positive effects of cocaine following 0, 6, and 12 mg of smoked cocaine. The visual analog scales (VAS) that make up the positive effects cluster include ratings of ‘high’, ‘stimulated’, and ‘good drug effect’. For each VAS the area under the curve (AUC) was calculated. The average of these three ratings was used to derive the AUC for positive effects. No difference in any of these measures was seen between the 0 and 6 mg doses. However, ratings of the 12 mg dose were significantly higher than the 0 or 6 mg doses.
The averaged cocaine plasma levels for the different doses were as follows: 1.26±3.06 ng ml−1 for the 0 mg dose, 16.63±18.61 ng ml−1 for the 6 mg dose, and 32.53±32.35 ng ml−1 for the 12 mg dose. The difference between the levels obtained for each dose was significant for each comparison (0 vs 6 vs 12 mg). A minimal detectable cocaine level was seen for the 0 mg dose, which most likely resulted from the fact that the dose order was counterbalanced, such that some subjects had received a 6 or 12 mg dose prior to the 0 mg dose. However, the cocaine levels were low for the 0 mg does and were unlikely to affect the VAS scales obtained for this dose. No correlation was seen between the plasma levels of cocaine drawn at 4 min following the dose and the positive effects of cocaine (0 mg dose: _r_=0.2, _p_=0.51: 6 mg dose: _r_=0.04, _p_=0.90, and 12 mg dose: _r_=0.32, _p_=0.27).
Multiple choices sessions
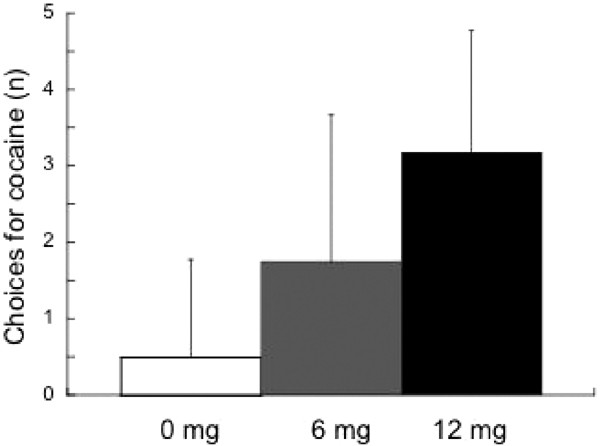
One subject did not undergo choice sessions due to a scheduling problem. Thus, 16 of the cocaine-dependent subjects completed the choice sessions. The results of the choice sessions are shown in Figure 7. Out of five possible choices, the 0 mg dose was chosen 0.50±1.26 times, the 6 mg dose was chosen 1.75±1.81 times, and the 12 mg dose was chosen 3.69±1.49 times. There was a significant difference between each of the three doses: the 12 mg dose was chosen more frequently than both the 0 mg (p<0.0001) and the 6 mg dose (_p_=0.0002). The 6 mg dose was also chosen more frequently than the 0 mg dose (_p_=0.01). No correlation was seen between the positive effects reported by each individual subject during the single-dose session and their choice for either the 6 mg (_r_=0.02, _p_=0.94) or the 12 mg dose (_r_=0.32, _p_=0.23).
Figure 7
Choices for cocaine over monetary reward following a priming dose of cocaine. Subjects were presented this choice five times, and the number of doses of cocaine chosen is on the _y_-axis (range is 0–5). Subjects chose both the 6 and 12 mg doses more frequently than the 0 mg dose (_p_=0.01 and p<0.0001, respectively). There was also a significant difference in the choice for the 12 mg dose vs 6 mg (_p_=0.0002).
The rationale for using low doses of cocaine in the self-administration sessions was to ensure enough variability between subjects to allow comparison with the [11C]raclopride data. The highest variance was seen for the 6 mg dose, (3.27) compared to the 0 mg (1.60) and 12 mg (2.23) doses. Therefore, the 6 mg was chosen for comparison with the PET data.
Relationships between Scan, Behavioral, and Clinical Data
No association was seen between LST [11C]raclopride _V_3″ and the positive effects of 12 mg cocaine (_r_=0.26, _p_=0.31), craving for cocaine (_r_=0.49, _p_=0.85), or with cocaine-taking behavior (choice frequency for the 6 mg dose of cocaine over money, _r_=0.18, _p_=0.51). No relationship was seen between LST [11C]raclopride _V_3″ and years of cocaine exposure (Table 7), which was also true when age was entered into the model (age factor, _p_=0.42, years of abuse factor, _p_=0.99). Similarly, no relationships were observed between [11C]raclopride _V_3″ in other regions and each of these behavioral variables (positive effects of cocaine, primed cocaine-seeking behavior, and years of exposure, Table 7).
Table 7 Relationship Between [11C]Raclopride _V_3″ and Cocaine Use Parameters in Chronic Cocaine-Dependent Subjects
DISCUSSION
The results of this study replicate the observation that striatal D2 receptor availability is decreased in recently detoxified chronic cocaine abusers (Volkow et al, 1990, 1993, 1997). This decrease was of a modest magnitude, and affected to the same extent the limbic, associative, and sensorimotor regions of the striatum. In CCD subjects, low D2 receptor availability in the limbic striatum (or in the other regions of the striatum) was not predictive of the positive effects of smoked cocaine, cocaine-induced cocaine-taking behavior, nor of the duration of cocaine abuse. Thus, while this study replicated the previous observations of low striatal D2 receptors in cocaine abuse, it failed to detect an anatomical selectivity of this alteration within the striatum, and failed to detect the behavioral significance of this abnormality.
D2 Receptor Availability and Cocaine Dependence
In this data set, cocaine dependence was associated with a reduction in both BP and _V_3″. No between-group difference was seen in nonspecific binding (_V_2), free fraction of the plasma (_f_1), or free fraction of the cerebellum (_f_2). Under these conditions, results derived with BP and _V_3″ should be in accordance (which was the case here). Furthermore, the decrease in binding parameters was not an artifact due to lower striatal volume in CCD subjects and partial volume effects. First, CCD subjects did not show differences in striatal volume compared to controls (a finding that contrasts with previous observation of increased striatal volume in CCD, Jacobsen et al, 2001). Second, partial volume effect correction produced results that enhanced the difference between the two groups. Thus, the decrease in binding parameters BP and _V_3″ can be attributed with confidence to a decrease in the D2 receptor Bmax/_K_D′ ratio. Since this study was performed only with tracer doses [11C]raclopride, it is not possible to separate changes in _B_max from changes in _K_D′. In theory, a reduction in the number of D2 receptors available to bind to [11C]raclopride could be due to elevated synaptic DA levels (which would translate into higher _K_D′ under a competitive model, lower _B_max under a noncompetitive model, or both under a mixed model). However, previous studies have demonstrated that cocaine abuse is associated with a reduction in [18F]6-FDOPA uptake (Wu et al, 1997) as well as a blunted DA response following a psychostimulant challenge (Volkow et al, 1997). Based on these findings, it is expected that DA synaptic concentration, if altered, would be lower in CCD. In this case, the reduction in D2 receptor availability observed here would actually represent an underestimation of the true effect.
Previous PET studies have shown a decrease in D2 receptor availability of a similar magnitude in the striatum. Volkow et al previously reported a decrease of 11% in striatal [11C]raclopride binding (Volkow et al, 1997) and decreases of 38% (Volkow et al, 1990), and 14% (Volkow et al, 1993) in [18F]_N_-methylspiroperidol striatal binding in CCD subjects. Whether this decreased D2 receptor expression represents a vulnerability factor or a consequence of long-term cocaine exposure cannot be determined from these studies. Rodent studies investigating the long-term effects of cocaine exposure on D2 receptor density have yielded inconsistent findings: studies have reported unchanged (Dwoskin et al, 1988; Alburges et al, 1993; Neisewander et al, 1994; Claye et al, 1995), increased (Taylor et al, 1979; Trulson and Ulissey, 1987; Zeigler et al, 1991) and decreased (Goeders and Kuhar, 1987; Kleven et al, 1990) D2 receptor densities in the striatum. Studies in nonhuman primates are less numerous, but more consistent. D2 receptor density is unaffected by short-term administration of cocaine (Farfel et al, 1992; Nader et al, 2002), but is decreased following prolonged exposure (Moore et al, 1998; Nader et al, 2002). Thus, the hypothesis that the decrease in D2 receptor availability observed in CCD subjects is a consequence of prolonged exposure to cocaine is supported by nonhuman primate data and cannot be ruled out.
Yet, the alternate hypothesis (low D2 receptor availability is a risk factor for the development of cocaine addiction) is supported by several indirect lines of evidence. First, decreases in D2 receptor availability of a similar magnitude have been shown in PET and SPECT studies of other addictive behaviors, including heroin addiction (Wang et al, 1997), alcohol dependence (Hietala et al, 1994; Volkow et al, 1996), methamphetamine abuse (Volkow et al, 2001), and obesity (Wang et al, 2001). Together, these studies suggest that low D2 receptor availability might be a general risk factor for addiction. Low D2 receptor availability might be associated with low sensitivity to naturally occurring reinforcers, and a propensity to depend on pharmacological stimulation or excessive consumption to experience reward. Low striatal D2 receptor availability is also found in other conditions, such as social phobia (Schneier et al, 2000) and social detachment in healthy control subjects (Farde et al, 1997; Breier et al, 1998), conditions which might be conceptualized as resulting from a low reinforcing effect of social interactions. An additional line of evidence supporting a role for low D2 receptor availability as a risk factor for addiction includes studies in healthy controls, which showed that low D2 receptor availability is associated with a more pleasurable experience following the administration of methylphenidate (Volkow et al, 1999, 2002). However, these results were not observed with amphetamine: in healthy subjects, D2 receptor availability measured with [11C]raclopride or [123I]IBZM were not predictive of the pleasurable effects reported following amphetamine administration (Abi-Dargham et al, 2003; Martinez et al, 2003). Finally, low D2 receptor availability is associated with a propensity to self-administer cocaine in nonhuman primates, an observation that also supports the hypothesis that low D2 receptor availability might constitute a risk factor for the development of addiction (Morgan et al, 2002). In conclusion, the literature provide data consistent with both hypotheses (toxic effect or vulnerability factor), and this issue cannot currently be settled.
D2 Receptor Availability in Functional Subdivisions of the Striatum
In the present study, the use of a high-resolution PET camera (ECAT EXACT HR+) allowed measurement of D2 receptor availability in the limbic, associative, and sensori-motor subdivisions of the striatum. Given the number of preclinical studies that have shown the connection between reinforcement and DA transmission in the nucleus accumbens (Nestler et al, 1990; Terwilliger et al, 1991; Striplin and Kalivas, 1993; Porrino et al, 2002), we formulated the hypothesis that, if low D2 receptor availability is associated with a vulnerability to develop addiction, this alteration might be more pronounced in the limbic compared to other subdivisions of the striatum of human cocaine abusers. The method used here to measure [11C]raclopride activity in the functional subdivisions of the striatum has been shown to have good test/retest reproducibility (Mawlawi et al, 2001), and to reliably detect between region differences of the effects of amphetamine on [11C]raclopride binding (Drevets et al, 2001; Martinez et al, 2003). In addition, the present data were analyzed with PVE correction, to remove cross-regional contamination of the signals. The relative regional distribution of D2 receptors measured in this study in HC subjects was similar to that previously reported in other control groups (Mawlawi et al, 2001; Martinez et al, 2003). The reduction in [11C]raclopride binding measured in the LST of CCD subjects was similar to that measured in the AST and SMST. Thus, these data do not support a selective decrease in D2 receptor availability within the striatal subregions. The only region in which this decrease failed to reach significance was the postCA. The lack of significance in the postCA might be due to the noise involved in measuring this small structure. However, the fact that the coefficient of variation in the post-CA was not increased compared to that of other regions, suggest a possible preservation of D2 receptors in this brain region.
D2 Receptor Availability and the Effects of Psychostimulants
In the present study, no correlation was observed between D2 receptor availability and the positive subjective effects of cocaine in CCD subjects. This result was consistent with the observation that D2 receptor availability is not associated with the pleasurable effects of amphetamine in HC (Abi-Dargham et al, 2003; Martinez et al, 2003), although it might be associated with the pleasurable effects of methylphenidate in HC (Volkow et al, 1999, 2002). The difference between results observed with methylphenidate and amphetamine in HC might be related to differences in the mode of action of methylphenidate, an uptake blocker, and amphetamine, an uptake blocker and DA releaser. The difference between results obtained in HC with methylphenidate and in CCD with cocaine (both drugs being uptake blockers) is more likely to be due to differences in patient populations. Thus, low D2 receptor availability might be related to a positive psychostimulant experience in HC subjects, but not in CCD subjects. In this scenario, low D2 receptor availability might play a role in the initial development of the addictive behavior, but once addiction is established, it might not affect the maintenance of drug-seeking behavior.
D2 receptor availability and cocaine-taking behavior
The most difficult aspect of treating cocaine abusers is their propensity to relapse after a period of abstinence. In fact, about 75% of ‘detoxified’ cocaine abusers relapse within a year of withdrawal (Carroll et al, 1994; Whiters et al, 1995). Cocaine abusers often describe their relapse as being precipitated by cocaine craving, which might be triggered by environmental stimuli associated with cocaine use, or by a ‘priming’ dose of cocaine itself (Childress et al, 1993). Thus, the identification of neurobiological factors that would confer vulnerability to relapse might provide important avenues for treatment. The cocaine-primed drug-taking behavior in the presence of an alternative reinforcer, as measured in the laboratory, provides a behavioral measure that, combined with brain imaging, might help to identify the neurobiological factors associated with the vulnerability to relapse.
The results from the cocaine self-administration study revealed the difference between drug liking and drug-taking behavior. Subjects reported no differences in the positive effects of a 6 mg dose of smoked cocaine and that of placebo. However, when asked to choose between this 6 mg dose and a $5 voucher (an amount worth more than the dose of drug), they chose the doses of cocaine more often than when given the choice between money and placebo. Furthermore, no correlation was seen between the positive effects reported by each subject from the sample dose and their subsequent choice for that dose in the multiple dose session.
These findings are in line with other behavioral studies of substance abuse which show that the reinforcing effects of drugs of abuse are more complex than simply the pleasurable or euphorigenic effects they produce (Fischman et al, 1990; Robinson and Berridge, 1993; Foltin and Fischman, 1996). Fischman (1989), have previously shown that the reinforcing effects of cocaine can be separated from the subjective effects in the laboratory. In a study of chronic cocaine abusers, subjects presented with a dose of cocaine that was too low to produce subjective effects, still chose cocaine over placebo (Fischman, 1989). In a similar study, Lamb et al (1991) demonstrated that opiate-dependent subjects would work to self-administer morphine, despite the fact that the dose was too low to be distinguished from placebo. Previous studies have also shown that medications that decrease the positive subjective effects of cocaine do not necessarily decrease its consumption (Fischman et al, 1990; Haney et al, 1999; Evans et al, 2001). Overall, these studies demonstrate that the reinforcing effects of cocaine involve neural pathways beyond those mediating drug-induced euphoria.
In this study, D2 receptor availability in the LST was not predictive of the drug-taking behavior following a priming dose of cocaine. To the extent that this laboratory paradigm adequately models subject's behavior in the natural environment, this result indicate that this neurobiological parameter does not significantly affect the risk of relapse following initial exposure to cocaine in CCD subjects.
CONCLUSION
The data from this study, by replicating the results of previous studies (Volkow et al, 1990, 1993, 1997), add to a growing body of evidence that low D2 receptor availability is associated with chronic cocaine abuse in human subjects. This study expanded on previous studies by demonstrating that D2 receptor availability is decreased in each functional subdivision of the striatum. Whether this decreased density is a consequence of chronic cocaine exposure or represents a vulnerability to develop this addiction remains to be firmly established. In addition, this study did not detect a relationship between D2 receptor availability and the positive subjective effects of cocaine or drug-taking behavior following a priming dose of cocaine within the CCD group. Thus, D2 receptor availability per se might not play a significant role in the maintenance of the addictive behavior. Additional studies are warranted to unravel neurobiological factors that might affect the risk of relapse.
References
- Abi-Dargham A, Kegeles L, Martinez D, Innis R, Laruelle M (2003). Dopamine mediation of positive reinforcing effects of amphetamine in stimulant naïve healthy volunteers: results from a large cohort. Eur Neuropsychopharmacol 13: 459–468.
Article CAS PubMed Google Scholar - Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS et al (2000). Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA 97: 8104–8109.
CAS PubMed PubMed Central Google Scholar - Alburges ME, Narang N, Wamsley JK (1993). Alterations in the dopaminergic receptor system after chronic administration of cocaine. Synapse 14: 314–323.
Article CAS PubMed Google Scholar - Battle G (1987). A block spin construction of ondelettes 1. Lemarie Functions. Commun Math Phys 110: 601–615.
Article Google Scholar - Beck A, Steer R, Brown G (1996). BDIII Beck Depression Inventory Manual, 2nd ed. Harcourt Brace & Company: San Antonio, TX.
Google Scholar - Breier A, Adler CM, Weisenfeld N, Su TP, Elman I, Picken L et al (1998). Effects of NMDA antagonism on striatal dopamine release in healthy subjects: application of a novel PET approach. Synapse 29: 142–147.
Article CAS PubMed Google Scholar - Carroll K, Rounsaville B, Nich C, Gordon L, Wirtz P, Gawin F (1994). One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Arch Gen Psych 51: 989–997.
Article CAS Google Scholar - Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP (1993). Cue reactivity and cue reactivity interventions in drug dependence. Nida Res Monogr 137: 73–95.
CAS PubMed Google Scholar - Claye LH, Akunne HC, Davis MD, DeMattos S, Soliman KF (1995). Behavioral and neurochemical changes in the dopaminergic system after repeated cocaine administration. Mol Neurobiol 11: 55–66.
Article CAS PubMed Google Scholar - Di Chiara G (1999). Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol 375: 13–30.
Article CAS PubMed Google Scholar - Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA et al (2001). Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry 49: 81–96.
Article CAS PubMed Google Scholar - Dwoskin LP, Peris J, Yasuda RP, Philpott K, Zahniser NR (1988). Repeated cocaine administration results in supersensitivity of striatal D-2 dopamine autoreceptors to pergolide. Life Sci 42: 255–262.
Article CAS PubMed Google Scholar - Evans SM, Haney M, Foltin RW (2002). The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 159: 397–406.
Article CAS Google Scholar - Evans SM, Walsh SL, Levin FR, Foltin RW, Fischman MW, Bigelow GE (2001). Effect of flupenthixol on subjective and cardiovascular responses to intravenous cocaine in humans. Drug Alcohol Depend 64: 271–283.
Article CAS PubMed Google Scholar - Farde L, Gustavsson JP, Jonsson E (1997). D2 dopamine receptors and personality traits. Nature 385: 590.
Article CAS PubMed Google Scholar - Farfel GM, Kleven MS, Woolverton WL, Seiden LS, Perry BD (1992). Effects of repeated injections of cocaine on catecholamine receptor binding sites, dopamine transporter binding sites and behavior in rhesus monkey. Brain Res 578: 235–243.
Article CAS PubMed Google Scholar - First M, Spitzer R, Gibbon M, Williams J (1995). Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P, Version 2.0). Biometrics Research Dept., New York State Psychiatric Institute: New York.
Google Scholar - First MB, Spitzer RL, Gibbon M, Williams JBW, Benjamin L (1994). Structured Clinical Interview for DSM-IV Axis II personality disorders (SCID-II, Version 2.0). Biometrics Research Department, New York State Psychiatric Department: New York.
Google Scholar - Fischman MW (1989). Relationship between self-reported drug effects and their reinforcing effects: studies with stimulant drugs. NIDA Res Monogr 92: 211–230.
CAS PubMed Google Scholar - Fischman MW, Foltin RW, Nestadt G, Pearlson GD (1990). Effects of desipramine maintenance on cocaine self-administration by humans. J Pharmacol Exp Ther 253: 760–770.
CAS PubMed Google Scholar - Foltin RW, Fischman MW (1996). Effects of methadone or buprenorphine maintenance on the subjective and reinforcing effects of intravenous cocaine in humans. J Pharmacol Exp Ther 278: 1153–1164.
CAS PubMed Google Scholar - Foltin RW, Fischman MW, Nestadt G, Stromberger H, Cornell EE, Pearlson GD (1990). Demonstration of naturalistic methods for cocaine smoking by human volunteers. Drug Alcohol Depend 26: 145–154.
Article CAS PubMed Google Scholar - Foltin RW, Ward AS, Collins ED, Haney M, Hart CL, Fischman MW (2003). The effects of venlafaxine on the subjective, reinforcing, and cardiovascular effects of cocaine in opioid-dependent and non-opioid-dependent humans. Exp Clin Psychopharmacol 11: 123–130.
Article CAS PubMed Google Scholar - Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frakowiak RSJ (1995). Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapping 2: 189–210.
Article Google Scholar - Gandelman MS, Baldwin RM, Zoghbi SS, Zea-Ponce Y, Innis RB (1994). Evaluation of ultrafiltration for the free fraction determination of single photon emission computerized tomography (SPECT) radiotracers: β-CIT, IBF and iomazenil. J Pharmaceut Sci 83: 1014–1019.
Article CAS Google Scholar - Goeders NE, Kuhar MJ (1987). Chronic cocaine administration induces opposite changes in dopamine receptors in the striatum and nucleus accumbens. Alcohol Drug Res 7: 207–216.
CAS PubMed Google Scholar - Haber SN, Fudge JL (1997). The primate substantia nigra and VTA: integrative circuitry and function. Crit Rev Neurobiol 11: 323–342.
Article CAS PubMed Google Scholar - Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L (1994). Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology 11: 245–256.
Article CAS PubMed Google Scholar - Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW (1999). Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration in humans. Psychopharmacology (Berl) 143: 102–110.
Article CAS Google Scholar - Hietala J, West C, Syvalahti E, Nagren K, Lehikoinen P, Sonninen P et al (1994). Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology (Berl) 116: 285–290.
Article CAS Google Scholar - Jacobsen LK, Giedd JN, Gottschalk C, Kosten TR, Krystal JH (2001). Quantitative morphology of the caudate and putamen in patients with cocaine dependence. Am J Psychiatry 158: 486–489.
Article CAS PubMed Google Scholar - Joel D, Weiner I (2000). The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience 96: 451–474.
Article CAS PubMed Google Scholar - Kleven MS, Perry BD, Woolverton WL, Seiden LS (1990). Effects of repeated injections of cocaine on D1 and D2 dopamine receptors in rat brain. Brain Res 532: 265–270.
Article CAS PubMed Google Scholar - Lamb RJ, Preston KL, Schindler C, Meisch RA, Davis F, Katz JL et al (1991). The reinforcing and subjective effects of morphine in post-addicts: a dose–response study. J Pharmacol Exp Ther 259: 1165–1173.
CAS PubMed Google Scholar - Laruelle M, DSouza CD, Baldwin RM, AbiDargham A, Kanes SJ, Fingado CL et al (1997). Imaging D-2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology 17: 162–174.
Article CAS PubMed Google Scholar - Laruelle M, van Dyck C, Abi-Dargham A, Zea-Ponce Y, Zoghbi SS, Charney DS et al (1994). Compartmental modeling of iodine-123-iodobenzofuran binding to dopamine D2 receptors in healthy subjects. J Nucl Med 35: 743–754.
CAS PubMed Google Scholar - Lemarie PG (1988). Ondelettes with exponential localization. J Math Pure Appl 67: 227–236.
Google Scholar - Mallat SG (1989). A theory for multiresolution signal decomposition—the wavelet representation. IEEE Trans Pattern Ana Mach Intell 11: 674–693.
Article Google Scholar - Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y et al (2003). Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 23: 285–300.
Article CAS PubMed Google Scholar - Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR et al (2001). Imaging human mesolimbic dopamine transmission with PET: I. Accuracy and precision of D2 parameter: measurements in the ventral striatum. J Cerebral Blood Flow Metab 21: 1034–1057.
Article CAS Google Scholar - Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP (1998). Effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys. Synapse 30: 88–96.
Article CAS PubMed Google Scholar - Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O et al (2002). Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci 5: 169–174.
Article CAS PubMed Google Scholar - Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR et al (2002). Effects of cocaine self-administration on striatal dopamine systems in Rhesus monkeys. initial and chronic exposure. Neuropsychopharmacology 27: 35–46.
Article CAS PubMed Google Scholar - Neisewander JL, Lucki I, McGonigle P (1994). Time-dependent changes in sensitivity to apomorphine and monoamine receptors following withdrawal from continuous cocaine administration in rats. Synapse 16: 1–10.
Article CAS PubMed Google Scholar - Nestler EJ, Terwilliger RZ, Walker JR, Sevarino KA, Duman RS (1990). Chronic cocaine treatment decreases levels of the G protein subunits Gi alpha and Go alpha in discrete regions of rat brain. J Neurochem 55: 1079–1082.
Article CAS PubMed Google Scholar - Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB et al (2002). Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci 22: 7687–7694.
Article CAS PubMed PubMed Central Google Scholar - Robinson TE, Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18: 247–291.
Article CAS PubMed Google Scholar - Rousset OG, Ma Y, Evans AC (1998). Correction for partial volume effects in PET: principle and validation. J Nucl Med 39: 904–911.
CAS PubMed Google Scholar - Schneier FR, Liebowitz MR, Abi-Dargham A, Zea-Ponce Y, Lin SH, Laruelle M (2000). Low dopamine D(2) receptor binding potential in social phobia. Am J Psychiatry 157: 457–459.
Article CAS PubMed Google Scholar - Sokoloff P, Giros B, Martres M-P, Bouthenet M-L, Schwartz J-C (1990). Molecular cloning and characterization of a novel dopamine receptor D3 as a target for neuroleptics. Nature 347: 146–151.
Article CAS PubMed Google Scholar - Striplin CD, Kalivas PW (1993). Robustness of G protein changes in cocaine sensitization shown with immunoblotting. Synapse 14: 10–15.
Article CAS PubMed Google Scholar - Taylor DL, Ho BT, Fagan JD (1979). Increased dopamine receptor binding in rat brain by repeated cocaine injections. Commun Psychopharmacol 3: 137–142.
CAS PubMed Google Scholar - Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ (1991). A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res 548: 100–110.
Article CAS PubMed Google Scholar - Trulson ME, Ulissey MJ (1987). Chronic cocaine administration decreases dopamine synthesis rate and increases [3 H] spiroperidol binding in rat brain. Brain Res Bull 19: 35–38.
Article CAS PubMed Google Scholar - Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M et al (2001). Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158: 2015–2021.
Article CAS PubMed Google Scholar - Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ et al (1993). Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14: 169–177.
Article CAS PubMed Google Scholar - Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R et al (1990). Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry 147: 719–724.
Article CAS PubMed Google Scholar - Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A et al (1999). Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry 156: 1440–1443.
Article CAS PubMed Google Scholar - Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R et al (1997). Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386: 830–833.
Article CAS PubMed Google Scholar - Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS et al (1996). Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res 20: 1594–1598.
Article CAS PubMed Google Scholar - Volkow ND, Wang GJ, Fowler JS, Thanos P, Logan J, Gatley SJ et al (2002). Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse 46: 79–82.
Article CAS PubMed Google Scholar - Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ et al (1997). Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology 16: 174–182.
Article CAS PubMed Google Scholar - Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W et al (2001). Brain dopamine and obesity. Lancet 357: 354–357.
Article CAS PubMed Google Scholar - Whiters N, Pulivirenti L, Koob G, Gillin J (1995). Cocaine abuse and dependence. J Clin Psychopharmacol 15: 63–78.
Article Google Scholar - Wise R, Romprè P (1989). Brain dopamine and reward. Ann Rev Psychol 40: 199–225.
Article Google Scholar - Woods RP, Cherry SR, Mazziotta JC (1992). Rapid automated algorithm for aligning and reslicing PET images. J Comp Assist Tomogr 16(4): 620–633.
Article CAS Google Scholar - Woods RP, Mazziotta JC, Cherry SR (1993). MRI-PET registration with automated algorithm. J Compu Assist Tomogr 17(4): 536–546.
Article CAS Google Scholar - Wu JC, Bell K, Najafi A, Widmark C, Keator D, Tang C et al (1997). Decreasing striatal 6-FDOPA uptake with increasing duration of cocaine withdrawal. Neuropsychopharmacology 17: 402–409.
Article CAS PubMed Google Scholar - Zeigler S, Lipton J, Toga A, Ellison G (1991). Continuous cocaine administration produces persisting changes in brain neurochemistry and behavior. Brain Res 552: 27–35.
Article CAS PubMed Google Scholar
Acknowledgements
The authors would like to thank Julie Arcement, Jennifer Bae, Ingrid Gelbard-Stokes, Elizabeth Hackett, Kimchung Ngo, Chaka Peters, Beatriu Reig, Lyudmila Savenkova, Norman Simpson, and Kris Wolff for excellent technical assistance. Supported by the Public Health Service (NIDA 2- RO1-DA10219-01, NIDA PA50 DA 09236-06, NIDA K08 DA00483-01, and NIH M01RR00645).
Author information
Authors and Affiliations
- Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York, NY, USA
Diana Martinez, Allegra Broft, Richard W Foltin, Mark Slifstein, Dah-Ren Hwang, Yiyun Huang, Audrey Perez, W Gordon Frankel, Thomas Cooper, Herbert D Kleber, Marian W Fischman & Marc Laruelle - Department of Radiology, Columbia University College of Physicians and Surgeons, New York, NY, USA
Marc Laruelle
Authors
- Diana Martinez
- Allegra Broft
- Richard W Foltin
- Mark Slifstein
- Dah-Ren Hwang
- Yiyun Huang
- Audrey Perez
- W Gordon Frankel
- Thomas Cooper
- Herbert D Kleber
- Marian W Fischman
- Marc Laruelle
Corresponding author
Correspondence toDiana Martinez.
Rights and permissions
About this article
Cite this article
Martinez, D., Broft, A., Foltin, R. et al. Cocaine Dependence and D2 Receptor Availability in the Functional Subdivisions of the Striatum: Relationship with Cocaine-Seeking Behavior.Neuropsychopharmacol 29, 1190–1202 (2004). https://doi.org/10.1038/sj.npp.1300420
- Received: 25 August 2003
- Revised: 21 November 2003
- Accepted: 25 November 2003
- Published: 10 March 2004
- Issue Date: 01 June 2004
- DOI: https://doi.org/10.1038/sj.npp.1300420