Comparison of incubation period distribution of human infections with MERS-CoV in South Korea and Saudi Arabia (original) (raw)
Introduction
South Korea experienced the largest outbreak of Middle East respiratory syndrome coronavirus (MERS-CoV) infections outside the Arabian Peninsula with 186 laboratory-confirmed cases from May to July 2015[1](/articles/srep35839#ref-CR1 "The World Health Organization (WHO). Outbreaks and emergencies: Middle East respiratory syndrome coronavirus (MERS-CoV): WHO Western Pacific Region; 2015. Available at: http://www.wpro.who.int/outbreaks_emergencies/wpro_coronavirus/en/
. (Accessed: 15th July 2015)."). As of 26 April 2016, a total of 1,728 laboratory-confirmed cases of MERS-CoV infection have been diagnosed globally and reported to the World Health Organization since the first reported case in Saudi Arabia in 2012, of which more than 1,000 cases have occurred in Saudi Arabia[1](/articles/srep35839#ref-CR1 "The World Health Organization (WHO). Outbreaks and emergencies: Middle East respiratory syndrome coronavirus (MERS-CoV): WHO Western Pacific Region; 2015. Available at:
http://www.wpro.who.int/outbreaks_emergencies/wpro_coronavirus/en/
. (Accessed: 15th July 2015)."),[2](/articles/srep35839#ref-CR2 "The World Health Organization (WHO). Middle East respiratory syndrome coronavirus (MERS-CoV): WHO updates. Available at:
http://www.who.int/emergencies/mers-cov/en/
(Accessed: 1st June 2016)."),[3](/articles/srep35839#ref-CR3 "Cowling, B. J. et al. Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June 2015. Euro Surveill. 20 (2015)."),[4](/articles/srep35839#ref-CR4 "Zaki, A. M., van Boheemen, S., Bestebroer, T. M., Osterhaus, A. D. M. E. & Fouchier, R. A. M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 367, 1814–1820 (2012)."). Estimation of the incubation period distribution, which is defined as the period between exposure/infection and the appearance of the first symptoms[5](/articles/srep35839#ref-CR5 "Vynnycky, E. & Fine, P. E. Lifetime risks, incubation period, and serial interval of tuberculosis. Am. J. Epidemiol. 152, 247–263 (2000)."), is a key parameter in the transmission dynamics, and forms part of the case definition[6](/articles/srep35839#ref-CR6 "Centers for Disease Control and Prevention (CDC). Middle East respiratory syndrome (MERS): Interim Patient under Investigation (PUI) Guidance and Case Definitions. Available at:
http://www.cdc.gov/coronavirus/mers/case-def.html
. (Accessed: 1st May 2016)."), is used to define the appropriate quarantine period, and is one of the parameters used in mathematical modeling studies to predict the impact of different control strategies[7](/articles/srep35839#ref-CR7 "Chowell, G., Blumberg, S., Simonsen, L., Miller, M. A. & Viboud, C. Synthesizing data and models for the spread of MERS-CoV, 2013: key role of index cases and hospital transmission. Epidemics 9, 40–51 (2014)."). There are a few published reports of the incubation period distribution of MERS-CoV infection, with a median incubation period varying from 5.2 days (95% confidence interval, 1.9–14.7 days) in the Middle East[8](/articles/srep35839#ref-CR8 "Assiri, A. et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N. Engl. J. Med. 369, 407–416 (2013).") to 6.0 days (95% confidence interval, 4–7 days) and 6.3 days (95% credibility interval, 5.7–6.8 days) in the recent outbreak in South Korea[9](/articles/srep35839#ref-CR9 "Cowling, B. J. et al. Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June 2015. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 20 (2015)."),[10](/articles/srep35839#ref-CR10 "Park, H. Y. et al. Epidemiological investigation of MERS-CoV spread in a single hospital in South Korea, May to June 2015. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 20 (2015)."). The objective of our study was to describe alternative approaches for estimation of the incubation period of MERS-CoV infection and to investigate whether there was variability in the incubation period by age, sex and geographic location.Results
Data on defined exposure periods and onset dates for cases in South Korea of the 2015 outbreak were available from 115 (63%) of all 186 patients diagnosed with laboratory-confirmed MERS-CoV infection, and we identified published data on exposure periods and onset dates for 34 patients of the 1,456 patients in Saudi Arabia as of 4 May 2016[2](/articles/srep35839#ref-CR2 "The World Health Organization (WHO). Middle East respiratory syndrome coronavirus (MERS-CoV): WHO updates. Available at: http://www.who.int/emergencies/mers-cov/en/
(Accessed: 1st June 2016)."),[11](/articles/srep35839#ref-CR11 "Assiri, A. et al. Hospital Outbreak of Middle East Respiratory Syndrome Coronavirus. N. Engl. J. Med. 369, 407–416 (2013)."),[12](/articles/srep35839#ref-CR12 "Health Protection Agency (HPA) UK Novel Coronavirus Investigation team. Evidence of person-to-person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 18, 20427 (2013)."),[13](/articles/srep35839#ref-CR13 "Guery, B. et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet Lond. Engl. 381, 2265–2272 (2013)."),[14](/articles/srep35839#ref-CR14 "Cauchemez, S. et al. Middle East respiratory syndrome coronavirus: quantification of the extent of the epidemic, surveillance biases, and transmissibility. Lancet Infect. Dis. 14, 50–56 (2014)."). The characteristics of patients from Korea and from Saudi Arabia are reported in [Table 1](/articles/srep35839#Tab1). Among patients with exposure data, the mean age was 54 years and 63% were males, and the age and sex of patients were similar in South Korea and in Saudi Arabia. The risk of death was much higher in the cases from Saudi Arabia (16/34; 47%) compared to the cases from South Korea (26/115; 23%) (p = 0.01, chi-squared test).Table 1 Characteristics of cases of MERS-CoV infection in South Korea and Saudi Arabia.
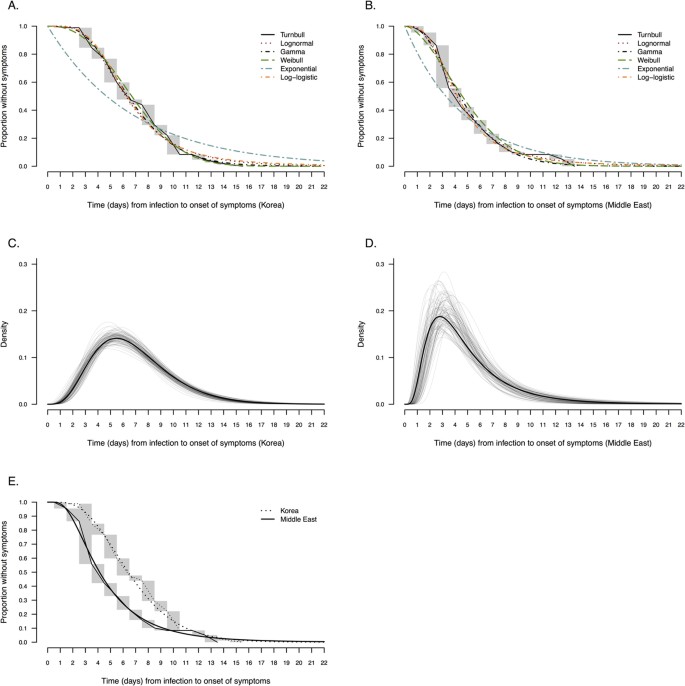
Figure 1A,B compare alternative parametric models with the non-parametric maximum likelihood estimator. Visual inspection of the parametric curves against the Turnbull estimate in Fig. 1A,B confirm that all of the two-parameter distributions provided reasonable fits, while the one-parameter exponential distribution was inferior. Among the cases in South Korea, the gamma and Weibull parametric models (Fig. 1C) had the best BIC value with an estimated mean of 6.9 days (95% credibility interval: 6.3–7.5) (Table 2). Among cases from Saudi Arabia, the lognormal distribution (Fig. 1D) had the best BIC value with an estimated mean of 5.0 days (95% credibility interval: 4.0–6.6) (Table 2). The other fitted two-parameter distributions had generally similar means, with 95th percentiles in the range 10–14 days and 99th percentiles in the range 14–22 days (Table 2). Except for the exponential distribution, the various two-parameter distributions had similar BIC values among the cases in each location.
Table 2 Alternative parametric estimates of the mean of the incubation distribution of MERS-CoV infection based on all available data.
Figure 1
Comparison of nonparametric and parametric estimates of the incubation period distribution in cases of MERS-CoV infection in South Korea and Saudi Arabia.
Panel (A,B) compare the Turnbull nonparametric estimate of the incubation period distribution with the fitted lognormal, Weibull, gamma, loglogistic and exponential distributions using data from (A) South Korea (n = 115) and Saudi Arabia (n = 34). Panel (C,D) present the probability density function of the parametric model with the best BIC value for the cases in South Korea (gamma distribution) and in Saudi Arabia (lognormal distribution). The solid line represents the uncertainty range estimated by bootstrapping with 1,000 resamples. Panel (E) compares the nonparametric (Turnbull) and parametric estimates of the incubation period distribution in South Korea (gamma distribution) and in Saudi Arabia (lognormal distribution).
Since a lognormal distribution gave a good fit to the data in both locations, we pooled information from both locations and fitted a log-linear regression model to the data. Using that model, we found that the mean incubation period was 1.40 times longer (95% credibility interval: 1.15–1.71) among cases in South Korea compared to Saudi Arabia without adjustment, and the estimate was almost the same after adjustment for age and sex (Table 3).
Table 3 Factors associated with the incubation period of MERS-CoV infection.
Discussion
Using all available data for the recent outbreak of MERS-CoV infections in South Korea, and published data from cases in Saudi Arabia, we estimated that the mean incubation period was 6.9 days for cases in South Korea and 5.0 days for cases in Saudi Arabia. In various parametric models, the 95th percentiles were in the range 10–14 days, which is consistent with the currently used case definitions[6](/articles/srep35839#ref-CR6 "Centers for Disease Control and Prevention (CDC). Middle East respiratory syndrome (MERS): Interim Patient under Investigation (PUI) Guidance and Case Definitions. Available at: http://www.cdc.gov/coronavirus/mers/case-def.html
. (Accessed: 1st May 2016)."). While it is difficult to estimate the right hand tail of the incubation period distribution based on small sample sizes, we estimated the 99th percentile could be as long as 14–22 days and this indicates that long incubation periods are possible. In South Korea, one of the 186 cases was reported to have an incubation period of 21 days or longer, although it has been suggested that immunosuppression in that person could potentially have delayed the onset of symptoms[15](/articles/srep35839#ref-CR15 "South Korean woman has MERS - a week after quarantine over. The Straits Time (2015).").Our estimates for the incubation period distribution of MERS-CoV infections in Saudi Arabia are consistent with the previous estimates of Assiri et al.8. in a hospital outbreak in the eastern province of Saudi Arabia based on 23 cases with an estimated median incubation period of 5.2 days (95% confidence interval: 1.9–14.7 days). Our estimates for cases in South Korea are also close to other reports with an estimated mean incubation period of 6.7 days (95% credibility interval: 6.1–7.3 days)9, and a median of 6 days in one hospital9,10.
We found a significant difference in mean incubation periods between the cases in South Korea and in Saudi Arabia (Table 3). This difference could be related to the transmission dynamics of MERS-CoV infection with only secondary cases and longer transmission chains in the outbreak in South Korea9, compared with cases in Saudi Arabia included in this study where a majority (74%) came from the same hospital8 where it has already been shown that the transmission chain was shorter with multiple separate animal-to-human infections16,17. Potential direct transmission could be related to a higher infecting dose and higher virulence of the strain that could lead to a shorter incubation period18. A recent studies on MERS-CoV transmission during the outbreak in South Korea reported different estimates of the incubation period depending on the intensity of exposure and/or inoculation route19. Indeed, the authors showed that the incubation period was significantly shorter among patients that were exposed to the index case in the same zone of the emergency room (median: 5 days; interquartile range (IQR): 4–8 days) compared with patients from different zones (median: 11 days; IQR: 6–12 days). These results strengthen the hypothesis that a higher infecting dose could have been transmitted by the index case leading to a shorter incubation period compared with cases associated with “indirect” transmission that may have been responsible for transmission in different zones of the emergency room. Further investigation on human-to-human and human-to-animal transmission dynamics would improve our understanding of the potential role of the exposure route on the incubation period. It is also possible that this difference is an artifact of different approaches to data collection or reporting in South Korea and in Saudi Arabia.
Our study had some limitations. First, we did not have access to original patient records, and our data on MERS-CoV infections in South Korea were based on publicly available information while we relied on published data for a relatively small number of cases in Saudi Arabia. There is a potential concern that symptoms and symptom onset dates might be reported differentially in the two locations. In the cases of MERS-CoV infection reported by Assiri et al. from Saudi Arabia, 20/23 cases had fever on the day of symptom onset11. In the outbreak in South Korea, fever was part of the case definition and symptom onset may reflect the date of onset of fever rather than other symptoms20,21. Secondly, regarding patients from Saudi Arabia, only 34 patients among the 1,456 patients (2%) diagnosed with MERS-CoV infection since 2012 in Saudi Arabia had publicly-available exposure data and most of these patients (25/34, 73%) came from the same hospital8. However, a larger proportion of patients from the outbreak in South Korea (N = 115; 63%) had publicly-available exposure data. Third, the outbreak in South Korea occurred 2 years after the first officially announced case of MERS-CoV infection in Saudi Arabia during which time the virus may have evolved somewhat, including changes in the transmission and pathogenesis characteristics. Fourth, no information about inoculation route was available for the patients included in this study as the data were retrieved from publicly available data or published studies.
In conclusion, accurate and rapid estimates of the length of incubation period are required during an outbreak to advise public health policy, to specify case definitions, and to facilitate robust mathematical modeling. In this paper, we assessed precisely the length of incubation period of MERS-CoV infections using two different datasets from Saudi Arabia and from South Korea and showed that the incubation period of MERS-CoV infections appeared to vary depending on the location of the outbreak.
Methods
Sources of data
For the outbreak in South Korea, we retrieved publicly available data from multiple sources, including the Korea Center for Disease Control and Prevention, the Korean Ministry of Health and Welfare, the World Health Organization, and local Korean news reports to compile a line list of all confirmed cases that had been reported by 27 July 2015. We used the most updated information from official reports that have been published by the Center for Disease Control and Prevention and the Ministry of Health and Welfare on a daily basis during the outbreak. The official reports included a brief description of each of all confirmed cases, including demographic characteristics (e.g., age and sex), dates of exposure, onset of symptoms and outcome. The information on exposure was mostly recorded as intervals of 2 to 15 days during which transmission was thought to have occurred rather than exact dates of presumed transmission.
Information on cases of MERS-CoV infection in the Middle East were retrieved from four published studies that provided individual patient data from Saudi Arabia11,12,13,14. We selected only the cases with available exposure information and collected data including demographic characteristics (e.g. age and sex), dates of exposure and onset of symptoms, geographical location of the exposure, and final outcome. For both locations, the day of symptoms onset was defined as the day when clinical symptoms related to MERS-CoV infection first occurred, including non-specific symptoms such as fever, chills, shortness of breath, cough, sputum, sore throat, myalgia, diarrhea, nausea and vomiting[1](/articles/srep35839#ref-CR1 "The World Health Organization (WHO). Outbreaks and emergencies: Middle East respiratory syndrome coronavirus (MERS-CoV): WHO Western Pacific Region; 2015. Available at: http://www.wpro.who.int/outbreaks_emergencies/wpro_coronavirus/en/
. (Accessed: 15th July 2015)."),[11](/articles/srep35839#ref-CR11 "Assiri, A. et al. Hospital Outbreak of Middle East Respiratory Syndrome Coronavirus. N. Engl. J. Med. 369, 407–416 (2013)."),[12](/articles/srep35839#ref-CR12 "Health Protection Agency (HPA) UK Novel Coronavirus Investigation team. Evidence of person-to-person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 18, 20427 (2013)."),[13](/articles/srep35839#ref-CR13 "Guery, B. et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet Lond. Engl. 381, 2265–2272 (2013)."),[14](/articles/srep35839#ref-CR14 "Cauchemez, S. et al. Middle East respiratory syndrome coronavirus: quantification of the extent of the epidemic, surveillance biases, and transmissibility. Lancet Infect. Dis. 14, 50–56 (2014)."),[20](/articles/srep35839#ref-CR20 "Korea Centers for Disease Control and Prevention. Middle East Respiratory Syndrome Coronavirus Outbreak in the Republic of Korea, 2015. Osong Public Health Res. Perspect. 6, 269–278 (2015)."),[21](/articles/srep35839#ref-CR21 "Kim, K. M. et al. Epidemiologic features of the first MERS outbreak in Korea: focus on Pyeongtaek St. Mary’s Hospital. Epidemiol. Health 37, e2015041 (2015).").Statistical analyses
The incubation period Tk for each case k is defined as Tk = Sk–Xk, where Sk is the symptom onset time and Xk the infection time. Infection events are rarely observed but rather interval-censored. If case k reported that exposure to infection occurred in a period between times Lk and Uk, where Lk≤Xk≤U_k_, the incubation time therefore is bounded by the interval (Sk-Uk, Sk-Lk). These interval-censored data are a special type of survival data, and it is possible to “reverse” the time axis considering Sk as the origin and Xk as the outcome time, if the density function for infection is uniform in chronologic time22. This condition should be reasonable here in the setting of MERS-CoV infections, with each exposure interval being relatively short, and reversing the time axis allowed us to use standard approaches for interval-censored data. We added +0.5 to each upper bound and −0.5 to each lower bound to give appropriate intervals in continuous time and to account account for uncertainty in the reported exposure times23. For example, an exposure that was reported two and three days before illness onset would be written as an incubation period censored on the interval (1.5, 3.5) instead of (2,3).
To deal with interval-exposure data, the most basic approach is to impute the infection dates as the midpoint of exposure intervals, but this leads to overestimation of the incubation period distribution, which tends to be right-skewed24. Non-parametric estimation of a distribution based on interval-censored data can be done with the generalized non-parametric maximum likelihood estimator developed by Turnbull25. The incubation period can often be appropriately characterized by different parametric distributions that have been previously used such as gamma9,26, Weibull9,27,28, lognormal9,18, log-logistic, and exponential distributions. We fitted five different distributions and estimated the parameters of each distribution using Markov Chain Monte Carlo (MCMC) in a Bayesian framework. The incubation period distribution was estimated using first the interval-censored data and compared between the different parametric models (using Bayesian Information Criterion) and the Turnbull non-parametric estimate25.
To evaluate potential factors such as age, sex and geographic location that could be associated with the length of the incubation period, we used a linear regression model on the log of the incubation period (assuming that incubation periods generally followed lognormal distributions), which can also be referred to as a log-linear model. The multiple linear regression model used in this study is based on the following equation:

where _IncP_i is the length of incubation period for individual i, β _i_’s are the regression coefficients, estimated with MCMC using flat priors, X i ’s the explanatory variables labeled directly in the equation above and ε Ithe disturbance factor, normally distributed, independently and identically with E(εi) = 0 and V(εi) = _σ_2 for all i. We used two different approaches to estimate model parameters, including an exact likelihood method and a resampling method29.
Approach 1: exact likelihood approach
The equation (1) above can be written as:
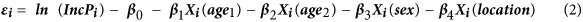
and consequently using equation (2) we can define the following pdf of the normal distribution:
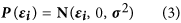
We defined the probability q i as:
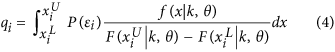
where  is the range of incubation period for case i and where (k, θ) is the couple of parameters of the gamma distribution.
is the range of incubation period for case i and where (k, θ) is the couple of parameters of the gamma distribution.
We estimated θ = (β 0, β 1, β 2, β 3, β 4, k, _θ, σ_2) simultaneously using MCMC and the following likelihood:

where f and F are the pdf and cdf of the gamma distribution with parameters k and θ, respectively.
Approach 2: resampling approach
We defined another multiple linear regression model using incubation times resampled from the 10,000 posterior samples. In this approach, the probability P(ε i) was similarly defined as in equation (3) and for each patient with interval-censored exposure data, we estimated 10,000 posterior samples for the incubation time using MCMC in order to simulate the incubation period distribution for each patient. We used the same likelihood as defined in equation (5) using the resampled incubation time for each patient.
In this analysis and the analyses described above, we used a Metropolis-Hasting algorithm, specified flat priors for each parameter, and drew 10,000 samples from the posterior distributions after a burn-in of 5,000 iterations. All analyses presented here were conducted using R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Additional Information
How to cite this article: Virlogeux, V. et al. Comparison of incubation period distribution of human infections with MERS-CoV in South Korea and Saudi Arabia. Sci. Rep. 6, 35839; doi: 10.1038/srep35839 (2016).
References
- The World Health Organization (WHO). Outbreaks and emergencies: Middle East respiratory syndrome coronavirus (MERS-CoV): WHO Western Pacific Region; 2015. Available at: http://www.wpro.who.int/outbreaks_emergencies/wpro_coronavirus/en/. (Accessed: 15th July 2015).
- The World Health Organization (WHO). Middle East respiratory syndrome coronavirus (MERS-CoV): WHO updates. Available at: http://www.who.int/emergencies/mers-cov/en/ (Accessed: 1st June 2016).
- Cowling, B. J. et al. Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June 2015. Euro Surveill. 20 (2015).
- Zaki, A. M., van Boheemen, S., Bestebroer, T. M., Osterhaus, A. D. M. E. & Fouchier, R. A. M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 367, 1814–1820 (2012).
Article CAS Google Scholar - Vynnycky, E. & Fine, P. E. Lifetime risks, incubation period, and serial interval of tuberculosis. Am. J. Epidemiol. 152, 247–263 (2000).
Article CAS Google Scholar - Centers for Disease Control and Prevention (CDC). Middle East respiratory syndrome (MERS): Interim Patient under Investigation (PUI) Guidance and Case Definitions. Available at: http://www.cdc.gov/coronavirus/mers/case-def.html. (Accessed: 1st May 2016).
- Chowell, G., Blumberg, S., Simonsen, L., Miller, M. A. & Viboud, C. Synthesizing data and models for the spread of MERS-CoV, 2013: key role of index cases and hospital transmission. Epidemics 9, 40–51 (2014).
Article Google Scholar - Assiri, A. et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N. Engl. J. Med. 369, 407–416 (2013).
Article CAS Google Scholar - Cowling, B. J. et al. Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June 2015. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 20 (2015).
- Park, H. Y. et al. Epidemiological investigation of MERS-CoV spread in a single hospital in South Korea, May to June 2015. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 20 (2015).
- Assiri, A. et al. Hospital Outbreak of Middle East Respiratory Syndrome Coronavirus. N. Engl. J. Med. 369, 407–416 (2013).
Article CAS Google Scholar - Health Protection Agency (HPA) UK Novel Coronavirus Investigation team. Evidence of person-to-person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 18, 20427 (2013).
- Guery, B. et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet Lond. Engl. 381, 2265–2272 (2013).
Article Google Scholar - Cauchemez, S. et al. Middle East respiratory syndrome coronavirus: quantification of the extent of the epidemic, surveillance biases, and transmissibility. Lancet Infect. Dis. 14, 50–56 (2014).
Article Google Scholar - South Korean woman has MERS - a week after quarantine over. The Straits Time (2015).
- Cotten, M. et al. Transmission and evolution of the Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive genomic study. Lancet Lond. Engl. 382, 1993–2002 (2013).
Article Google Scholar - Madani, T. A., Azhar, E. I. & Hashem, A. M. Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 371, 1360 (2014).
PubMed Google Scholar - Virlogeux, V. et al. Incubation Period Duration and Severity of Clinical Disease Following Severe Acute Respiratory Syndrome Coronavirus Infection. Epidemiol. Camb. Mass (2015).
- Cho, S. Y. et al. MERS-CoV outbreak following a single patient exposure in an emergency room in South Korea: an epidemiological outbreak study. Lancet Lond. Engl. (2016).
- Korea Centers for Disease Control and Prevention. Middle East Respiratory Syndrome Coronavirus Outbreak in the Republic of Korea, 2015. Osong Public Health Res. Perspect. 6, 269–278 (2015).
- Kim, K. M. et al. Epidemiologic features of the first MERS outbreak in Korea: focus on Pyeongtaek St. Mary’s Hospital. Epidemiol. Health 37, e2015041 (2015).
Article Google Scholar - De Gruttola, V. & Lagakos, S. W. Analysis of doubly-censored survival data, with application to AIDS. Biometrics 45, 1–11 (1989).
Article CAS MathSciNet Google Scholar - Farewell, V. T., Herzberg, A. M., James, K. W., Ho, L. M. & Leung, G. M. SARS incubation and quarantine times: when is an exposed individual known to be disease free? Stat. Med. 24, 3431–3445 (2005).
Article CAS MathSciNet Google Scholar - Sartwell, P. E. The distribution of incubation periods of infectious disease. Am. J. Hyg. 51, 310–318 (1950).
CAS PubMed Google Scholar - Turnbull, B. & Bechhofer, R. The Empirical Distribution Function with Arbitrarily Grouped, Censored and Truncated Data. J. R. Stat. Soc. 38, 290–295 (1976).
MathSciNet MATH Google Scholar - Cauchemez, S. et al. Middle East respiratory syndrome coronavirus: quantification of the extent of the epidemic, surveillance biases, and transmissibility. Lancet Infect. Dis. 14, 50–56 (2014).
Article Google Scholar - Virlogeux, V. et al. Estimating the Distribution of the Incubation Periods of Human Avian Influenza A(H7N9) Virus Infections. Am. J. Epidemiol. 182, 723–729 (2015).
Article Google Scholar - Kuk, A. Y. C. & Ma, S. The estimation of SARS incubation distribution from serial interval data using a convolution likelihood. Stat. Med. 24, 2525–2537 (2005).
Article MathSciNet Google Scholar - Virlogeux, Victor, Park, Minah, Wu, Joseph T. & Benjamin, J. Cowling Association between Severity of MERS-CoV Infection and Incubation Period. Emerg. Infect. Dis. J. 22 (2016).
Acknowledgements
This research was supported by the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558), and a commissioned grant from the Health and Medical Research Fund, Food and Health Bureau, Government of the Hong Kong Special Administrative Region. The funding bodies had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish.
Author information
Authors and Affiliations
- Department of Biology, Ecole Normale Supérieure de Lyon, Lyon, France
Victor Virlogeux - WHO Collaborating Centre for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China
Victor Virlogeux, Vicky J. Fang, Minah Park, Joseph T. Wu & Benjamin J. Cowling - Cancer Research Center of Lyon, UMR Inserm U1052, CNRS, 5286, Lyon, France
Victor Virlogeux
Authors
- Victor Virlogeux
You can also search for this author inPubMed Google Scholar - Vicky J. Fang
You can also search for this author inPubMed Google Scholar - Minah Park
You can also search for this author inPubMed Google Scholar - Joseph T. Wu
You can also search for this author inPubMed Google Scholar - Benjamin J. Cowling
You can also search for this author inPubMed Google Scholar
Contributions
Conceived of the study: V.V. Collected data: M.P. Performed statistical analysis: V.V. Wrote the first draft: V.V. Edited the manuscript and approved the final version: V.V., V.J.F., M.P., J.T.W. and B.J.C.
Ethics declarations
Competing interests
BJC reports receipt of research funding from MedImmune Inc. and Sanofi Pasteur, and consults for Crucell NV. The authors report no other potential conflicts of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Virlogeux, V., Fang, V., Park, M. et al. Comparison of incubation period distribution of human infections with MERS-CoV in South Korea and Saudi Arabia.Sci Rep 6, 35839 (2016). https://doi.org/10.1038/srep35839
- Received: 09 May 2016
- Accepted: 05 October 2016
- Published: 24 October 2016
- DOI: https://doi.org/10.1038/srep35839