The magnitude of hedgehog signaling activity defines skin tumor phenotype (original) (raw)
Introduction
Basal cell carcinomas (BCCs) are the most common cancers in light‐skinned individuals, with >1 000 000 new cases diagnosed every year in the USA. The identification of PTCH mutations in human BCCs (Hahn et al., 1996; Johnson et al., 1996) provided the first clue to the molecular pathogenesis of these tumors. Ptch encodes a cell surface receptor for Sonic hedgehog (Shh), a secreted signaling molecule that regulates embryonic growth and patterning of various structures, including hair follicles (reviewed in Chuang and Kornberg, 2000; Ingham and McMahon, 2001). In addition to its many roles during development, transient activation of Shh signaling in postnatal skin has been implicated in the regulation of hair follicle growth (Sato et al., 1999; Wang et al., 2000).
Ptch normally maintains the hedgehog pathway in an inactive state by inhibiting the signaling effector Smo (reviewed in Ingham and McMahon, 2001). Shh activates signaling in target cells by inhibiting Ptch, resulting in derepression of Smo and activation of hedgehog target genes such as Ptch and Gli1. Whereas this pathway normally is regulated by the spatially and temporally restricted expression of Shh, loss‐of‐function PTCH mutations are associated with constitutive hedgehog signaling in human cancers, particularly BCCs (reviewed in Ruiz i Altaba et al., 2002). In addition, activating mutations in SMO, rendering SMO protein largely resistant to inhibition by PTCH (Murone et al., 1999; Taipale et al., 2000), have been identified in tumors that do not contain detectable alterations in PTCH (Reifenberger et al., 1998; Xie et al., 1998; Lam et al., 1999). One of these mutant SMO alleles, designated M2SMO, was isolated from a human BCC and can induce ‘basal cell‐like’ proliferations in newborn mouse skin when driven by a keratin 5 (K5) promoter (Xie et al., 1998). This phenotype is similar to that seen in K14‐SHH transgenic mice, in which hedgehog signaling was constitutively activated using a keratin 14 promoter to drive an SHH cDNA (Oro et al., 1997). However, the lethal phenotype of both of these transgenic strains has complicated analysis beyond the perinatal period. In particular, it is not known whether M2SMO expression in keratinocytes is sufficient for the development and maintenance of full‐blown BCCs, or other skin tumors, in adult animals.
In an effort to study the consequences of deregulated Shh signaling in adult mouse skin, we generated transgenic mice expressing the human M2SMO mutant (Xie et al., 1998) using a truncated, 1.3 kb K5 promoter (Casatorres et al., 1994; Brown et al., 1998) that is expressed in a subset of cells expressing the full‐length K5 promoter (Ramirez et al., 1994). This promoter has been used previously to drive the human H‐RAS gene in skin, successfully circumventing the lethal phenotype seen with the full‐length K5 promoter (Bailleul et al., 1990) to yield viable, tumor‐bearing mice (Brown et al., 1998). The epidermis of adult Δ_K5‐M2SMO_ mice exhibits several features in common with BCC keratinocytes; however, BCCs do not develop even in mice surviving for >1 year. Instead, there is an accumulation of primitive, hair follicle‐like structures that appear remarkably similar to slow‐growing, benign human skin tumors called basaloid follicular hamartomas. Comparison of BCCs arising in K5‐Gli2 mice (Grachtchouk et al., 2000) with follicular hamartomas revealed marked upregulation of Shh target genes only in BCCs. In addition, only BCCs exhibited upregulation of the G1 cyclins D1 and D2, which recently have been implicated as direct targets of hedgehog signaling with central roles in the control of cell growth and proliferation (Kenney and Rowitch, 2000; Long et al., 2001; Duman‐Scheel et al., 2002; Yoon et al., 2002). Our data point to a role for deregulated Shh signaling in the genesis of basaloid follicular hamartomas, and strongly support the concept that different levels of Shh signaling activity can result in formation of distinct tumor types in skin. Although the mutant M2SMO allele has been detected in a sizeable fraction of human BCCs, expression of this putative oncogene in cutaneous epithelium is not sufficient to drive BCC development in this mouse model.
Results
Progressive alopecia in ΔK5‐M2SMO transgenic mice
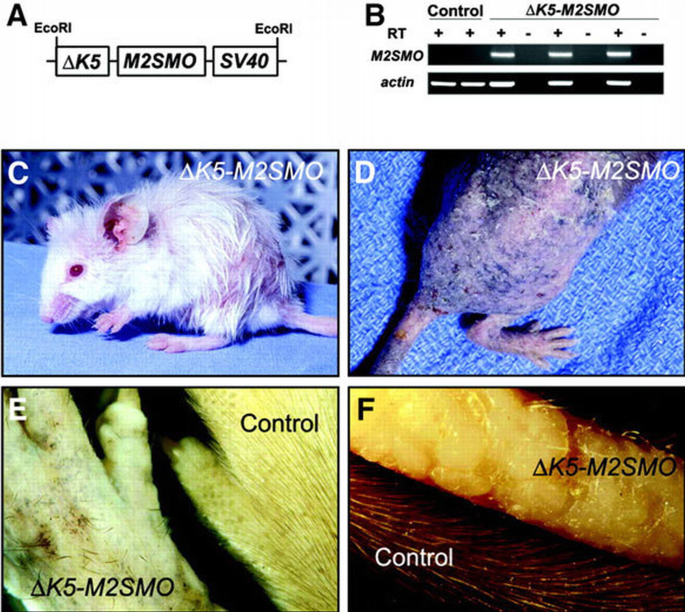
Injection of the Δ_K5‐M2SMO_ transgene (Figure 1A) into oocytes yielded 14 transgene‐positive mice from 58 potential founders, and RT–PCR confirmed the presence of M2SMO mRNA in skin from multiple founders (Figure 1B). Five of the 14 founders developed a grossly visible phenotype affecting their skin and/or hair. In the two most severely affected founders, several regions of skin never produced normal hairs, resulting in the appearance of hairless patches within 10 days of birth. Over time, the hair loss progressed (Figure 1C), and in the most severely affected mice resulted in generalized alopecia by 5 months of age (Figure 1D). Additional changes in the skin included a dry and scaly appearance, excoriations and focally increased pigmentation (Figure 1D). Other mice exhibited a similar but less dramatic phenotype resulting in varying degrees of hair loss. In older mice exhibiting a strong phenotype, the few hairs remaining were disoriented and arranged in a haphazard manner, in striking contrast to the regular patterning and uniform orientation of hair shafts in control skin (Figure 1E). The phenotype in tail skin (Figure 1F) was typically more severe and appeared earlier than in other regions of the body. Interestingly, there was a conspicuous absence of tumors resembling BCCs (Grachtchouk et al., 2000; Nilsson et al., 2000) in Δ_K5‐M2SMO_ mice.
Figure 1
Transgene design, characterization and gross phenotype of Δ_K5‐M2SMO_ mice. (A) M2SMO was subcloned into a transgenic cassette containing 1.3 kb of bovine K5 promoter and SV40 small t poly(A) sequence with intron. (B) Expression of M2SMO mRNA in transgenic mouse skin detected by RT–PCR using transgene‐specific primers, with β‐actin used as an internal control. RT, reverse transcriptase. (C) Three‐month‐old Δ_K5‐M2SMO_ founder with patchy hair loss. (D) An F2 Δ_K5‐M2SMO_ 601 mouse exhibiting severe phenotype at ∼5 months of age, with generalized alopecia, patchy pigmentation and dry, scaly skin. (E) Close‐up of dorsal paw from a Δ_K5‐M2SMO_ founder, revealing the haphazard arrangement of the few remaining hair shafts at ∼1 year of age. (F) Extensive alopecia and minute elevations on the surface of the tail from a Δ_K5‐M2SMO_ founder at 4.5 months of age, compared with an age‐matched control tail.
Although the truncated K5 promoter enabled prolonged survival of multiple Δ_K5‐M2SMO_ transgenic mice into adulthood, founders gradually developed a wasted appearance, and three died of unknown causes by 6 months of age. Of three founders that produced transgene‐positive offspring, only one transgenic line (Δ_K5‐M2SMO_ 601) was established successfully. Mice from this line have a phenotype similar to that seen in multiple founders as described above, and also never produced macroscopic tumors grossly resembling BCCs. Interestingly, the survival of Δ_K5‐M2SMO_ 601 mice was influenced by genetic background. Offspring from initial crosses with C57BL/6 breeders (litters F1–F2) were smaller than control littermates and exhibited impaired viability. However, backcrossing onto an FVB background beginning with the F2 generation gradually produced healthier offspring with a less severe skin phenotype, and these mice currently are robust breeders in the F9 generation.
Impaired terminal differentiation and hyperplasia in adult ΔK5‐M2SMO mouse epidermis
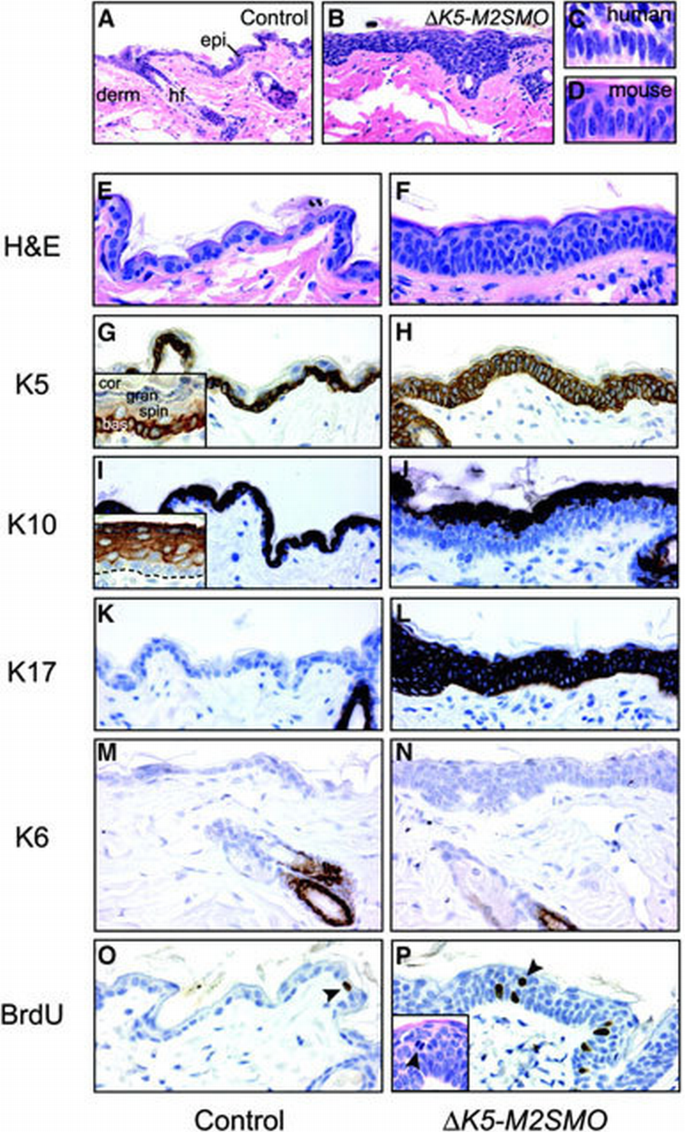
Proliferating cells in the basal layer of the epidermis give rise to several post‐mitotic, differentiated cell types in the overlying spinous, granular and cornified layers (Figure 2G, inset) (Fuchs and Raghavan, 2002; Niemann and Watt, 2002). Alterations in terminal differentiation are seen characteristically in a variety of cancers (Hanahan and Weinberg, 2000), including BCCs (reviewed in Miller, 1995). Examination of histological sections from several Δ_K5‐M2SMO_ founders revealed regions of thickened epidermis containing undifferentiated, basaloid‐appearing cells with a high nuclear to cytoplasmic ratio (Figure 2A and B), reminiscent of BCC keratinocytes. A similar but more widespread phenotype was seen in descendents of founder 601, which were characterized in greater detail. The epidermis of Δ_K5‐M2SMO_ 601 mice was appreciably thicker than in control littermates as early as 8 days after birth, and by 3 weeks of age was strikingly hyperplastic (Figure 2E and F). Interestingly, in some regions of transgenic epidermis, the nuclei of cells abutting the basement membrane were vertically elongated and crowded together in a pattern known as palisading, a characteristic feature of cells seen at the periphery of human BCC nodules (Figure 2C and D).
Figure 2
Altered epidermal growth and differentiation in Δ_K5‐M2SMO_ transgenic mice. Epidermal hyperplasia (B) was seen in several Δ_K5‐M2SMO_ founders, when compared with age‐matched controls (A). epi, epidermis; derm, dermis; hf, hair follicle. Epidermal basal cells in some regions of Δ_K5‐M2SMO_ mouse skin were crowded together and elongated (D) in a pattern resembling the peripheral palisading characteristically seen at the edge of human BCC tumor nodules (C). Altered growth and differentiation in skin from Δ_K5‐M2SMO_ mice (F, H, J, L, N and P) compared with littermate controls (E, G, I, K, M and O). Note the thickened epidermis and expansion of basal layer‐like cells into upper strata (E and F). Expression of K5 is limited to the basal layer in control epidermis (G), but is expressed in multiple suprabasal cell layers in epidermis of Δ_K5‐M2SMO_ mice (H). The inset in (G) illustrates normal K5 expression largely limited to the basal layer compartment, even in hyperplastic newborn mouse epidermis. bas, basal cell layer; spin, spinous layers; gran, granular cell layers; cor, cornified cells. The appearance of the suprabasal cell marker K10 is delayed in Δ_K5‐M2SMO_ mice (J) compared with controls (I). The inset in (I) shows a normal K10 expression pattern in all suprabasal cell layers of newborn mouse epidermis, but not in the basal cell layer. The dashed line indicates basement membrane separating the epidermis from the dermis. K17 is ectopically expressed in epidermis of Δ_K5‐M2SMO_ mice (L) but not controls (K), which exhibit expression limited to the hair follicle outer root sheath. K6 is expressed in the same subset of hair follicle keratinocytes in control (M) and transgenic (N) skin sections. Increased proliferation in Δ_K5‐M2SMO_ mouse epidermis (O and P). Note the increased number of BrdU‐labeled nuclei (arrowheads) in transgenic (P) versus control (O) mouse skin. BrdU‐labeled nuclei and mitotic figures (inset) are seen in suprabasal layers of Δ_K5‐M2SMO_ epidermis (P).
We further characterized the epidermal phenotype of Δ_K5‐M2SMO_ mice by examining the expression pattern of several protein markers. K5 normally is expressed almost exclusively in the basal layer of the epidermis and outermost layers of the hair follicle, the outer root sheath. The basal layer‐specific expression of K5 is best appreciated in newborn mouse epidermis (Figure 2G, inset), which is several times thicker than adult epidermis (Figure 2G) and therefore has more conspicuous cellular compartments. In Δ_K5‐M2SMO_ 601 mice, K5 expression was expanded from the basal layer to all living layers of the epidermis (Figure 2H). The expression pattern of K10 in epidermis is reciprocal to that of K5: K10 is detected in all epidermal spinous cells, beginning immediately above the basal layer (Figure 2I). However, K10 was not detected in the lower spinous cell layers of hyperplastic Δ_K5‐M2SMO_ 601 epidermis, although it was present in the upper spinous cell compartment (Figure 2I and J). The persistent expression of K5 and delayed appearance of K10 suggest that M2SMO impairs the ability of epidermal keratinocytes to terminally differentiate. Expression of K17 and K6 normally is restricted to the outer root sheath (see Figure 3Q) of hair follicles, with K6 limited to the differentiated cell compartment (Rothnagel and Roop, 1995; McGowan and Coulombe, 1998); in addition, K17 is detected consistently in BCCs. K17 was highly expressed throughout the thickened epidermis of Δ_K5‐M2SMO_ 601 mice, but K6 expression was undetectable in both control and transgenic epidermis (Figure 2K–N). The keratin expression profile in the lower layers of the hyperplastic Δ_K5‐M2SMO_ 601 epidermis is thus strikingly similar to that reported in human and mouse BCCs, which express K5 and K17 but little or no K10 and K6 (Yoshikawa et al., 1998; Grachtchouk et al., 2000).
Figure 3
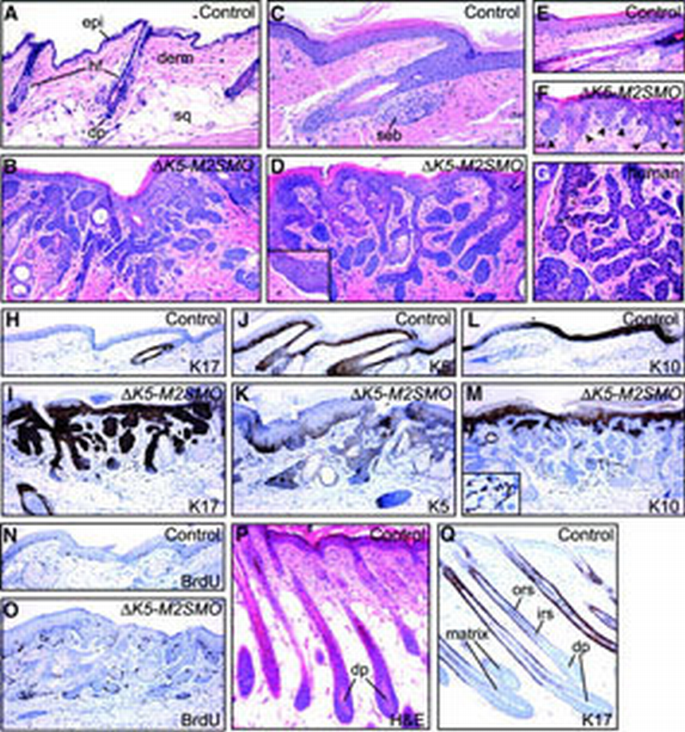
Histology, marker expression and increased proliferation rate of basaloid follicular hamartomas in Δ_K5‐M2SMO_ mice. H&E‐stained skin sections from control (A, C and E) and Δ_K5‐M2SMO_ (B, D and F) mice at ∼14 weeks (A–D) and 23 days (E and F) of age. Basaloid follicular hamartomas were detected throughout the integument, including dorsal (A and B) and tail (C–F) skin. In some areas, a cleft separated abnormal epithelium from the surrounding mesenchyme (inset in D), mimicking changes seen in human BCCs. Hair follicles in age‐matched control skin (A and C) are in the resting phase of the hair cycle, telogen, with compact clusters of dermal papilla cells (dp) at the tips of the follicles. epi, epidermis; derm, dermis; hf, hair follicle; sq, subcutaneous fat; dp, dermal papilla; seb, sebaceous gland. Analysis of tail skin from young mice (23 days) revealed multiple epithelial downgrowths originating directly from Δ_K5‐M2SMO_ epidermis (arrowheads in F, compared with control in E exhibiting a single hair follicle). Note the similar morphology of mouse lesions and human basaloid follicular hamartoma in (G). Marker analysis and BrdU labeling in control (H, J, L and N) and Δ_K5‐M2SMO_ transgenic (I, K, M and O) tail skin. Note the widespread expression of K17 in hamartomas (I); expansion of K5 expression (K), which is more evident in immunofluorescence studies (not shown); and focal expression of K10 (M). Scattered pigmented cells frequently are present in the dermis of Δ_K5‐M2SMO_ mice (inset in M). Immunostaining for BrdU revealed focally increased proliferation in transgenic mouse skin (O) compared with control (N). (P and Q) Normal growing (anagen) hair follicles. H&E‐stained section (P) reveals dermal papilla cells with abundant extracellular material enveloped by hair matrix cells. K17 is expressed in the outer root sheath (Q), but not matrix cells or inner root sheath. ors, outer root sheath; irs, inner root sheath.
To assess the consequences of M2SMO expression on epidermal proliferation, we identified cells undergoing DNA synthesis by immunostaining for bromodeoxyuridine (BrdU) following a 1 h exposure. As expected, cell proliferation in control epidermis was restricted to the basal layer but, in the skin of Δ_K5‐M2SMO_ 601 mice, BrdU‐labeled cells were detected well into the spinous cell layers (Figure 2O and P). In addition, mitotic cells were seen occasionally in suprabasal epidermal layers of Δ_K5‐M2SMO_ 601 mice (Figure 2P, inset). Thus, the impaired capacity for terminal differentiation in the epidermis of Δ_K5‐M2SMO_ 601 mice is coupled to inappropriate cell division in suprabasal cell layers.
ΔK5‐M2SMO mice develop widespread hair follicle hamartomas, but not BCCs
Since allegedly oncogenic SMO mutations have been reported in up to 21% of human BCCs (Lam et al., 1999), we were surprised by the lack of grossly evident tumors in multiple Δ_K5‐M2SMO_ founders observed for at least 8 months. Even microscopic BCCs were not detected, based on hematoxylin and eosin (H&E) analysis of sections from multiple mice ranging in age from newborn to 14 months. These findings are in striking contrast to those obtained by _K5_‐driven expression of the hedgehog transcriptional effectors GLI1 (Nilsson et al., 2000) or Gli2 (Grachtchouk et al., 2000): BCCs arose spontaneously in both of these transgenic models by several months of age.
Although macroscopic tumors did not develop in Δ_K5‐M2SMO_ mice, there was a gradual loss of normal‐appearing hair follicles and their replacement by immature follicle‐like structures consisting of strands, cords and/or sheets of squamous epithelial cells filling the dermis (Figure 3A–F). Other histological findings included cysts, enlarged sebaceous glands and masses of undifferentiated, basophilic epithelial cells with scant cytoplasm, resembling hair matrix cells, located at the tips of epithelial downgrowths. Hair shafts or other morphological signs of advanced hair follicle differentiation were rarely seen in advanced lesions. In some of the deepest portions of these lesions, tumor epithelium was separated from surrounding mesenchyme by a cleft (Figure 3D, inset): similar retraction spaces are seen frequently surrounding human BCC nodules (Kirkham, 1997). Histological abnormalities were detected in tail skin earlier than other sites, and clearly revealed numerous epithelial invaginations originating directly from the epidermis (Figure 3E and F). Thus, in addition to activating expression of certain hair follicle markers in epidermis (Figure 2), M2SMO appears capable of triggering widespread morphological changes reminiscent of early stages in hair follicle development. Interestingly, the gross appearance and histological features of skin lesions arising in Δ_K5‐M2SMO_ mice are strikingly similar to those reported for human basaloid follicular hamartomas (Figure 3G) (Brownstein, 1992; Nelson et al., 1993; Walsh and Ackerman, 1993). Little is known presently about the molecular pathogenesis of these benign, slow‐growing tumors.
Immunohistochemical analysis revealed expression of the follicle outer root sheath marker K17 in nearly all epithelial cells in the follicular hamartomas, with the exception of cells in the deepest portions that resemble hair matrix cells which are also negative for K17 in control hair follicles (Figure 3H, I and Q). K5 was also detected throughout the follicular hamartomas, but the distribution was not as widespread as for K17 (Figure 3J and K). The differentiation‐specific keratin K10, expressed in inner root sheath cells of hair follicles, was detected in a subset of cells within the hamartomas (Figure 3L and M). This result suggests that follicular hamartomas contain cells with a greater potential for terminal differentiation than BCC cells, which rarely express K10. Immunostaining for BrdU to identify proliferating cells revealed significant heterogeneity with follicular hamartomas. While some regions contained numerous cells with labeled nuclei, other regions contained very few labeled cells (Figure 3N and O).
In addition to alterations involving epidermis and hair follicle epithelium, other cell types were affected in skin of Δ_K5‐M2SMO_ transgenic mice including the mesenchymal dermal papilla required for proper hair follicle growth (reviewed in Jahoda and Reynolds, 1996), and neural crest‐derived melanocytes that provide pigment to growing hairs. Hair follicles in adult mice are usually in the resting phase of the hair cycle (reviewed in Stenn and Paus, 2001) called telogen (e.g. Figure 3A and C): in these quiescent follicles, the dermal papilla is a small cluster of cells at the tip of the follicle epithelium (Figure 3A). In actively growing anagen hair follicles, dermal papilla cells are embedded within abundant extracellular material and are surrounded by proliferating matrix cells of the hair follicle bulb (Figure 3P and Q). Established follicular hamartomas in Δ_K5‐M2SMO_ mice did not contain morphologically recognizable dermal papillae, suggesting a requirement for normal follicle architecture in maintenance of this critical cell population. In normal dorsal mouse skin, pigmented melanocytes are localized almost exclusively to the anagen hair bulb, where they transfer pigment to hair shaft progenitor cells. They regress together with the follicle epithelium during the catagen phase of the hair cycle, and are not detected in resting, telogen, hair follicles (Slominski et al., 1994). In striking contrast, and consistent with the gross appearance of adult Δ_K5‐M2SMO_ mice (Figure 1D), the dermis surrounding follicular hamartomas frequently contained pigmented cells, and many of these exhibited a dendritic morphology reminiscent of melanocytes (Figure 3M, inset, and Figure 5I).
M2SMO upregulates Shh target genes in transgenic mouse skin in vivo
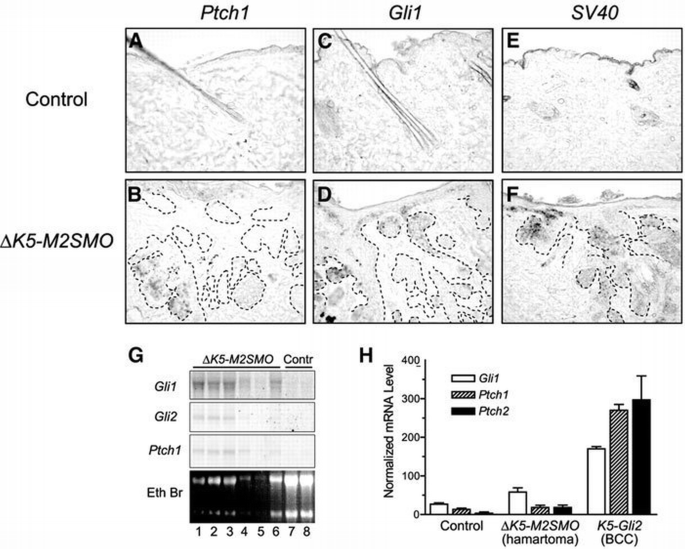
To assess whether the M2SMO transgene was functioning to activate Shh signaling in adult mouse skin, we examined expression of Shh target genes by in situ hybridization, northern blotting and real‐time PCR (Figure 4). In situ analysis revealed upregulation of Ptch1 and Gli1 in follicular hamartomas in a patchy distribution (Figure 4B and D). To investigate the potential basis for this patchy expression of Shh target genes, we generated a transgene‐specific probe against the SV40 small t poly(A) sequence (Brown et al., 1998). In situ analysis revealed that only some of the epithelial downgrowths expressed the M2SMO transgene (Figure 4F), which is in keeping with the expected expression pattern for the truncated K5 promoter (Brown et al., 1998). Essentially no signal was detected for Ptch1, Gli1 or the SV40 poly(A) sequence in skin from control mice (Figure 4A, C and E). Northern blot analysis revealed elevated expression of Gli1, Gli2 and Ptch1 in skin from Δ_K5‐M2SMO_ mice when compared with controls (Figure 4G), confirming increased Shh signaling in transgenic skin. Since high‐level expression of Shh target genes is a hallmark of BCCs, we also performed real‐time PCR to compare Shh pathway activity in control skin, follicular hamartomas arising in Δ_K5‐M2SMO_ mice and BCCs from K5‐Gli2 mice (Grachtchouk et al., 2000). Interestingly, while BCCs exhibited the expected upregulation of Shh target genes Gli1, Ptch1 and Ptch2, only a marginal increase was seen in Δ_K5‐M2SMO_ samples that was not statistically different from controls (Figure 4H). The discrepancy between northern blotting and real‐time PCR results, which were performed using different sets of RNA samples, reflects the low level and variable expression of Shh target genes in Δ_K5‐M2SMO_ transgenic mice.
Figure 4
Expression of transgene and Shh target genes in follicular hamartomas arising in Δ_K5‐M2SMO_ transgenic mice. In situ hybridization of control (A, C and E) and Δ_K5‐M2SMO_ skin sections (B, D and F), with epithelial cell islands outlined using dotted lines. Antisense riboprobes hybridizing to the Shh target genes Ptch1 (A and B) and Gli1 (C and D), as well as the transgene‐encoded mRNA which contains SV40‐derived poly(A) sequence (E and F), revealed patchy expression (blue‐purple staining) in a fraction of epithelial cells in follicular hamartomas. Control skin containing resting (telogen) hair follicles did not contain detectable levels of any of these mRNAs. (G) Northern blot analysis revealed elevated expression of Gli1, Gli2 and Ptch1 in RNA samples from Δ_K5‐M2SMO_ mouse skin (lanes 1–6), compared with control skin (lanes 7 and 8). (H) Expression of Shh target genes Gli1, Ptch1 and Ptch2, measured by quantitative PCR (TaqMan). RNA was isolated from control skin (n = 3), follicular hamartomas arising in Δ_K5‐M2SMO_ mice (n = 6) and BCCs from K5‐Gli2 mice (n = 3). Note the dramatic increase in Shh target gene expression in BCCs, with only modest elevations in follicular hamartomas. Standard errors are indicated by bars: the increase in mRNA levels is statistically significant for BCCs (P < 0.05), but not follicular hamartomas.
Figure 5
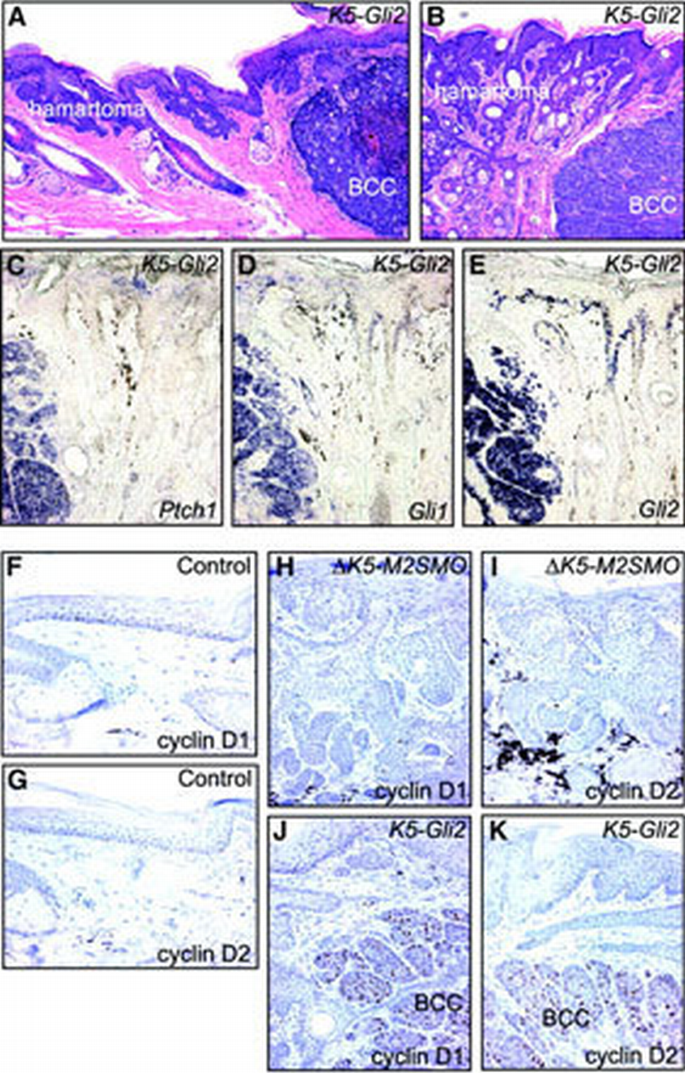
Distinct skin tumor phenotypes (basaloid follicular hamartoma versus BCC) are associated with different expression levels of Shh target genes and G1 cyclins. (A and B) H&E staining showing basaloid follicular hamartomas, on the left side of each panel, and BCCs, on the right, in sections from K5‐Gli2 mice. (C–E) In situ analysis reveals abundant expression of Shh target genes Ptch1 and Gli1, and the transgene Gli2, in BCCs on the left side of each panel, but not follicular hamartomas on the right. Negligible expression level of G1 cyclins in control (F and G) and Δ_K5‐M2SMO_ (H and I) tail skin. In K5‐Gli2 mouse skin, only the BCCs expressed high levels of cyclin D1 (J) and cyclin D2 (K).
The magnitude of Shh signaling activity defines skin tumor phenotype
Given the relatively low expression level of Shh target genes in follicular hamartomas arising in Δ_K5‐M2SMO_ mice, additional studies were performed to explore more rigorously the potential relationship between Shh signaling activity and skin tumor phenotype. We previously reported that mice overexpressing the transcription factor Gli2 using a K5 promoter developed multiple BCCs by 3–4 months of age (Grachtchouk et al., 2000). We have generated additional K5‐Gli2 founders and established three transgenic mouse lines that spontaneously develop BCCs similar to those described in our initial report, but these mice are healthier and have therefore allowed a more complete characterization well into adulthood. As these mice age, some of them develop abnormal‐appearing skin on their tails and ears even in the absence of grossly evident BCCs, and H&E analysis revealed follicular hamartomas similar to those arising in Δ_K5‐M2SMO_ mice. The appearance of follicular hamartomas in K5‐Gli2 mice suggests that upregulation of endogenous Gli activity, albeit at a relatively low level (Figure 4G and H), is responsible for development of follicular hamartomas in Δ_K5‐M2SMO_ mice. The focal appearance of BCCs in K5‐Gli2 mouse lines enabled analysis of both BCCs and follicular hamartomas in the same tissue section (Figure 5A and B). In situ hybridization revealed high levels of Ptch1 and Gli1 expression in the BCCs, with markedly weaker or undetectable signals in adjacent follicular hamartomas (Figure 5C and D). Upregulation of Shh target genes in BCCs is associated with high transgene expression levels, based on the abundance of Gli2 mRNA in these tumors relative to the adjacent follicular hamartomas (Figure 5E).
Several reports have implicated G1 cyclins in hedgehog‐mediated growth control, in both Drosophila (Duman‐Scheel et al., 2002) and vertebrates (Kenney et al., 2000; Long et al., 2001; Yoon et al., 2002). We therefore examined cyclin D1 and D2 levels to see whether differences in their expression could help explain the relatively slow growth of follicular hamartomas when compared with BCCs. Immunostaining of control skin or follicular hamartomas revealed cyclin D1 and D2 levels to be low or undetectable (Figure 5F–I); in contrast, BCC keratinocytes expressed both D‐type cyclins (Figure 5J and K). These data suggest that enhanced expression of cyclin D1 and D2, driven by high levels of Shh signaling activity, may be required for the sustained, expansive tumor growth seen in BCCs.
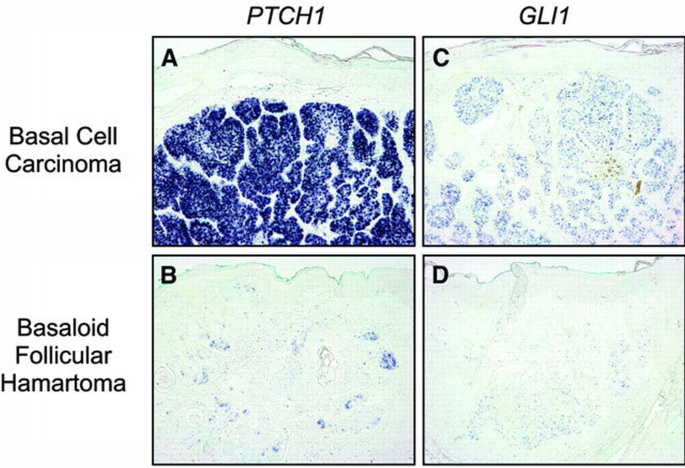
Given the fact that basaloid follicular hamartomas arise in both Δ_K5‐M2SMO_ and K5‐Gli2 mice, we were interested in assessing whether deregulation of SHH signaling may also play a role in the genesis of the corresponding human lesions. To explore this possibility, we examined the expression of PTCH1 and GLI1 mRNA in a series of human BCCs (n = 5) and basaloid follicular hamartomas (n = 5). Expression of both SHH target genes was readily detected in essentially all BCC tumor cells (Figure 6A and C). In contrast, PTCH1 was detected at low levels and in only a fraction of cells comprising the follicular hamartomas (Figure 6B). GLI1 mRNA was also detected in follicular hamartomas at substantially lower levels than in BCCs (Figure 6D). Collectively, our findings strongly support the notion that modestly deregulated hedgehog signaling plays a role in the development of basaloid follicular hamartomas in both mice and humans.
Figure 6
Low level expression of SHH target genes in human basaloid follicular hamartomas relative to BCCs. (A and B) In situ analysis for PTCH1 mRNA reveals high expression in essentially all tumor cells in human BCC (A), with low expression levels detected in a fraction of cells comprising basaloid follicular hamartoma (B). (C and D) Low expression of GLI1 mRNA in human follicular hamartoma (D) compared with BCC (C). Note the absence of PTCH1 or GLI1 signal in overlying epidermis of all samples. In situ analysis of five BCCs and five basaloid follicular hamartomas revealed substantially lower PTCH1 and GLI1 expression in all follicular hamartomas.
Discussion
The consistently elevated expression of Shh target genes in BCCs suggests that uncontrolled Shh signaling plays a crucial role in the initiation and/or maintenance of these tumors. We have shown that different levels of Shh pathway activity are associated with different epithelial tumor types in the skin of mice and humans, and provide evidence implicating modestly deregulated Shh signaling in the genesis of basaloid follicular hamartomas. Despite the occurrence of activating SMO mutations in humans BCCs, our findings argue that expression of M2SMO in keratinocytes is not sufficient for full‐blown BCC development.
The diffuse epidermal phenotype seen in Δ_K5‐M2SMO_ mice (Figure 2) suggests that deregulated Shh signaling can dramatically alter the differentiation program of interfollicular keratinocytes, in keeping with previous studies both in transgenic mice and in human skin grafts (Fan et al., 1997; Oro et al., 1997; Xie et al., 1998). Interestingly, the expression pattern of certain keratins in epidermis of Δ_K5‐M2SMO_ mice is distinctly different from that seen in epidermal hyperplasia triggered by other stimuli, such as wound‐healing or tumor‐promoting phorbol esters. While K17 and K6 are normally upregulated coordinately in these settings, K6 is conspicuously missing in epidermis of Δ_K5‐M2SMO_ mice. Together with the expanded distribution of K5 into suprabasal cell layers and delayed appearance of K10, this keratin profile mimics that which is seen in both BCCs and the proliferating compartment of follicle outer root sheath (Yoshikawa et al., 1998; Grachtchouk et al., 2000). Since Shh signaling is activated in both of these settings, albeit only transiently in the anagen outer root sheath, additional studies are warranted to investigate the potential role of Shh signaling in bringing about these characteristic changes in marker expression.
The absence of BCCs in Δ_K5‐M2SMO_ mice is particularly interesting in light of the well‐documented resistance of mice to BCC development. While mice treated with chemical or physical carcinogens readily develop squamous tumors such as papillomas and carcinomas, BCCs are almost never seen; in contrast, BCCs are common in rats exposed to a variety of carcinogens (reviewed in Dlugosz et al., 2002). The resistance of adult mouse skin to BCC formation has only been circumvented recently by genetically modulating the Shh pathway. K5 promoter‐driven overexpression of the transcription factors GLI1 (Nilsson et al., 2000) or Gli2 (Grachtchouk et al., 2000; Sheng et al., 2002) results in the spontaneous development of grossly visible tumors within several months. K14‐SHH mice produce BCC‐like tumors at birth (Oro et al., 1997), but it is not yet known whether SHH is sufficient for BCC tumorigenesis in adult mice. While macroscopic BCCs arise in Ptch+/− mice, they do so after prolonged intervals and require exposure to either UV or ionizing radiation (Aszterbaum et al., 1999). Thus, while nuclear mediators of Shh signaling readily produce BCCs when targeted to mouse skin, deregulation of this pathway at a proximal level may be less effective at generating skin tumors. These data are consistent with the intriguing notion that mice harbor a defect in responsiveness to pathological Shh signaling that lies downstream of Ptch and Smo.
The appearance of basaloid follicular hamartomas in skin of K5‐Gli2 mice strongly suggests that the development of these lesions in Δ_K5‐M2SMO_ mice is due to increased transcriptional activity of endogenous Gli proteins. In keeping with this idea, Gli1 and Gli2 mRNA levels are increased in skin from Δ_K5‐M2SMO_ mice (Figure 4G). However, upregulation of Shh target genes is far greater in BCCs than in follicular hamartomas both in mice (Figures 4H, and 5C and D) and in humans (Figure 6), suggesting that a critical threshold of signaling activity must be achieved for full‐blown BCC development and growth. These results also suggest that reduction of Shh signaling activity, rather than total suppression, may be sufficient to reverse the locally invasive behavior of BCC. Although the downstream effectors driving proliferation in cancers caused by deregulated hedgehog signaling are not yet known, recent studies suggest a role for the G1 cyclins in physiological cell growth (Kenney et al., 2000; Long et al., 2001; Duman‐Scheel et al., 2002) and transformation (Yoon et al., 2002) mediated by the hedgehog pathway. In addition, cell cycle withdrawal normally associated with terminal differentiation is blocked in human keratinocytes overexpressing SHH (Fan and Khavari, 1999). Upregulation of cyclins D1 and D2 in mouse BCCs, compared with follicular hamartomas and normal skin, suggests that modulation of G1 cyclin expression contributes to tumor growth associated with hedgehog pathway activation.
Follicular hamartomas in humans may be solitary or multiple, and either congenital or acquired; generalized forms, inherited as an autosomal dominant trait, have also been described (reviewed in Requena et al., 1999; Morohashi et al., 2000). Lesions may be associated with extensive hair loss, reminiscent of changes seen in Δ_K5‐M2SMO_ mice. The slow growth of human follicular hamartomas and absence of local invasion are also mimicked by the murine lesions described in this report. In many cases, the epithelium of human follicular hamartomas is connected to epidermis rather than hair follicles, suggesting that these lesions arise in a manner similar to those in mice. Their appearance in both Δ_K5‐M2SMO_ and K5‐Gli2 mice strongly implicates deregulated Shh signaling in the development of murine basaloid follicular hamartomas, and the presence of low‐level PTCH1 and GLI1 mRNA in human follicular hamartomas points to a role for aberrant Shh signaling in the genesis of these lesions as well. If this hypothesis is correct, future studies may yield insight into a specific molecular defect underlying the development of these interesting skin lesions.
The absence of BCCs in Δ_K5‐M2SMO_ mice was unanticipated given the proposed role for this mutant allele as an oncogene capable of driving human BCC development. The simplest interpretation is that M2SMO expression is not sufficient for BCC formation either in humans or in mice, but the inherent resistance of mice to BCC development remains an important caveat. Transgenic studies designed to express M2SMO in rats would get around this issue, and provide a direct way of testing for species‐specific differences in susceptibility to BCC development using a genetic approach. Another potential explanation for the lack of BCCs is that sufficiently high transgene expression levels were not achieved in Δ_K5‐M2SMO_ mice, but this may not be a valid argument since only a single copy of mutant M2SMO is proposed to be sufficient for human tumorigenesis. Although the human and mouse Smo proteins exhibit 93% identity (Akiyama et al., 1997), it is possible that the human protein is incapable of signaling at sufficiently high levels to drive tumorigenesis in mice. Future studies using a mouse mutant analogous to M2SMO, designated SmoA1 (Taipale et al., 2000), should help resolve this issue. The lack of BCCs is probably not due to use of the Δ_K5_ promoter, since we have seen the same phenotype in adult mice that conditionally express M2SMO under the control of the full‐length K5 promoter (M.Allen, M.Grachtchouk, V.Grachtchouk, E.DeLassus, A.Wang, L.Wei and A.A.Dlugosz, manuscript in preparation). It is conceivable that the absence of BCCs relates to differences in the manner in which SMO activation takes place in humans versus mice. During human BCC development, SMO mutation is likely to occur in a single keratinocyte in adult skin, not in a large number of cells beginning during embryogenesis as occurs in many conventional transgenic mouse strains. The importance of timing with regard to Shh responsiveness was elegantly demonstrated in a previous study using chick skin, which showed that ectopic hedgehog signaling produces vastly different results based on when it is activated during development (Morgan et al., 1998). To model human BCC development more accurately, an inducible transgenic model enabling activation of M2SMO in a limited number of adult skin cells will be needed.
Materials and methods
Production of transgenic mice and tissue harvests
The M2SMO cDNA (Xie et al., 1998), encoding a constitutively active human SMO protein, was subcloned into the _Bgl_II site of the K5pola transgenic cassette containing the 1.3 kb bovine K5 promoter and SV40 small t poly(A) signal with intron (Brown et al., 1998). Subcloning was verified by sequencing. The insert was released using _Eco_RI, and DNA was microinjected into C57/BL6 × SJL F2 embryos by members of the University of Michigan Transgenic Core. Founders were crossed onto either C57/BL6 or FVB/n breeders (Charles River), as discussed in the Results. Animals were housed and maintained according to institutional guidelines and sacrificed by CO2 asphyxiation. Some mice were injected intraperitoneally with BrdU (100 μg/g) 1 h prior to sacrifice (Dlugosz et al., 1995). Tissue was fixed overnight in either neutral‐buffered formalin (NBF) or Carnoy's solution for H&E staining and routine immunohistochemistry. Mouse tissue harvested for in situ analysis was fixed in 4% paraformaldehyde at 4°C overnight, transferred to 30% sucrose at 4°C for 24–48 h, and frozen in OCT embedding medium (Miles). Additional tissue samples for RNA isolation were homogenized in TriZol solution (Invitrogen) according to the manufacturer's instructions.
Immunostaining
Immunostaining was performed with the following antibodies at the indicated concentrations: K5 and K10 (Covance), 1:2000; K6 (Covance), 1:1000; K17 (kindly provided by Pierre Coulombe), 1:2000; BrdU (Zymed), 1:1000; cyclin D1 (Neomarkers Ab‐4), 1:100; cyclin D2 (Santa Cruz, sc‐181), 1:100. All antibodies are rabbit polyclonals except anti‐BrdU, which is a mouse monoclonal. Sections from Carnoy's‐fixed tissues were used for keratin antibodies (K5, K6, K10 and K17). NBF‐fixed tissue sections were used for cyclin D1, cyclin D2 and BrdU immunostaining, following antigen retrieval using boiling citrate buffer (Chiang et al., 1999). Immunostaining was performed with Vectastain ABC kits (Vector) using 3,3′‐diaminobenzidine (DAB) to detect horseradish peroxidase (HRP)‐conjugated streptavidin bound to biotinylated secondary antibodies. Immunostained sections were counterstained with hematoxylin.
In situ hybridization
Detailed protocols for in situ hybridization using mouse and human tissue, obtained according to University of Michigan Institutional Guidelines, are included in the Supplementary data available at The EMBO Journal Online. Probes for mouse Gli1 and Ptch1 were as previously described (Hui et al., 1994; Goodrich et al., 1996), and were kindly provided by Dr C.‐c.Hui. An ∼0.5 kb transgene‐specific riboprobe was designed to detect the SV40 small t poly(A), using T3 or T7 promoters and primers just downstream of the small t intron (5′‐attccaacctatggaactgat‐3′) and near the 3′ end (5′‐gcattcattttatgtttc‐3′). For in situ analysis of human tissue, T3 or T7 promoters were used to generate riboprobes against human GLI1 (nucleotides 3285–3598) with the following primers: forward primer (5′‐cagctctggacatacccc‐3′), reverse primer (5′‐gatgcagttcctttattatc‐3′). For the human PTCH1 probe (nucleotides 216–805), a forward primer with flanking _Eco_RI site (5′‐acggaattccggaaagcgcc‐3′) and a reverse primer with an internal _Eco_RI site (5′‐ttaactcttccaggaattccaaagg‐3′) were used for PCR amplification of reverse‐transcribed human RNA. The resultant fragment was subcloned following _Eco_RI digestion into pBluescript II SK (Stratagene). Digoxigenin‐UTP‐labeled riboprobes were generated by in vitro transcription (Roche).
Northern blotting, semi‐quantitative RT–PCR and real‐time PCR analysis
Northern blot analysis was performed using total RNA isolated from TriZol lysates. A 20 μg aliquot of total RNA was loaded per lane on 1% agarose gels containing 2.2 M formaldehyde and a MOPS‐based buffer system. RNA was transferred onto Zeta‐probe (Bio‐Rad) nylon membranes, and pre‐hybridization performed for at least 3 h at 42°C in buffer containing 6× SSC, 5× Denhardt's solution, 0.5% SDS, 100 μg/ml salmon sperm DNA and 50% formamide. Hybridization was performed overnight at 42°C using probes labeled with 32P by random priming (Roche), according to the manufacturer's instructions. Gli1 probe for northern blotting was a 500 bp _Nco_I 3′ cDNA fragment (Park et al., 2000); Gli2 probe was a 1 kb _Eco_RI fragment (Hui et al., 1994); and the Ptch1 probe was the same 0.8 kb fragment used for in situ hybridization studies. Filters were washed at a final stringency of 0.1× SSC with 0.1% SDS, at 68°C. Methods for semi‐quantitative RT–PCR and real‐time PCR analysis are described in the Supplementary data.
References
- Akiyama H, Shigeno C, Hiraki Y, Shukunami C, Kohno H, Akagi M, Konishi J and Nakamura T (1997) Cloning of a mouse smoothened cDNA and expression patterns of hedgehog signalling molecules during chondrogenesis and cartilage differentiation in clonal mouse EC cells, ATDC5. Biochem Biophys Res Commun, 235, 142–147.
Google Scholar - Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit PE, Scott MP and Epstein EHJr (1999) Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med, 5, 1285–1291.
Google Scholar - Bailleul B, Surani MA, White S, Barton SC, Brown K, Blessing M, Jorcano J and Balmain A (1990) Skin hyperkeratosis and papilloma formation in transgenic mice expressing a ras oncogene from a suprabasal keratin promoter. Cell, 62, 697–708.
Google Scholar - Brown K, Strathdee D, Bryson S, Lambie W and Balmain A (1998) The malignant capacity of skin tumours induced by expression of a mutant H‐ras transgene depends on the cell type targeted. Curr Biol, 8, 516–524.
Google Scholar - Brownstein MH (1992) Basaloid follicular hamartoma: solitary and multiple types. J Am Acad Dermatol, 27, 237–240.
Google Scholar - Casatorres J, Navarro JM, Blessing M and Jorcano JL (1994) Analysis of the control of expression and tissue specificity of the keratin 5 gene, characteristic of basal keratinocytes. Fundamental role of an AP‐1 element. J Biol Chem, 269, 20489–20496.
Google Scholar - Chiang C et al. (1999) Essential role for sonic hedgehog during hair follicle morphogenesis. Dev Biol, 205, 1–9.
Google Scholar - Chuang PT and Kornberg TB (2000) On the range of hedgehog signaling. Curr Opin Genet Dev, 10, 515–522.
Google Scholar - Dlugosz AA, Glick AB, Tennenbaum T, Weinberg WC and Yuspa SH (1995) Isolation and utilization of epidermal keratinocytes for oncogene research. Methods Enzymol, 254, 3–20.
Google Scholar - Dlugosz A, Merlino G and Yuspa SH (2002) Progress in cutaneous cancer research. J Invest Dermatol Symp Proc, 7, 17–26.
Google Scholar - Duman‐Scheel M, Weng L, Xin S and Du W (2002) Hedgehog regulates cell growth and proliferation by inducing cyclin D and cyclin E. Nature, 417, 299–304.
Google Scholar - Fan H and Khavari PA (1999) Sonic hedgehog opposes epithelial cell cycle arrest. J Cell Biol, 147, 71–76.
Google Scholar - Fan H, Oro AE, Scott MP and Khavari PA (1997) Induction of basal cell carcinoma features in transgenic human skin expressing Sonic Hedgehog. Nat Med, 3, 788–792.
Google Scholar - Fuchs E and Raghavan S (2002) Getting under the skin of epidermal morphogenesis. Nat Rev Genet, 3, 199–209.
Google Scholar - Goodrich LV, Johnson RL, Milenkovic L, McMahon JA and Scott MP (1996) Conservation of the hedgehog/patched signaling pathway from flies to mice: Induction of a mouse patched gene by Hedgehog. Genes Dev, 10, 301–312.
Google Scholar - Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC and Dlugosz AA (2000) Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet, 24, 216–217.
Google Scholar - Hahn H et al. (1996) Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell, 85, 841–851.
Google Scholar - Hanahan D and Weinberg RA (2000) The hallmarks of cancer. Cell, 100, 57–70.
Google Scholar - Hui CC, Slusarski D, Platt KA, Holmgren R and Joyner AL (1994) Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli‐2 and Gli‐3, in ectoderm‐ and mesoderm‐derived tissues suggests multiple roles during postimplantation development. Dev Biol, 162, 402–413.
Google Scholar - Ingham PW and McMahon AP (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev, 15, 3059–3087.
Google Scholar - Jahoda CA and Reynolds AJ (1996) Dermal–epidermal interactions. Adult follicle‐derived cell populations and hair growth. Dermatol Clin, 14, 573–583.
Google Scholar - Johnson RL et al. (1996) Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science, 272, 1668–1671.
Google Scholar - Kenney AM and Rowitch DH (2000) Sonic hedgehog promotes G1 cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol Cell Biol, 20, 9055–9067.
Google Scholar - Kirkham N (1997) Tumors and cysts of the epidermis. In Elder,D., Elenitsas,R., Jaworsky,C. and Johnson,B.J. (eds), Lever's Histopathology of the Skin. Lippincott‐Raven, Philadelphia, PA, pp. 685–746.
- Lam CW et al. (1999) A frequent activated smoothened mutation in sporadic basal cell carcinomas. Oncogene, 18, 833–836.
Google Scholar - Long F, Zhang XM, Karp S, Yang Y and McMahon AP (2001) Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development, 128, 5099–5108.
Google Scholar - McGowan KM and Coulombe PA (1998) Expression of keratin 17 coincides with the definition of major epithelial lineages during skin development. J Cell Biol, 143, 469–486.
Google Scholar - Miller SJ (1995) Etiology and pathogenesis of basal cell carcinoma. Clin Dermatol, 13, 527–536.
Google Scholar - Morgan BA, Orkin RW, Noramly S and Perez A (1998) Stage‐specific effects of sonic hedgehog expression in the epidermis. Dev Biol, 201, 1–12.
Google Scholar - Morohashi M, Sakamoto F, Takenouchi T, Hashimoto T, Tago O and Ito M (2000) A case of localized follicular hamartoma: an ultrastructural and immunohistochemical study. J Cutaneous Pathol, 27, 191–198.
Google Scholar - Murone M, Rosenthal A and de Sauvage FJ (1999) Sonic hedgehog signaling by the patched–smoothened receptor complex. Curr Biol, 9, 76–84.
Google Scholar - Nelson BR, Johnson TM, Waldinger T, Gillard M and Lowe L (1993) Basaloid follicular hamartoma: a histologic diagnosis with diverse clinical presentations. Arch Dermatol, 129, 915–917.
Google Scholar - Niemann C and Watt FM (2002) Designer skin: lineage commitment in postnatal epidermis. Trends Cell Biol, 12, 185–192.
Google Scholar - Nilsson M, Unden AB, Krause D, Malmqwist U, Raza K, Zaphiropoulos PG and Toftgard R (2000) Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI‐1. Proc Natl Acad Sci USA, 97, 3438–3443.
Google Scholar - Oro AE, Higgins KM, Hu ZL, Bonifas JM, Epstein EHJr, and Scott MP (1997) Basal cell carcinomas in mice overexpressing sonic hedgehog. Science, 276, 817–821.
Google Scholar - Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M and Joyner AL (2000) Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development, 127, 1593–1605.
Google Scholar - Ramirez A, Bravo A, Jorcano JL and Vidal M (1994) Sequences 5′ of the bovine keratin 5 gene direct tissue‐ and cell‐type‐specific expression of a lacZ gene in the adult and during development. Differentiation, 58, 53–64.
Google Scholar - Reifenberger J, Wolter M, Weber RG, Megahed M, Ruzicka T, Lichter P and Reifenberger G (1998) Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res, 58, 1798–1803.
Google Scholar - Requena L, Farina MC, Robledo M, Sangueza OP, Sanchez E, Villanueva A, Marquina A and Tamarit R (1999) Multiple hereditary infundibulocystic basal cell carcinomas: a genodermatosis different from nevoid basal cell carcinoma syndrome. Arch Dermatol, 135, 1227–1235.
Google Scholar - Rothnagel JA and Roop DR (1995) Hair follicle companion layer: reacquainting an old friend. J Invest Dermatol, 104, 42S–43S.
Google Scholar - Ruiz i Altaba A, Sanchez P and Dahmane N (2002) Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer, 2, 361–372.
Google Scholar - Sato N, Leopold PL and Crystal RG (1999) Induction of the hair growth phase in postnatal mice by localized transient expression of Sonic hedgehog. J Clin Invest, 104, 855–864.
Google Scholar - Sheng H et al. (2002) Dissecting the oncogenic potential of Gli2: deletion of an NH2‐terminal fragment alters skin tumor phenotype. Cancer Res, 62, 5308–5316.
Google Scholar - Slominski A, Paus R, Plonka P, Chakraborty A, Maurer M, Pruski D and Lukiewicz S (1994) Melanogenesis during the anagen–catagen–telogen transformation of the murine hair cycle. J Invest Dermatol, 102, 862–869.
Google Scholar - Stenn KS and Paus R (2001) Controls of hair follicle cycling. Physiol Rev, 81, 449–494.
Google Scholar - Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP and Beachy PA (2000) Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature, 406, 1005–1009.
Google Scholar - Walsh N and Ackerman AB (1993) Basaloid follicular hamartoma: solitary and multiple types. J Am Acad Dermatol, 29, 125–129.
Google Scholar - Wang LC et al. (2000) Regular articles: conditional disruption of hedgehog signaling pathway defines its critical role in hair development and regeneration. J Invest Dermatol, 114, 901–908.
Google Scholar - Xie J et al. (1998) Activating Smoothened mutations in sporadic basal‐cell carcinoma. Nature, 391, 90–92.
Google Scholar - Yoon JW, Kita Y, Frank DJ, Majewski RR, Konicek BA, Nobrega MA, Jacob H, Walterhouse D and Iannaccone P (2002) Gene expression profiling leads to identification of GLI1‐binding elements in target genes and a role for multiple downstream pathways in GLI1‐induced cell transformation. J Biol Chem, 277, 5548–5555.
Google Scholar - Yoshikawa K, Katagata Y and Kondo S (1998) Biochemical and immunohistochemical analyses of keratin expression in basal cell carcinoma. J Dermatol Sci, 17, 15–23.
Google Scholar