High-density lipoprotein’s vascular protective functions in metabolic and cardiovascular disease – could extracellular vesicles be at play? (original) (raw)
Article navigation
Cover Image
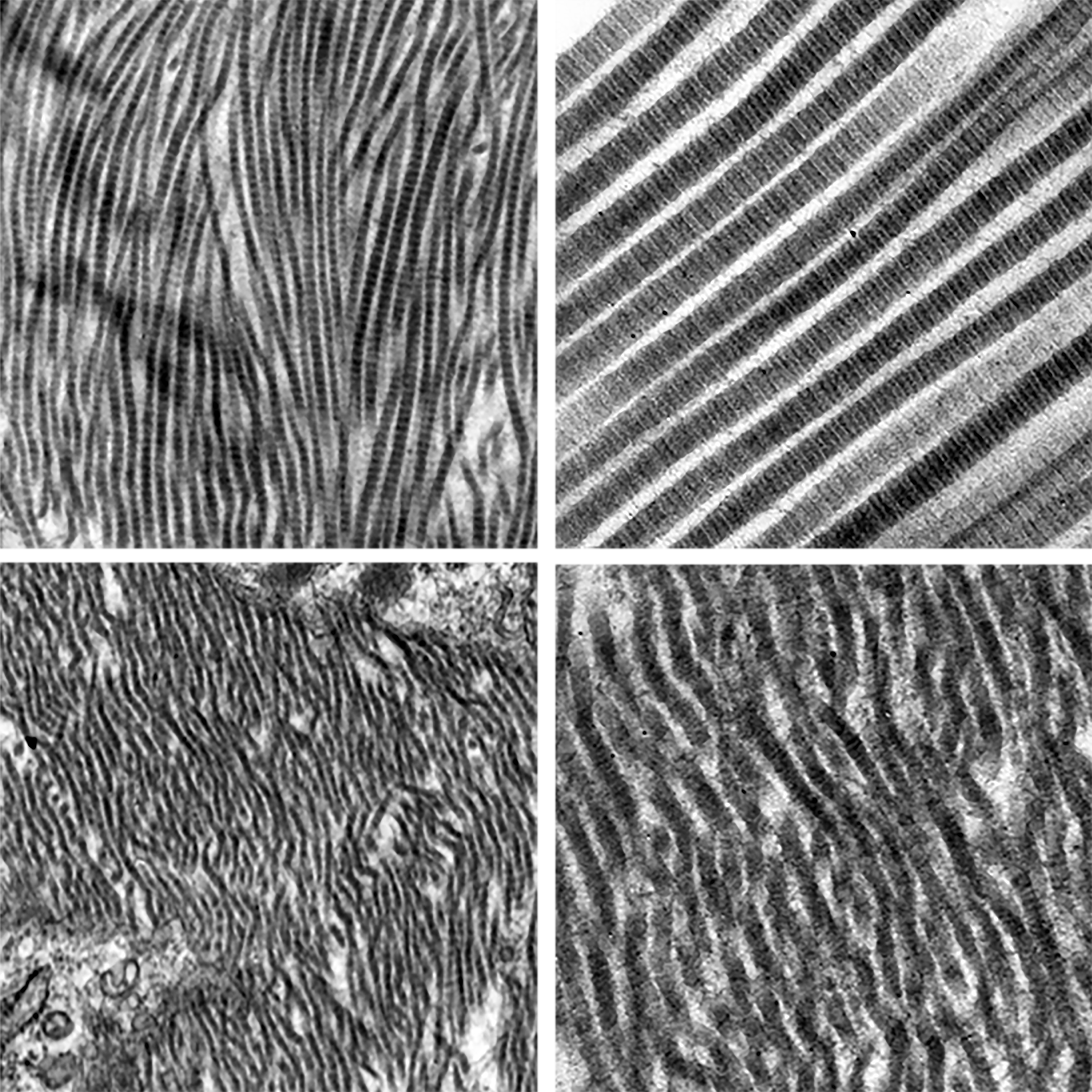
The cover image of this issue of Clinical Science (volume 134, issue 22) features representative transmission electron microscopy images of normal (top panel; AngII + DAPT) and abnormal collagen fibrils (bottom panel; AngII + vehicle) in the mouse aorta. In their study, Hans et al. highlight the novel therapeutic potentials of Notch inhibitor (DAPT) to regress an actively growing abdominal aortic aneurysm via divergent pathways.
Review Article| November 19 2020
;
1Institute of Cardiovascular and Medical Science, College of Medical Veterinary and Life Science, University of Glasgow, Wolfson Link Building, University Avenue, Glasgow G12 8QQ, U.K.
Search for other works by this author on:
1Institute of Cardiovascular and Medical Science, College of Medical Veterinary and Life Science, University of Glasgow, Wolfson Link Building, University Avenue, Glasgow G12 8QQ, U.K.
Search for other works by this author on:
1Institute of Cardiovascular and Medical Science, College of Medical Veterinary and Life Science, University of Glasgow, Wolfson Link Building, University Avenue, Glasgow G12 8QQ, U.K.
Search for other works by this author on:
1Institute of Cardiovascular and Medical Science, College of Medical Veterinary and Life Science, University of Glasgow, Wolfson Link Building, University Avenue, Glasgow G12 8QQ, U.K.
Search for other works by this author on:
2Occupational and Environmental Medicine Centre, Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden
Search for other works by this author on:
2Occupational and Environmental Medicine Centre, Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden
Search for other works by this author on:
3Section of Pharmacology, Vascular and Metabolic Diseases, Department of Internal Medicine, Erasmus University Medical Centre, Rotterdam, The Netherlands
Search for other works by this author on:
1Institute of Cardiovascular and Medical Science, College of Medical Veterinary and Life Science, University of Glasgow, Wolfson Link Building, University Avenue, Glasgow G12 8QQ, U.K.
Search for other works by this author on:
Publisher: Portland Press Ltd
Received: June 30 2020
Revision Received: October 19 2020
Accepted: November 09 2020
Online ISSN: 1470-8736
Print ISSN: 0143-5221
© 2020 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society
2020
Clin Sci (Lond) (2020) 134 (22): 2977–2986.
Revision Received:
October 19 2020
Accepted:
November 09 2020
Abstract
High-density lipoprotein (HDL) is a circulating complex of lipids and proteins known primarily for its role in reverse cholesterol transport and consequent protection from atheroma. In spite of this, therapies aimed at increasing HDL concentration do not reduce the risk of cardiovascular disease (CVD), and as such focus has shifted towards other HDL functions protective of vascular health – including vasodilatory, anti-inflammatory, antioxidant and anti-thrombotic actions. It has been demonstrated that in disease states such as CVD and conditions of insulin resistance such as Type 2 diabetes mellitus (T2DM), HDL function is impaired owing to changes in the abundance and function of HDL-associated lipids and proteins, resulting in reduced vascular protection. However, the gold standard density ultracentrifugation technique used in the isolation of HDL also co-isolates extracellular vesicles (EVs). EVs are ubiquitous cell-derived particles with lipid bilayers that carry a number of lipids, proteins and DNA/RNA/miRNAs involved in cell-to-cell communication. EVs transfer their bioactive load through interaction with cell surface receptors, membrane fusion and endocytic pathways, and have been implicated in both cardiovascular and metabolic diseases – both as protective and pathogenic mediators. Given that studies using density ultracentrifugation to isolate HDL also co-isolate EVs, biological effects attributed to HDL may be confounded by EVs. We hypothesise that some of HDL’s vascular protective functions in cardiovascular and metabolic disease may be mediated by EVs. Elucidating the contribution of EVs to HDL functions will provide better understanding of vascular protection and function in conditions of insulin resistance and potentially provide novel therapeutic targets for such diseases.
© 2020 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society
2020
You do not currently have access to this content.
Sign in
Sign in to your personal account
You could not be signed in. Please check your email address / username and password and try again.
Email address / Username ?
Password
Could not validate captcha. Please try again.
