Role of αvβ3 integrin in the activation of vascular endothelial growth factor receptor‐2 (original) (raw)
Introduction
Integrin‐mediated cell–matrix interactions regulate often divergent biological events including cell adhesion, migration, proliferation, differentiation and survival (Giancotti, 1997; Schwartz, 1997). Upon binding to matrix proteins, integrins transmit ‘outside‐in’ signals to the cell, which trigger a large array of intracellular signaling events. These include (i) activation of kinases, such as focal adhesion kinase, pp60src, mitogen‐activated protein (MAP) kinase, Jun kinase, protein kinase C; (ii) changes in cytosolic ions, such as proton and calcium; and (iii) production of lipid mediators (Clark and Brugge, 1995; Schwartz et al., 1995; Yamada and Miyamoto, 1995; Parsons, 1996; Frisch and Ruoslahti, 1997). Many of the integrin‐induced signaling pathways can also be activated by binding of soluble growth factors to their receptors, which suggests the existence of co‐ordinate mechanisms between integrins and growth factors in the control of cellular functions. Cell adhesion has been shown to increase autophosphorylation of the epidermal growth factor (EGF) and platelet‐derived growth factor (PDGF) receptors in response to their cognate ligands (Miyamoto et al., 1996). Ligation of specific integrins is followed by activation of the MAP kinase through the engagement of the adaptor molecule, Shc, and the Ras pathway (Wary et al., 1996), of the focal adhesion kinase (Schlaepfer et al., 1994), and engagement of phosphoinositide 3‐OH kinase (PI 3‐kinase) (King et al., 1997). Cell adhesion is also specifically required for the induction of cyclin D1 (Zhu et al., 1996). Within the integrins, αvβ3 engagement in cell–matrix interactions seems to be strictly involved in the co‐ordinated activation of tyrosine kinase receptors. Therefore, αvβ3 associates with the tyrosine‐phosphorylated PDGF and insulin receptor (Vuori and Ruoslahti, 1994; Rousseau et al., 1997) and its natural ligand vitronectin enhances the biological activity of PDGF‐β (Schneller et al., 1997; Woodard et al., 1998). Alternatively, this integrin complex can interact with tenascin‐C, thus favoring the clustering of EGF receptors and EGF‐dependent growth (Jones et al., 1997).
Interaction between integrin αvβ3 and the extracellular matrix has been identified as the most important survival system for nascent vessels. Integrin αvβ3 is expressed in high quantities on angiogenic endothelial cells (Brooks et al., 1994a), where it suppresses the activity of p53 and the p53‐inducible cell‐cycle inhibitor p21WAF1/CIP1 and increases the Bcl‐2:Bax ratio, with a consequent anti‐apoptotic effect (Stromblad et al., 1996). It is also involved in a late and sustained activation of mitogen‐activated protein kinase in the chorionallantoic membrane stimulated by basic fibroblast growth factor (Eliceiri et al., 1998). Thus, both the cell cycle and apoptotic signaling cascades are balanced by this integrin complex. Furthermore, αvβ3 antagonists induce apoptosis of growing endothelial cells and reduce the invasive behavior of breast carcinoma in human skin by blocking tumor angiogenesis (Brooks et al., 1994b, 1995). During vasculogenesis, angioblasts and early endothelial cells secrete into extracellular matrix Del1, which is a recently discovered ligand for αvβ3 involved in vascular remodeling (Hidai et al., 1998). In addition, ligation of this integrin complex also promotes a calcium influx required for endothelial motility (Leavesley et al., 1993). The expression of αvβ3 integrin is induced in microvascular endothelial cells by vascular endothelial growth factor‐A (VEGF‐A) (Senger et al., 1997), a highly specific activator of in vitro endothelial cell migration and proliferation and in vivo angiogenesis (Thomas, 1996; Ferrara and Davis‐Smyth, 1997).
VEGF‐A is a 40–45 kDa homodimer belonging to the cysteine knot family of growth factors. It exists as five different isoforms of 121, 145, 165, 189 and 206 amino acids (Thomas, 1996; Ferrara and Davis‐Smyth, 1997). On adult endothelial cells it exhibits high‐affinity binding sites corresponding to two distinct tyrosine kinase receptors, the VEGF receptor (VEGFR)‐1 encoded by Flt‐1 (De Vries et al., 1992) and VEGFR‐2 encoded by KDR/Flk‐1 (Terman et al., 1991; Millauer et al., 1993). The latter is also specifically activated by HIV‐1‐Tat protein (Albini et al., 1996b; Ganju et al., 1998), a viral molecule which contributes to angiogenesis associated with Kaposi's sarcoma (Ensoli et al., 1994). Although endothelial cells express both receptors, recent findings suggest that VEGFR‐2, but not VEGFR‐1, is able to mediate the mitogenic and motogenic effect of VEGF‐A (Millauer et al., 1993; Waltenberger et al., 1994).
In this report we present evidence that β3 integrin subunit associates with stimulated‐VEGFR‐2 and a mAb directed to β3 molecule blocks signaling events elicited by VEGF‐A critical to the induction of the angiogenic program in endothelial cells.
Results
Tyrosine phosphorylation of VEGFR‐2 is increased in endothelial cells adherent on vitronectin
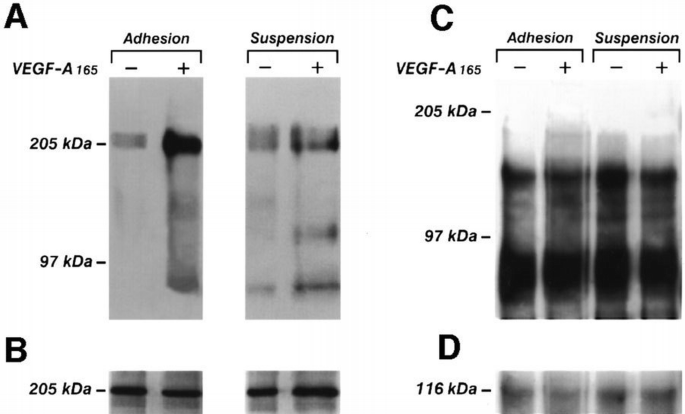
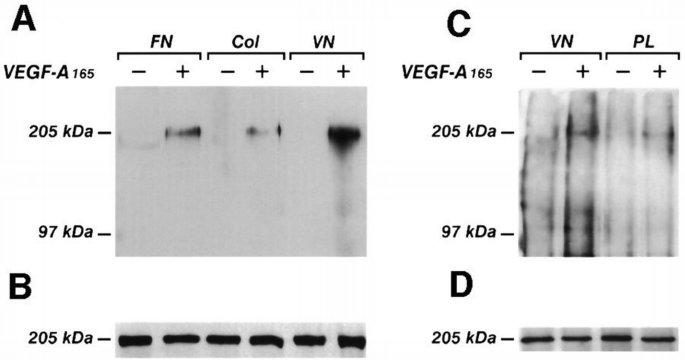
To examine the role of cell adhesion on VEGFR‐2 activation, we evaluated the phosphorylation of the receptor in adherent or suspended endothelial cells treated with VEGF‐A165. Confluent and quiescent cells were detached from the plastic surface by calcium chelant or left adherent and challenged with VEGF‐A165. Proteins were immunoprecipitated with an anti‐VEGFR‐2 antibody and then subjected to Western blotting using an anti‐phosphotyrosine mAb (Figure 1). The results show that the phosphorylation of the receptor was enhanced in adherent cells. In suspended cells, the effect of VEGF‐A165 was markedly lower. The phosphorylation level of CD31, a cell–cell interaction protein which is phosphorylated on tyrosine residues (Sagawa et al., 1997), was not increased by VEGF‐A165 in adherent or suspended cells (Figure 1). Subsequent experiments have been performed to test whether a specific matrix protein has a role in VEGFR‐2 activation. Endothelial cells were plated on vitronectin, fibronectin or collagen I, which are the ligands for αvβ3, α5β1 and α2β1, respectively, (Kuhn and Eble, 1994), or on the irrelevant substrate poly‐L‐lysine, and quiescent cells were stimulated with VEGF‐A165. Cell lysates were immunoprecipitated by an anti‐VEGFR‐2 antibody. Proteins were separated by SDS–PAGE and then blotted with anti‐phosphotyrosine mAb. Figure 2 shows that VEGFR‐2 is more phosphorylated in VEGF‐A165‐stimulated endothelial cells plated on vitronectin, than in cells plated on fibronectin, collagen or poly‐L‐lysine, suggesting a specific involvement of αvβ3 integrin in the activation of VEGFR‐2. The adhesion of endothelial cells to the different substrates was similar (not shown), thus excluding the possibility that the effect of vitronectin on VEGFR‐2 phosphorylation was caused by differences in cell attachment.
Figure 1
VEGF‐A165‐induced phosphorylation in adherent and suspended endothelial cells. Quiescent and confluent or suspended endothelial cells obtained as detailed in the Materials and methods were stimulated for 10 min with VEGF‐A165 (10 ng/ml) or vehicle alone for 10 min at 37°C. Cells were lysed and immunoprecipitated with anti‐VEGFR‐2 Ab (A and B) or with BV10 anti‐CD31 mAb. Immunoprecipitate was analyzed by SDS–PAGE followed by immunoblotting with anti‐phosphotyrosine mAb (A and C). Subsequently, blots were re‐probed with anti‐VEGFR‐2 Ab (B) or with BV10 mAb anti‐CD31. (D) Immunoreactive bands were detected by ECL technique. The results are representative of three identical experiments.
Figure 2
Effect of different endothelial cell adhesion substrates on the phosphorylation of VEGFR‐2. Endothelial cells plated at confluence on vitronectin, fibroncetin or collagen I (A and B) were made quiescent and then stimulated with VEGF‐A165 as in Figure 1. For the experiments with poly‐L‐lysine, quiescent endothelial cells were incubated for 2 h with cycloheximide (1 μM) and then for 1 h with monensin (2 μM) to block the synthesis of extracellular matrix. Then they were detached in cold PBS containing 2 mM EGTA, washed twice in M199 containing 1% FCS and then plated for 1 h on poly‐L‐lysine (PL) (C and D). Cells were lysed and immunoprecipitated with anti‐VEGFR‐2 Ab. Immunoprecipitates were analyzed by SDS–PAGE followed by immunoblotting with anti‐phosphotyrosine mAb (A and C). Subsequently, blots were re‐probed with anti‐VEGFR‐2 Ab (B and D). Immunoreactive bands were detected by ECL technique. The results are representative of two identical experiments. The different degree of receptor phosphorylation of cells plated on vitronectin between (A) and (C) was caused by the treatment with cycloheximide and monensin.
The mitogenic activity of VEGF‐A 165 is enhanced by α v β 3 ligand substrate
The evidence that vitronectin facilitates the phosphorylation of VEGFR‐2 prompted us to investigate whether the activation of endothelial cells by VEGF‐A, as well as by HIV‐1‐Tat, another ligand of VEGFR‐2 which stimulates migration and proliferation of endothelial and Kaposi's sarcoma cells (Albini et al., 1996b; Ganju et al., 1998), was enhanced by specific substrates. After plating on several matrix proteins, quiescent sub‐confluent cells were stimulated with either VEGF‐A165 or HIV‐1‐Tat and the mitogenic activity was evaluated by 5‐bromo‐2′‐deoxyuridine (BrdU) incorporation. Table I shows that the proliferation of endothelial cells after challenge with the two VEGFR‐2 ligands was higher on vitronectin (145 and 135% stimulation with VEGF‐A165 and HIV‐1‐Tat, respectively) and on fibrinogen, another specific ligand of αvβ3 (Kuhn and Eble, 1994) (168 and 141% stimulation with VEGF‐A165 and HIV‐1‐Tat, respectively) than on collagen I (99 and 57% stimulation with VEGF‐A165 and HIV‐1‐Tat, respectively), or on fibronectin (85 and 73% stimulation with VEGF‐A165 and HIV‐1‐Tat, respectively) or poly‐L‐lysine (36 and 17% stimulation with VEGF‐A165 and HIV‐1‐Tat, respectively).
Table I Effect of extracellular matrix and BV4 mAb antiβ3 integrin on BrdU incorporation in endothelial cells stimulated by VEGF‐A165 and HIV‐1‐Tat
VEGFR‐2 phosphorylation is inhibited by anti‐α v and anti‐β 3 antibodies
Next we evaluated whether mAbs raised against integrin subunits β3 and αv could interfere with the VEGFR‐2 phosphorylation stimulated by VEGF‐A165. We used BV4, an anti‐β3 subunit mAb. [This antibody was developed in the laboratory of Dr E.Dejana (Mario Negri Insitute, Milano, Italy) by immunizing mice with human endothelial cells.] BV4 is able to immunoprecipitate the 93 kDa β3 integrin subunit in human endothelial cells (G.Tarone, unpublished results; see also Figure 11). The effect of BV4 mAb on endothelial cell adhesion to vitronectin was compared with the effects elicited by mAbs B212 (anti‐β3; Thiagarajan et al., 1985), LM609 (anti‐αvβ3 integrin complex; Brooks et al., 1994a) and 0.165 [anti‐major histocompatibility complex (MHC) class I antigen]. Cells were incubated with increasing concentrations of mAbs for 20 min at 4°C and then were plated in the adhesion assay. Alternatively, confluent endothelial cells grown on vitronectin were incubated for 6 h with increasing concentrations of the mAbs. The results reported in Table II show that BV4 does not inhibit cell adhesion to substrate nor does it cause cell detachment, suggesting that it does not interfere in the mechanisms involved in endothelial cell adhesion to vitronectin.
Table II Effect of anti‐β3 integrin subunit mAbs on endothelial cell adhesion to and detachment from vitronectin
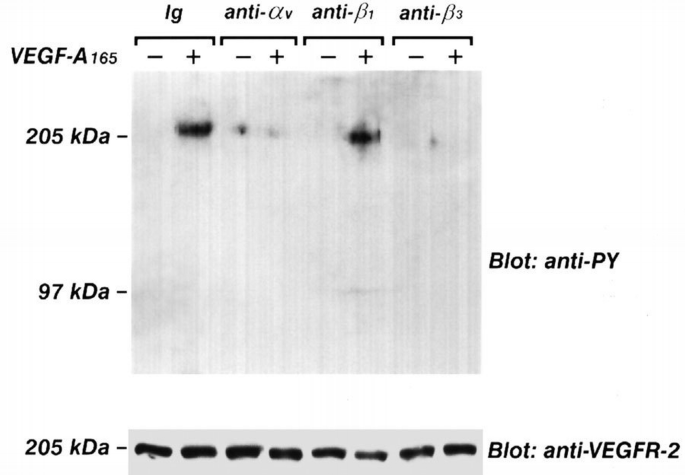
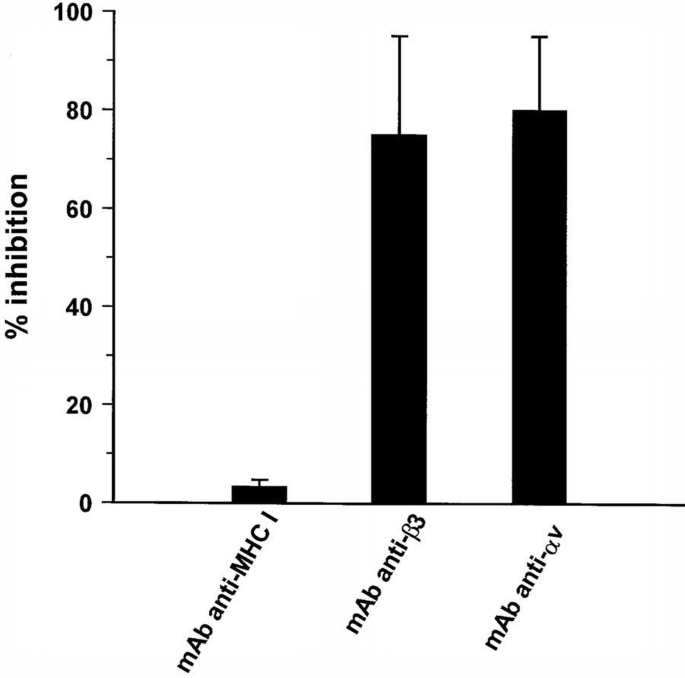
Confluent and quiescent endothelial cells were pre‐incubated for 20 min at 4°C with mAbs against αv, β3 and β1 integrin subunits, washed and then stimulated with VEGF‐A165 at 37°C. Cell lysates were immunoprecipitated by anti‐VEGFR‐2 Ab, and the proteins separated by SDS–PAGE were probed with anti‐phosphotyrosine mAb. Anti‐αv and anti‐β3 mAbs inhibited the VEGFR‐2 phosphorylation, but BV7 (anti‐β1 mAb) was ineffective (Figure 3). The inhibition of VEGFR‐2 phosphorylation caused by anti‐β3 and anti‐αv ranged from 100 to 50% (Figure 4). As a positive control, a neutralizing anti‐VEGFR‐2 antibody inhibited the receptor phosphorylation induced by VEGF‐A165 (data not shown). Thus, these experiments indicate that αvβ3 integrin, but not β1, is crucial in mediating tyrosine phosphorylation of VEGFR‐2.
Figure 3
Effect of BV4 mAb anti‐β3, BV7 mAb anti‐β1, and mAb anti‐αv on VEGF‐A165‐induced phosphorylation of VEGFR–2. Quiescent, confluent endothelial cells were pre‐incubated for 20 min at 4°C with specific or irrelevant Abs, washed and then stimulated with VEGF‐A165 as in Figure 1. Cells were lysed and immunoprecipitated with anti‐VEGFR‐2 Ab. Immunoprecipitate was analyzed by SDS–PAGE followed by immunoblotting with anti‐phosphotyrosine mAb. Subsequently, blots were re‐probed with anti‐VEGFR‐2 Ab. Immunoreactive bands were detected by ECL technique. The results are representative of six similar experiments.
Figure 4
Densitometric analysis of the inhibitory effect of BV4 mAb anti‐β3 and mAb anti‐ αv on VEGF‐A165‐induced phosphorylation of VEGFR‐2. The phosphorylated protein recognized by anti‐phospho‐ tyrosine mAb corresponding to VEGFR‐2 in the experiments detailed in Figure 3 were analyzed by densitometric analysis performed with a GS250 Molecular Imager (Bio‐Rad, Hercules, CA). Results (mean ± SD) indicate % inhibition induced by mAbs of the phosphorylation in tyrosine residues of VEGFR‐2 in endothelial cells stimulated by VEGF‐A165 in the presence of irrelevant Ig.
The β3 integrin is necessary for the activation of PI 3‐kinase in endothelial cells stimulated by VEGF‐A 165
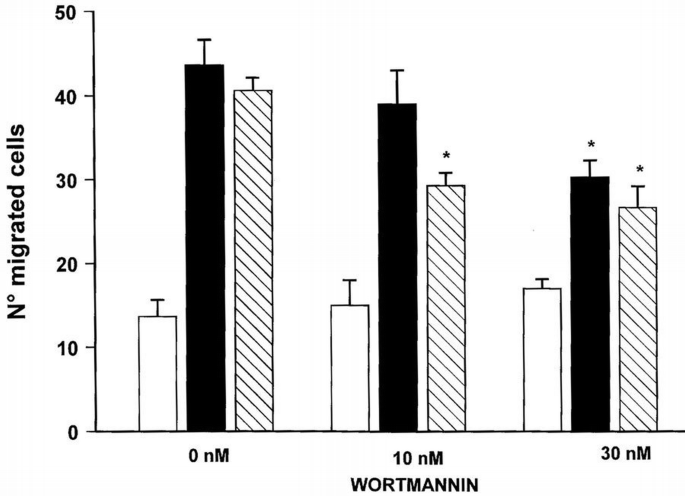
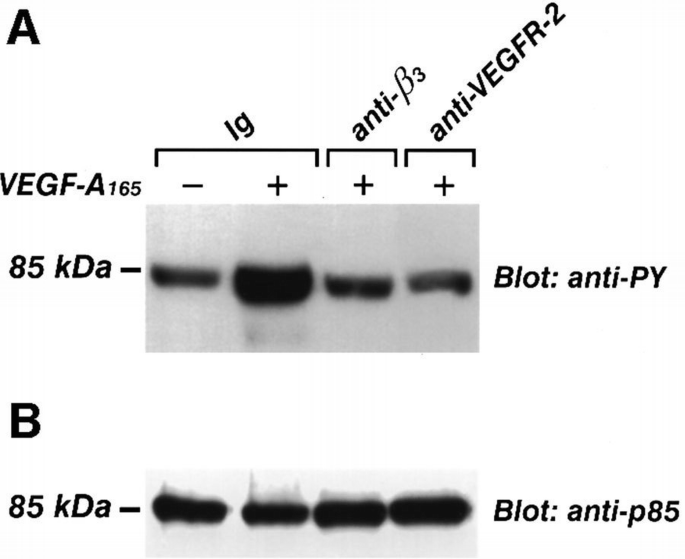
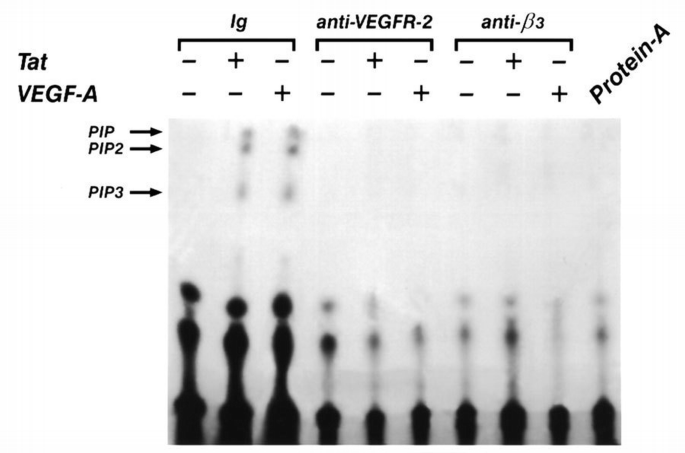
PI 3‐kinase belongs to the intracellular signals triggered by VEGF‐A in bovine endothelial cells, as demonstrated by the phosphorylation of its regulatory protein p85 (Guo et al., 1995). As shown in Figure 5, wortmannin used at nanomolar concentrations specific for PI 3‐kinase inhibition (Arcaro and Wymann, 1993), reduced the human endothelial cells migration induced by VEGF‐A165. To determine whether β3 integrin interferes in PI 3‐kinase activation occurring after VEGFR‐2 stimulation, we evaluated the effect of BV4 (anti‐β3 subunit mAb) on the phosphorylation in tyrosine residues of the p85 subunit and on the catalytic activity of the enzyme. After pre‐incubation with BV4 or with anti‐VEGFR‐2 Ab for 20 min at 4°C, cells were stimulated with VEGF‐A165. Cell lysates were immunoprecipitated with anti‐p85 mAb, and the separated proteins by SDS–PAGE were probed with anti‐phosphotyrosine mAb. VEGF‐A165 increased the tyrosine phosphorylation of p85, but it failed to phosphorylate the PI 3‐kinase subunit when endothelial cells were pre‐incubated with anti‐β3 or anti‐VEGFR‐2 antibodies (Figure 6). Similarly, BV4 and anti‐VEGFR‐2 inhibited the catalytic activity of PI‐3 kinase induced by VEGF‐A165 and by HIV‐1‐Tat (Figure 7). BV7, anti‐β1 mAb (not shown) and irrelevant Ig did not affect p85 phosphorylation and the increase of PI 3‐kinase activity triggered by both ligands (Figures 6 and 7).
Figure 5
Dose‐dependent effect of wortmannin on VEGF‐A165‐induced endothelial cell migration. Suspended endothelial cells were incubated for 15 min at 37°C with increasing concentrations of wortmannin and their ability to migrate across a 5 μm pore‐size polycarbonate filter in response to VEGF‐A165 (10 ng/ml) (black bar), HIV‐1‐Tat (hatched bar) or vehicle (open bar) was evaluated in a Boyden's chamber. At the end of the incubation (37°C, 4 h), filters were removed, stained with Diff‐Quik (Baxter Spa, Rome, Italy) and five high power oil‐immersion fields were counted. Results (mean ± SD) of one experiment (performed in triplicate) representative of at least three independent experiments are shown. *p <0.05 versus wortmannin‐untreated cells.
Figure 6
Inhibitory effect of BV4 anti‐β3 integrin subunit mAb and anti‐VEGFR‐2 Ab on VEGF‐A165‐induced p85 phosphorylation. Quiescent, confluent endothelial cells were pre‐incubated for 20 min at 4°C with specific or irrelevant Ig, washed and then stimulated with VEGF‐A165 (10 ng/ml) for 15 min at 37°C. Cells were lysed and immunoprecipitated with anti‐p85 mAb. Immunoprecipitate was analyzed by SDS–PAGE followed by immunoblotting with anti‐phosphotyrosine mAb (A). Subsequently, blots were re‐probed with anti‐p85Ab (B). Immunoreactive bands were detected by ECL technique. The results are representative of two similar experiments.
Figure 7
Inhibitory effect of BV4 anti‐β3 mAb and anti‐VEGFR‐2 Ab on VEGF‐A165‐induced PI 3‐kinase activation. Quiescent, confluent endothelial cells were pre‐incubated for 20 min at 4°C with specific or irrelevant mAb, washed and then stimulated with VEGF‐A165 (10 ng/ml) or with HIV‐1‐Tat (10 ng/ml) for 15 min at 37°C. PI 3‐kinase assay was performed on immune complexes done with anti‐phosphotyrosine mAb from lysates of endothelial cells. PIP, phosphatidylinositol; PIP2, phosphatidylinositol (4)P; PIP3, phosphatidylinositol (3,4)P2. Protein A indicates a PI 3‐kinase assay done on protein A alone.
The β3 integrin is required for the activation of the angiogenic program in endothelial cells
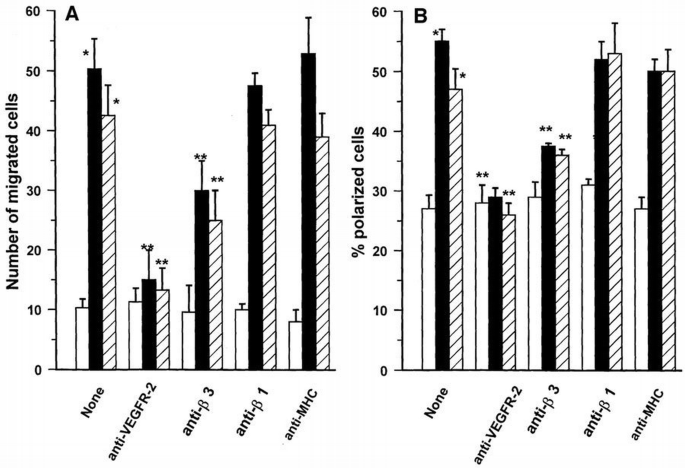
Through activation of VEGFR‐2, endothelial cells change their phenotype and start to proliferate and migrate (Thomas, 1996). To verify the biological relevance of the β3 integrin subunit on VEGF‐A165‐mediated endothelial cell activation, we have studied the effect of the anti‐β3 subunit mAb, BV4, on polarization, migration and proliferation of endothelial cells challenged with either VEGF‐A165 or HIV‐1‐Tat. BV4 inhibited by 40, 32 and 47%, respectively, the migration, polarization (Figure 8) and proliferation (Table I) induced by VEGF‐A165, and to a similar extent the activities triggered by HIV‐1‐Tat (Figure 8; Table I). BV7 (anti‐β1 integrin mAb) did not inhibit migration and polarization induced by both ligands (Figure 8). An anti‐MHC class I mAb was ineffective in all experimental conditions tested (Figure 8; Table I), whereas a neutralizing anti‐VEGFR‐2 antibody inhibited migration, polarization and proliferation (data not shown).
Figure 8
Effect antibodies specific for integrin subunits or VEGFR‐2 on migration (A) and polarization (B) of endothelial cells induced by VEGF‐A165 or HIV‐1‐Tat. Suspended endothelial cells were incubated for 20 min at 4°C with BV4 (anti‐β3 integrin mAb), BV7 (anti‐β1 integrin mAb), 0.165 (anti‐MHC class I mAb) and anti‐VEGFR‐2 antibody. Cells were washed and then stimulated with VEGF‐A165 (10 ng/ml) (black bar), HIV‐1‐Tat (10 ng/ml) (hatched bar) or vehicle alone (open bar). Migration was evaluated by Boyden's chamber technique as shown in detail in Figure 5. The percentage of polarized cells was obtained by counting bipolar cells in suspension by phase‐contrast microscopy at 400× magnification. Mean ± SD of four experiments performed in triplicate. Data were analyzed by one‐way analysis of variance [experiments shown in (A): F = 63.35; experiments shown in (B): F = 50.01) and Student–Newman‐Keuls test. *p <0.05 between unstimulated and stimulated cells; **p <0.05 between stimulated cells and cells stimulated after mAb‐pretreatment.
Effects of BV4 on binding displacement of [I 125 ]VEGF‐A 165 on endothelial cells
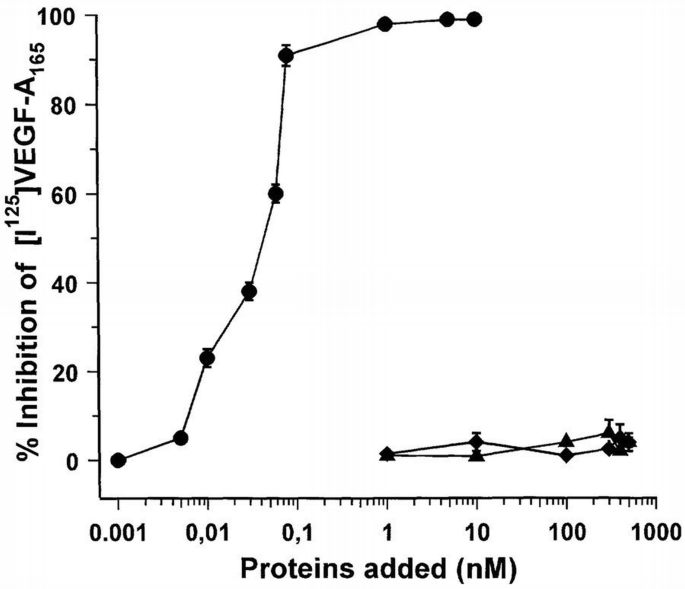
In order to assess the mechanisms leading to the inhibitory effect of BV4, we have evaluated the effect of this mAb on the binding of [I125]VEGF‐A165 on endothelial cells. Figure 9 shows that the pre‐incubation of endothelial cells with increasing concentrations of BV4 mAb or anti‐MHC class I mAb did not inhibit the binding of labeled VEGF‐A165 to the surface of endothelial cells, indicating that β3 integrin is not involved directly in the binding of VEGF‐ A165 to its specific binding sites, nor did it cause a steric hindrance. In contrast, cold VEGF‐A165 inhibited the binding to endothelial cells with an apparent IC50 of 60 pM.
Figure 9
Effect of BV4 mAb anti‐β3 integrin subunit on the binding at equilibrium of [125I]VEGF‐A165 to endothelial cells. Cell monolayers were incubated for 90 min at room temperature in 0.2 ml of binding buffer with 0.05 nM [125I]VEGF, with or without the indicated concentrations of unlabeled VEGF‐A165 (●), BV4 mAb (♦) and anti‐MHC class I antigen (▴). At the end of incubation, the cells were washed, solubilized with 2% SDS and the radioactivity was counted. Data are the mean ± SD of three determinations in one experiment out of two with similar results.
Integrin β3 becomes associated with VEGFR‐2 upon VEGF‐A 165 stimulation
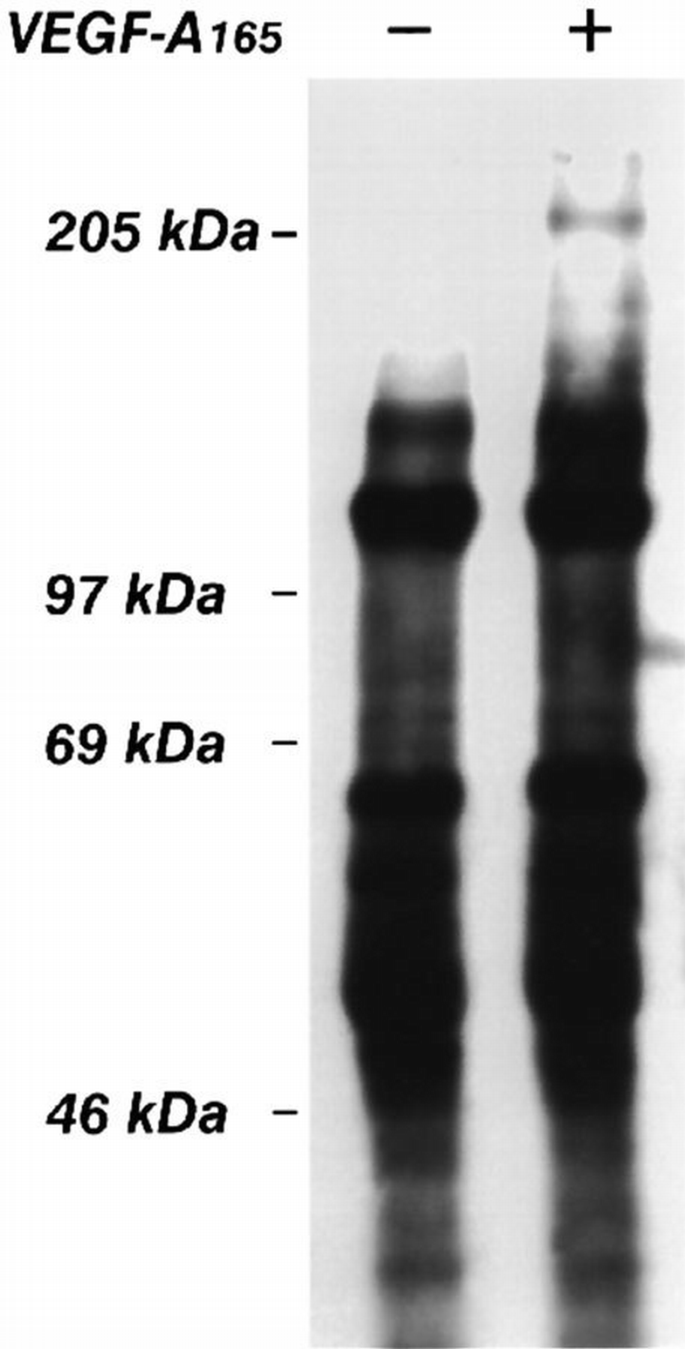
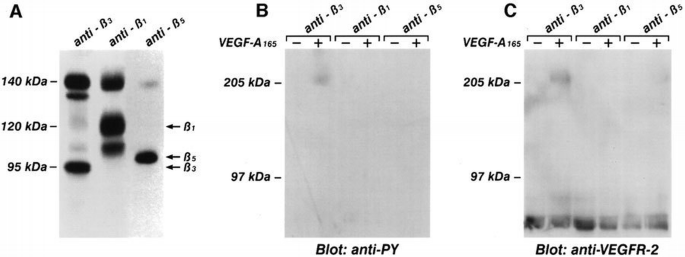
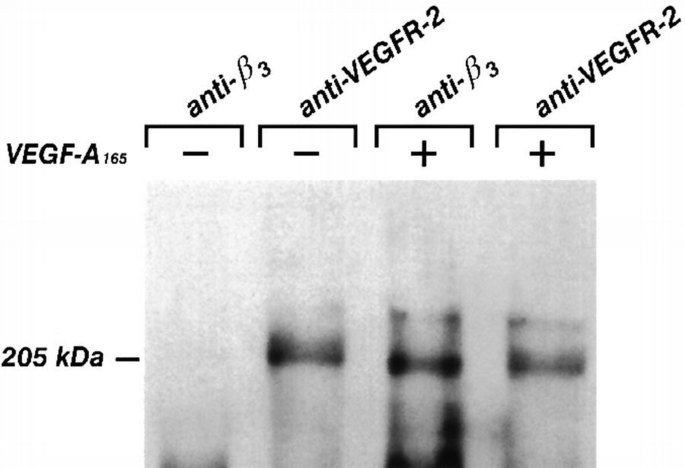
We next tested the possibility that VEGFR‐2 stimulation by its ligand induces the formation of a complex with β3 integrin. The membrane surface of endothelial cells was biotinylated and then cells were challenged with VEGF‐A165. Only in the β3 immune complexes from VEGF‐A165‐stimulated cells was a 205 kDa band detected (Figure 10), this being the molecular weight of VEGFR‐2 (Thomas, 1996). To identify this protein, cell lysates were immunoprecipitated with the BV4 mAb. The presence of VEGFR‐2 in the immune complexes was investigated by performing a second immunoprecipitation on solubilized proteins with an anti‐VEGFR‐2 antibody and immunoblotting with anti‐VEGFR‐2 or with anti‐phosphotyrosine Abs. As shown in Figure 11, in absence of the ligand, VEGFR‐2 was not present in the anti‐β3 immunoprecipitate. However, the receptor was associated in phosphorylated form with β3 integrin in the presence of VEGF‐A165. The β1 or β5 immunoprecipitates from stimulated and unstimulated endothelial cells, did not contain VEGFR‐2 (Figure 11). The amount of tyrosine‐phosphorylated receptor associated with β3 integrin visualized in double‐immunoprecipitation experiments seemed to be lower than the overall amount of phosphorylated receptor (i.e. Figure 1). A quantitative investigation of the amount of VEGFR‐2 associated with β3 integrin has been done on biotinylated endothelial cells. Figure 12 shows that the amount of a 205 kDa protein immunoprecipitated by anti‐VEGFR‐2 or by BV4 antibodies in VEGF‐A165‐stimulated cells was similar to that associated with β3 integrin. This experiment suggests that themajor fraction of VEGFR‐2 is associated with β3 integrin after ligand challenge. The discrepancy observed in the level of tyrosine phosphorylation studied by immunoprecipitating VEGFR‐2 from cell lysate or from β3 integrin, in double‐immunoprecipitation experiments, could be caused by a poor recovery of the receptor during the second step of immunoprecipitation.
Figure 10
Effect of VEGF‐A165 on association of biotinylated membrane proteins to β3 integrin subunit. Surface membrane proteins of confluent and quiescent endothelial cells were labelled with the membrane‐impermeable NHS.biotin and then incubated for 10 min at 37°C with VEGF‐A165 (10 ng/ml). Cells were lysed and immunoprecipitated with BV4 mAb anti‐β3 integrin. The biotinylated proteins were analyzed by SDS–PAGE followed by blotting stained with avidin–peroxidase and enhanced chemiluminescence technique. The figure is representative of two experiments which gave similar results.
Figure 11
Phosphorylated VEGFR‐2 becomes associated with β3, but not with β1 and β5 integrin subunits. (A) Cell lysate from quiescent and confluent endothelial cells labeled with [35S]methionine were immunoprecipitated with mAbs BV4 anti‐β3, BV7 anti‐β1 or with Ab anti‐β5 integrins. Soluble immune complexes were eluted by boiling beads in 1% SDS and analyzed by 6% SDS–PAGE in non‐reducing conditions. The pattern shown by this picture is not modified by cell treatment with VEGF‐A165 (not shown). (B and C) Quiescent and confluent endothelial cells were incubated for 10 min at 37°C with VEGF‐A165 (10 ng/ml) and cell lysate were immunoprecipitated with mAb BV4 anti‐β3, BV7 anti‐β1 or Ab anti‐β5. After protein solubilization from protein A–Sepharose, samples were subjected to a second immunoprecipitation with anti‐VEGFR‐2 antibody. Samples were analyzed by SDS–PAGE followed by immunoblotting with anti‐phosphotyrosine antibody (B). Subsequently, the blot was reprobed with anti‐VEGFR‐2 (C). The figure is representative of three similar experiments. For each experiment, the same batch of endothelial cells was used.
Figure 12
Recovery of VEGFR‐2 expressed on endothelial cell surface in immunocomplexes anti‐β3 and anti‐VEGFR‐2 antibodies. Surface membrane proteins of confluent and quiescent endothelial cells were labeled with the membrane‐impermeable NHS.biotin and then incubated for 10 min at 37°C with VEGF‐A165 (10 ng/ml). Cells were lysed and immunoprecipitated with BV4 mAb anti‐β3 integrin or with anti‐VEGFR‐2 Ab. The biotinylated proteins were analyzed by SDS–PAGE followed by blotting stained with avidin–peroxidase and enhanced chemiluminescence technique. The picture is representative of two experiments performed with similar results.
Discussion
VEGFR‐2 is a tyrosine kinase receptor which is responsible for the angiogenic activity of VEGF‐A (Millauer et al., 1993; Waltenberger et al., 1994). The tyrosine kinase activity of the receptor and its autophosphorylation begin when two molecules of VEGFR‐2 bind the N‐terminal 110 amino acids of two dimerized monomers of VEGF‐A (Keyt et al., 1996; Muller et al., 1997). At least for the interaction between VEGF‐A165 and VEGFR‐2, it has been demonstrated that neuropilin‐1 acts as a co‐receptor which enhances the affinity of the receptor for the ligand (Soker et al., 1998). Through its phosphorylated tyrosine residues, the activated VEGFR‐2 associates with the adapter molecules Shc, Grb2 and Nck, to Ras GTPase activating protein, p59_fyn_, pp62_yes_ and phospholipase Cγ, and to the tyrosine phosphatases SHP‐1 and SHP‐2 (Waltenberger et al., 1994; Kroll and Waltenberger, 1997; Takahashi and Shibuya, 1997). The formed transductosome mediates the activation of MAP kinase, PI 3‐kinase, Jun kinase, cGMP‐dependent kinase and focal adhesion kinase (Guo et al., 1995; Abedi and Zachary, 1997; Kroll and Waltenberger, 1997; Rousseau et al., 1997; Hood and Granger, 1998; Pedran et al., 1998), which are putative mediators of chemotaxis, mitogenicity, actin reorganization and gross morphology changes in endothelial cells.
The findings in this work add new insights to the mechanism of activation of an angiogenic program in vascular endothelial cells stimulated by VEGF‐A. Cell adhesion is critical for receptor activation based on two experimental observations: (i) VEGF‐A165‐dependent VEGFR‐2 phosphorylation was strongly activated in adherent cell, but showed a substantially reduced response in suspended cells; and (ii) the ligand‐induced phosphorylation of VEGFR‐2, migration and proliferation of endothelial cells were enhanced when the cells were plated on vitronectin or fibrinogen, which bind to αvβ3. Furthermore, β3 integrin is required for the full activation of VEGFR‐2, by a mechanism possibly independent of its role in cell adhesion. The BV4 anti‐β3 integrin mAb, which does not inhibit cell adhesion (see Table II and Materials and methods), markedly reduced the VEGFR‐2 phosphorylation triggered by VEGF‐A165, the activation of PI 3‐kinase, which is a downstream event to VEGFR‐2 dimerization (Guo et al., 1995). Furthermore, this mAb reduced the mitogenic and the motogenic effects of two ligands of VEGFR‐2: VEGF‐A165 and HIV‐1‐Tat. The lack of effect of the BV4 mAb on endothelial cell adhesion to vitronectin (Table II), and the observed inhibitory effects on VEGFR‐2, suggest that the β3 integrin subunit is involved directly in VEGFR‐2 activation. This hypothesis is strongly supported by the result that VEGF‐A165‐stimulated, tyrosine phosphorylated VEGFR‐2 was associated with β3 integrin. The specificity of the model was restricted to αvβ3 integrin. In fact, we did not observe anti‐β1 mAb inhibition of endothelial cell migration and polarization induced by VEGF‐A165 or HIV‐1‐Tat. Fibronectin, the ligand of α5β1, and collagen I, the ligand of α2β1, were also unfavorable substrates for the ligand‐dependent phosphorylation of VEGFR‐2. Furthermore, the observed selectivity of VEGFR‐2 for β3 integrin is not simply due to its high level of expression in endothelial cells. In our experimental conditions, the levels of β3 and β5 were similar and lower than that of β1 (Figure 11). Furthermore, it has been reported previously that the amount of αvβ3 in cultured endothelial cells is lower than that of α2β1 and α5β1(Defilippi et al., 1991b) and similar to that of αvβ5 (Bhattacharya et al., 1995).
The relevance of αvβ3 integrin in the activation of tyrosine kinase receptors has been achieved in three different models. In rat vascular smooth muscle cells, the clustering of β3 integrin favor the EGF‐dependent tyrosine phosphorylation of EGF receptor and cell growth (Jones et al., 1997). Furthermore, a neutralizing anti‐αvβ3 mAb inhibits the effect of EGF in terms of receptor phosphorylation and proliferation induction (Jones et al., 1997). In human foreskin fibroblasts, αvβ3 is associated with activated insulin and platelet‐derived growth factor (PDGF)‐β receptors and potentiates the growth and the motility response to PDGF‐BB (Schneller et al., 1997). Similar results have been reported by Woodard and colleagues on rat endothelial cells (Woodard et al., 1998). The nature of the connection between αvβ3 and VEGFR‐2 remains to be elucidated. The β3 integrin subunit is associated with the phosphorylated form of VEGFR‐2. This effect is specific, because β5, which has been demonstrated to be involved in angiogenic response to VEGF‐A (Friedlander et al., 1995), and β1 integrins did not associate to VEGFR‐2. Furthermore, the pre‐treatment of quiescent endothelial cells with BV4 anti‐β3 mAb, but not with BV7 anti‐β1 mAb, reduces the receptor activation as well as the activation of downstream signals (i.e. activation of PI 3–kinase). These data suggest that αvβ3 is a molecular component specifically required for a correct function of the receptor. The lack of inhibition exerted by BV4 on the binding of VEGF‐A165 to endothelial cells and on cell adhesion excludes the possibility that the inhibitory effect of the BV4 mAb was due to interference in ligand‐receptor interaction or to a block of adhesion machinery. The chemical basis of the association between β3 integrin subunit and VEGFR‐2 is unknown. We can not exclude the possibility that molecules associated with VEGFR‐2, such as a insulin receptor substrate‐1‐like molecule that has been demonstrated to bind αvβ3 (Vuori and Ruoslahti, 1994), or that neuropilin‐1, the co‐receptor of VEGFR‐2 (Soker et al., 1998), have docking sites for β3 integrin. It has been reported that β3 integrin can be phosphorylated on tyrosine residues (Blystone et al., 1996; Law et al., 1996). Indeed the SH2 or PTB domains of different signaling molecules associated with VEGFR‐2 (Waltenberger et al., 1994; Kroll and Waltenberger, 1997; Takahashi and Shibuya, 1997) may also bind directly to β3 cytoplasmic tail. In endothelial cells, an anti‐apoptotic signal is started by αvβ3 clustering (Stromblad et al., 1996). However, we have shown that the clustering of β3 integrin obtained by subsequent cell incubation with a specific mAb followed by a secondary goat anti‐mouse IgG did not enhance the VEGFR‐2 phosphorylation induced by VEGF‐A165 (S.Mitola and F.Bussolino, unpublished results). This suggests that in our model the role of αvβ3 can not be limited to the formation of specialized cytoskeleton structures enriched with signaling molecules, which collaborate with growth factor‐dependent signaling (Miyamoto et al., 1996; Giancotti, 1997; Jones et al., 1997; Schwartz, 1997). One possibility is that β3 association might protect VEGFR‐2 against the activity of phosphatases. Consistent with this, protein tyrosine phosphatases SHP‐1 and SHP‐2 are associated with VEGFR‐2 (Kroll and Waltenberger, 1997). Furthermore, it is possible that β3 is needed to ensure the correct subcellular juxtaposition of cytoplasmic tails of the dimerized VEGFR‐2. Both hypotheses agree with the observation that the activation of the catalytic activity of PI 3‐kinase and the tyrosine phosphorylation of its regulatory subunit p85 induced by VEGF‐A165 and HIV‐1‐Tat also required β3 integrin, as inferred by the inhibitory role of BV4 mAb. PI 3‐kinase is at least involved in cell survival and in cytoskeleton rearrangement by co‐operating with the integrin system (Khwaja et al., 1997; Toker and Cantley, 1997), and therefore the observed activation in endothelial cells challenged with VEGF‐A165 and HIV‐1‐Tat agrees with its known functions. In this study, a relationship between PI 3‐kinase activation and biological activation of endothelial cells stimulated by VEGFR‐2 ligands has been achieved by the use of wortmannin, employed at low doses considered specific for PI 3‐kinase inhibition (Arcaro and Wymann, 1993). In bovine aortic endothelial cells, Guo and co‐workers reported that VEGF‐A was capable of inducing the phosphorylation of PI 3–kinase (Guo et al., 1995), but successively it has been reported that VEGF‐A did not activate the catalytic activity of the enzyme in human endothelial cells (Abedi and Zachary, 1997). We believe that this discrepancy could be due to differences in experimental conditions, in particular to a different time‐course or to a difference in conditions of cell culture.
Consistent results have demonstrated a crucial role for extracellular matrix in the development of vasculature in physiological and pathological conditions (Ingber and Folkman, 1989; Bussolino et al., 1997), and in particular, a prominent role is attributed to αvβ3 integrin (Brooks et al., 1994a,b; Friedlander et al., 1996; Stromblad et al., 1996; Senger et al., 1997; Eliceiri et al., 1998), at least in adult life (Bader et al., 1998). Besides being expressed in vivo on endothelial cells of angiogenic vessels (Brooks et al., 1994a; Friedlander et al., 1996) and in vitro by microvascular endothelial cells after treatment with VEGF‐A (Senger et al., 1997), αvβ3 integrin takes part in the in vitro endothelial angiogenic program with different and temporally distinct roles. First, as demonstrated here, αvβ3 co‐operates with an angiogenic receptor (i.e. VEGFR‐2) for its full activation triggered by the ligand; next it is necessary for the second wave of activation of MAP kinase induced by an angiogenic activator (Eliceiri et al., 1998); and finally, most probably through the MAP kinase pathway, it regulates the expression of genes which control the cell cycle (Stromblad et al., 1996).
Materials and methods
Cells
Human endothelial cells from umbilical cord veins, prepared and characterized as previously described (Bussolino et al., 1992), were grown in M199 medium (Gibco‐BRL, Grand Island, NY) supplemented with 20% fetal calf serum (FCS) (Irvine, Santa Ana, CA), endothelial cell growth factor (100 μg/ml) (Sigma Chemical Co., St Louis, MO) and porcine heparin (Sigma) (100 μg/ml). Cells were used at second passage and growth on a plastic surface coated with porcine gelatin (Sigma), unless specified.
Antibodies
The rabbit polyclonal antibody against the C‐terminus peptide of VEGFR‐2 was purchased from Santa Cruz Biotecnology Inc. (Santa Cruz, CA; C‐1158), the rabbit neutralizing (Albini et al., 1996a) polyclonal anti‐VEGFR‐2 antibody was kindly provided by Dr H.Weich, (Gesellschaft fuer Biotechologische Forschung, Braunschweig, Germany). The rabbit polyclonal anti‐p85 antibody and the anti‐phosphotyrosine mAb (clone G410) were purchased from Upstate Biotechnology Incorporated (Lake Placid, NY). mAb BV7 to β1 integrin (class IgG1) (Martin‐Padura et al., 1994), BV4 to β3 integrin (class IgG1) and BV10 to CD31 (class IgG1) (Lampugnani et al., 1992) were originally described by Dr E.Dejana (Istituto Mario Negri, Milano, Italy) and obtained by Bioline (Torino, Italy). B212 mAb to β3 integrin (Thiagarajan et al., 1985) was a gift from Dr P.Thiagarajan (Jefferson Medical Collge, PA). Anti‐αv integrin subunit mAb was purchased from American Type Cell Collection (Bethesda, MD). The polyclonal anti‐β5 integrin subunit Ab was raised against the C‐terminal sequence (NH2‐KTFNKFNKSYNGTVD‐COOH) (Bioline). A mAb anti‐MHC class I antigen (0.165 mAb, class IgG1) was kindly provided by Professor F.Malavasi (Istituto di Biologia e Genetica, Ancona, Italy). LM609, which recognizes αvβ3 integrin complex, was a gift from Dr D.A.Cheresh (The Scripps Research Institute, La Jolla, CA).
Endothelial cell labeling with [ 35 S]methionine
Endothelial cell were grown at confluence and labeled with [35S]methionine (800 Ci/mmol; Amersham, UK) by overnight incubation in methionine‐free medium (Sigma) with 5% FCS and 40 μCi/ml of radioisotope.
Endothelial cell surface biotinylation
Confluent endothelial cells were incubated for 20 min at room temperature with Sulfo‐NHS‐biotin (Pierce, Rockford, IL) dissolved in phosphate‐buffered saline (PBS) at 1 mg/ml. After washing the cells once with PBS, the residual NHS groups were reacted with 0.1 M glycine in PBS on ice for 15 min. After several washes, the cells were processed for specific immunoprecipitation followed by electrophoresis and blotting as specified below. Biotinylated proteins were visualized with avidin‐peroxidase and developed using the enhanced chemiluminescence technique (Amersham).
Immunoprecipitation and immunoblotting
Confluent endothelial cells (1×107 cells/ 150 cm2 dish) were made quiescent by 20 h starvation in M199 containing 0.5% FCS and 0.1% human serum albumin (Farma Biagini, Lucca, Italy), pre‐incubated for 15 min at 37°C with 1 mM Na3V and then stimulated with VEGF‐A165 (Dr H.Weich, Gesellschaft für Biotechologische Forschung, Braunschweig, Germany) or with HIV‐1‐Tat (Intracel, London, U.K.) in presence of heparin (1 U/ml). Cells were lysed in a 50 mM Tris–HCl buffer pH 7.4, containing 150 mM NaCl, 1% Triton X‐100, and protease and phosphatase inhibitors (50 μg/ml pepstatin, 50 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM PMSF; 500 μg/ml soybean trypsin inhibitor, 100 μM ZnCl2, 1 mM Na3VO4, Sigma). After centrifugation (20 min, 10 000 g), supernatants were pre‐cleared by incubation for 1 h with protein A–Sepharose or with anti‐mouse Ig‐agarose (Sigma). Samples (1 mg of protein) were incubated with rabbit polyclonal anti‐VEGFR‐2 (Santa Cruz), or BV4 (anti‐β3 integrin subunit) mAbs, BV7 (anti‐β1 integrin), anti‐p85, anti‐phosphotyrosine, anti‐CD31 (5–10 μg/ml) and anti‐β5 integrin for 1 h at 4°C and immune complexes were recovered on protein A–Sepharose or anti‐mouse Ig–agarose. Immunoprecipitates were washed four times with lysis buffer, twice with the same buffer without Triton X‐100 and once with TBS. In some experiments, anti‐β3 or anti‐β1 integrin immune complexes recovered on agarose beads were boiled for 2 min in a solubilization buffer containing 0.4% SDS, 50 mM triethanolamine chloride (pH 7.4), 100 mM NaCl, 2 mM EDTA, 10% glycerol and protease and phosphatase inhibitors, and 2 mM 2β‐mercaptoethanol. After boiling, iodacetamide was added to a concentration of 10 mM (Soldi et al., 1997). These extracts were again immunoprecipitated with anti‐VEGFR‐2 antibody (Santa Cruz). Proteins were solubilized under reducing or non‐reducing conditions (Laemmli, 1970), separated by SDS–PAGE (8 or 10%), and transferred to Immobilon‐P sheets (Millipore, Bedford, MA) and probed with anti‐phosphotyrosine, or with anti‐VEGFR‐2 mAbs (Santa Cruz). The enhanced chemiluminescence technique (Amersham) was used for detection.
PI 3‐kinase
PI 3‐kinase assay was performed directly on anti‐phosphotyrosine immunoprecipitates as described before (Auger et al., 1989; Soldi et al., 1994) except that the beads were also washed twice with Tris‐buffered saline containing 0.5 M LiCl. Briefly, immunoprecipitates were incubated with 40 μM ATP, 50–100 μCi [γ‐32P]ATP (Amersham), and a presonicated mixture of phosphatidylinositol (4,5)P2 and phosphatidylserine (50 μg/ml final concentration of both lipids, Sigma), in 25 mM HEPES pH 7.4, and 1 mM EGTA. The reaction was stopped after 10 min incubation at room temperature by the addition of 1 vol. of 1 M HCl and 2 vol. chloroform/methanol (1:1). The lipids in the organic phase were separated by thin‐layer chromatography (Merck, Darmstadt, Germany, Silica Gel 60) in 1‐propanol:2 M acetic acid 65:35 (v/v) and visualized by autoradiography.
Migration, polarization and proliferation assays
Endothelial cell motility was studied using a modified Boyden's chamber technique as previously described (Bussolino et al., 1992; Albini et al., 1996b). To study endothelial cell polarization, cells were detached in cold PBS containing 2 mM EGTA and washed twice in M199 containing 1% FCS. Endothelial cells (106/ml M199 containing 1% human serum albumin) were pre‐warmed in polypropilene tubes at 37°C for 5 min and then exposed to 10 ng/ml of VEGF‐A165 or HIV‐1‐Tat for 10 min. The reaction was stopped by adding an equal volume (1 ml) of ice‐cold phosphate‐buffered formaldehyde (10% v/v; pH 7.2). The percentage of cells with bipolar configuration (front‐tail) was determined in at least 200 cells for each tube by phase‐contrast microscopy at 400× magnification (Mitola et al., 1997).
The evaluation of endothelial cells proliferation was performed with Biotrak cell proliferation ELISA system (Amersham). Cells (2.5×103) were plated in 96‐well plate coated with human serum albumin (0.1%), or human vitronectin, or human fibronectin, or with poly‐L‐lysine (Sigma) or human fibrinogen (Kabivitrum Stockholm, Swedish) (0.001%), and grown for 12 h in M199 containing 5% FCS. Then, they were washed and maintained for 12 h at 37°C in serum‐free M199 containing 5% human serum albumin, and subsequently stimulated with 10 ng/ml of VEGF‐A165 or HIV‐1‐Tat for 24 h. BrdU was added to the cells for an additional time of 3 h. Fixed cells were treated according to the manufacturer's instruction and the resultant color developed by the anti‐BrdU peroxidase‐labeled immune complexes was read at 450 nm in microtiter plate spectrophotometer (EL340, Bio‐tek Instruments).
Treatment of endothelial cells with specific antibodies
To study the effect of specific antibodies on the experimental designs above (experiments of VEGFR‐2 phosphorylation, migration and polarization), endothelial cells were incubated in M199 with 10 μg anti‐β3 (BV4 mAb), anti‐β1 (BV7 mAb) or anti‐MHC class I antigen (0.165 mAb), or with a neutralizing anti‐VEGFR‐2 antibody, or with irrelevant mouse or rabbit IgG (Sigma) at 4°C for 20 min. Cells were washed twice in cold M199 containing 1% human serum albumin and then exposed to stimuli. To evaluate the effect of the above mAbs in BrdU incorporation, the antibodies (20 μg/ml) were added in the last 27 h.
Binding displacement assay
In a previous study, we demonstrated that the equilibrium of binding of labelled VEGF‐A with its high affinity sites on endothelial cells is reached after 90 min at room temperature (Albini et al., 1996b). On the basis of this data, binding displacement studies with mAb anti‐β3 integrin were performed. Cell monolayers on 24‐well plates were incubated for 90 min at room temperature in 0.2 ml M199 containing 20 mM (_n_‐[2‐hydroxyethyl]piperazine‐_N_′‐[4‐butanesulfonic acid]), pH 7.4, 0.1% BSA, 100 μg/ml soybean trypsin inhibitor (Sigma) with 0.05 nM [125I]VEGF (specific activity 140 μCi/μg) (Amersham) and increasing concentrations of cold BV4 mAb anti‐β3, mAb anti‐MHC class I or VEGFA165. The cells were washed twice with PBS, solubilized with 2% SDS in PBS and the radioactivity was measured.
References
- Abedi H and Zachary I (1997) Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem, 272, 15442–15451.
Google Scholar - Albini A, Benelli R, Presta M, Rusnati M, Ziche M, Rubartelli A, Paglialunga G, Bussolino F and Noonan D (1996a) HIV‐tat protein is a heparin‐binding angiogenic growth factor. Oncogene, 12, 289–297.
Google Scholar - Albini A et al. (1996b) The angiogenesis induced by HIV‐1 tat protein is mediated by the Flk‐1/KDR receptor on vascular endothelial cells. Nature Med, 2, 1371–1375.
Google Scholar - Arcaro A and Wymann MP (1993) Wortmannin is a potent phosphatidylinositol 3‐kinase inhibitor: the role of phosphatidylinositol 3,4,5‐triphosphate in neutrophil responses. Biochem J, 296, 297–301.
Google Scholar - Auger KR, Serunian LA, Soltoff SP, Libby P and Cantley LC (1989) PDGF‐dependent tyrosine phosphorylation stimulates production of novel phosphoinositides in intact cells. Cell, 57, 167–175.
Google Scholar - Bader BL, Rayburn H, Crowley D and Hynes R (1998) Extensive vasculogenesis, angiogenesis and organogenesis precede lethality in mice lacking all αv integrins. Cell, 95, 507–519.
Google Scholar - Bhattacharya S, Fu C, Bhattacharya J and Greenberg S (1995) Soluble ligands of the integrin mediate enhanced tyrosine phosphorylation of multiple proteins in adherent bovine pulmonary artery endothelial cells. J Biol Chem, 270, 16781–16787.
Google Scholar - Blystone SD, Lindberg FP, Williams MP, McHugh KP and Brown EJ (1996) Inducible tyrosine phosphorylation of the β3 integrin requires αv integrin cytoplasmic tail. J Biol Chem, 271, 31458–31462.
Google Scholar - Brooks PC, Clark RAF and Cheresh DA (1994a) Requirement of vascular integrin αvβ3 for angiogenesis. Science, 264, 569–571.
Google Scholar - Brooks PC, Montgomery AMP, Rosenfeld M, Reisfeld RA, Hu T, Klier G and Cheresh DA (1994b) Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell, 79, 1157–1164.
Google Scholar - Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH and Cheresh DA (1995) Antiintegrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest, 96, 1815–1822.
Google Scholar - Bussolino F et al. (1992) Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol, 119, 629–641.
Google Scholar - Bussolino F, Mantovani A and Persico G (1997) Molecular mechanisms of blood vessel formation. Trends Biochem Sci, 22, 251–256.
Google Scholar - Clark EA and Brugge JS (1995) Integrins and signal transduction pathways: the road taken. Science, 268, 233–239.
Google Scholar - De Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N and Williams LT (1992) The fms‐like tyrosine kinase, a receptor for vascular endothelial growth factor. Science, 255, 989–991.
Google Scholar - Defilippi P, Truffa G, Stefanutto G, Altruda F, Silengo L and Tarone G (1991a) Tumor necrosis factor α and interferon γ modulates the epression of the vitronectin receptor (integrin β3) in human endothelial cells. J Biol Chem, 266, 7638–7645.
Google Scholar - Defilippi P, van Hinsberg V, Bertolotto A, Rossino P, Silengo L and Tarone G (1991b) Differential distribution and modulation of expression of α1/β1 integrin on human endothelial cells. J Cell Biol, 114, 855–863.
Google Scholar - Eliceiri BP, Klemke R, Stromblad S and Cheresh DA (1998) Integrin αvβ3 requirement for sustained mitogen‐activated protein kinase activity during angiogenesis. J Cell Biol, 140, 1255–1263.
Google Scholar - Ensoli B et al. (1994) Synergy between basic fibroblast growth factor and HIV‐1 Tat protein in induction of Kaposi's sarcoma. Nature, 371, 674–680.
Google Scholar - Ferrara N and Davis‐Smyth T (1997) The biology of vascular endothelial growth factor. Endocrinol Rev, 18, 4–25.
Google Scholar - Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA and Cheresh DA (1995) Definition of two angiogenic pathways by distinct αv integrins. Science, 270, 1500–1502.
Google Scholar - Friedlander M, Theesfeld CL, Sugita M, Fruttiger M, Thomas MA, Chang S and Cheresh DA (1996) Involvement of integrins αvβ3 and αvβ5 in ocular neovascular diseases. Proc Natl Acad Sci USA, 93, 9764–9769.
Google Scholar - Frisch SM and Ruoslahti E (1997) Integrins and anoikis. Curr Opin Cell Biol, 9, 701–706.
Google Scholar - Ganju RK, Munshi N, Nair BC, Zy L, Gill P and Groopman JE (1998) Human immunodeficiency virus Tat modulates the Flk‐1/KDR receptor, mitogen‐activated protein kinases and components of focal adhesion in Kaposi's sarcoma cells. J Virol, 72, 6131–6137.
Google Scholar - Giancotti FG (1997) Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol, 9, 691–700.
Google Scholar - Guo D, Jia Q, Song HY, Warren RS and Donner DB (1995) Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation. J Biol Chem, 270, 6729–6733.
Google Scholar - Hidai C et al. (1998) Cloning and characterization of developmental endothelial locus‐1: an embryonic endothelial cell protein that binds the αvβ3 integrin receptor. Genes Dev, 12, 21–33.
Google Scholar - Hood J and Granger HJ (1998) Protein kinase G mediates vascular endothelial growth factor‐induced Raf‐1 activation and proliferation in human endothelial cells. J Biol Chem, 273, 23504–23508.
Google Scholar - Ingber D and Folkman J (1989) How does extracellular matrix control capillary morphogenesis? Cell, 58, 803–805.
Google Scholar - Jones RL, Crack J and Rabinovitch M (1997) Regulation of Tenascin‐C, a vascular smooth muscle cell survival factor that interacts with the αvβ3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol, 139, 279–293.
Google Scholar - Keyt BA, Berleau LT, Nguyen HV, Chen H, Heinsohn H, Vandlen R and Ferrara N (1996) The carboxyl‐terminal domain (111–165) of vascular endothelial growth factor is critical for its mitogenic potency. J Biol Chem, 271, 7788–7795.
Google Scholar - Khwaja A, Rodriguez‐Viciana P, Wennstrom S, Warne PH and Downward J (1997) Matrix adhesion and Ras transformation both activate a phosphoinositide 3‐OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J, 16, 2783–2793.
Google Scholar - King WG, Mattaliano MD, Chan TO, Tsichilis PM and Brugge PS (1997) PI3‐kinase is required for integrin‐stimulated AKT and Raf‐1/MAP kinase pathway activation. Mol Cell Biol, 17, 4406–4418.
Google Scholar - Kroll J and Waltenberger J (1997) The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem, 272, 32521–32527.
Google Scholar - Kuhn K and Eble J (1994) The structural bases of integrin–ligand interactions. Trends Cell Biol, 4, 256–261.
Google Scholar - Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 230, 680–682.
Google Scholar - Lampugnani MG, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, Ruco LP and Dejana E (1992) A novel endothelial‐specific membrane protein is a marker of cell–cell contacts. J Cell Biol, 118, 1511–1522.
Google Scholar - Law DA, Nannizzi‐Alaimo L and Phillps DR (1996) Outside–in integrin signal transduction. Alpha Ib beta3‐ (GP IIb IIIa) tyrosine phosphorylation induced by platelet aggregation. J Biol Chem, 271, 10811–10815.
Google Scholar - Leavesley PI, Schwartz MA, Rosenfeld M and Cheresh DA (1993) Integrin β1‐ and β3‐mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol, 121, 737–743.
Google Scholar - Martin‐Padura I, Bazzoni G, Zanetti A, Bernasconi S, Elices MJ, Mantovani A and Dejana E (1994) A novel mechanisms of colon carcinoma cell adhesion to the endothelium triggered by beta 1 integrin chain. J Biol Chem, 269, 6124–6132.
Google Scholar - Millauer B, Wizigmann‐Voos S, Schnurch H, Martinez R, Moller NP, Risau W and Ullrich A (1993) High affinity VEGF binding and developmental expression suggest Flk‐1 as a major regulator of vasculogenesis and angiogenesis. Cell, 72, 835–846.
Google Scholar - Mitola S, Sozzani S, Luini W, Arese M, Borstatti A, Weich HA and Bussolino F (1997) Tat‐HIV‐1 induces human monocyte chemotaxis by activation of vascular endothelial growth factor receptor‐1. Blood, 90, 1365–1372.
Google Scholar - Miyamoto S, Teramoto H, Gutkind JS and Yamada KM (1996) Integrins can collaborate with growth factor for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol, 135, 1633–1642.
Google Scholar - Muller YA, Li B, Christinger HW, Cunningham BC and de Vos AM (1997) Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proc Natl Acad Sci USA, 94, 7192–7197.
Google Scholar - Parsons JT (1996) Integrin‐mediated signaling‐regulation by protein‐tyrosine kinases and small GTP‐binding proteins. Curr Opin Cell Biol, 8, 146–152.
Google Scholar - Pedran A, Razandi M and Levin ER (1998) Extracellular signal‐regulated protein kinase/Jun kinase cross‐tal underlies vascular endothelial cell growth factor‐induced endothelial cell proliferation. J Biol Chem, 273, 26722–26728.
Google Scholar - Rousseau S, Houle F, Landry J and Huot J (1997) p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migtaion in human endothelial cells. Oncogene, 15, 2169–2177.
Google Scholar - Sagawa K, Kimura T, Swieter M and Siraganian RP (1997) The protein‐tyrosine phosphatase SHP‐2 associates with tyrosine phosphorylated adhesion molecules PECAM‐1 (CD31). J. Biol Chem, 272, 31086–31091.
Google Scholar - Schlaepfer DD, Hanks SK, Hunter T and van der Geer P (1994) Integrin‐mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature, 372, 786–791.
Google Scholar - Schneller M, Vuori K and Ruoslahti E (1997) αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO J, 16, 5600–5607.
Google Scholar - Schwartz MA (1997) Integrins, oncogenes and anchorage independence. J Cell Biol, 139, 575–578.
Google Scholar - Schwartz MA, Schaller MD and Ginsberg MH (1995) Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol, 11, 549–599.
Google Scholar - Senger DR, Ledbetter SR, Claffey KP, Papadopoulos‐Sergiou A, Peruzzi CA and Detmar M (1997) Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the αvβ3 integrin, osteopontin and thrombin. Am J Pathol, 149, 293–305.
Google Scholar - Soker S, Takashima S, Miao HQ, Neufeld G and Klagsbrun M (1998) Neuropilin‐1 is expressed by endothelial and tumor cells as an isoform‐specific receptor for vascular endothelial growth factor. Cell, 92, 735–745.
Google Scholar - Soldi R, Graziani A, Benelli R, Ghigo D, Bosia A, Albini A and Bussolino F (1994) Oncostatin M activates phosphatidylinositol‐3‐kinase in Kaposi's sarcoma cells. Oncogene, 9, 2253–2260.
Google Scholar - Soldi R, Primo L, Brizzi MF, Sanavio F, Polentarutti N, Pegoraro P, Mantovani A and Bussolino F (1997) Activation of JAK2 in human vascular endothelial cells by granulocyte‐macrophage colony stimulating factor. Blood, 89, 863–872.
Google Scholar - Stromblad S, Becker JC, Yebra M, Brooks PC and Cheresh DA (1996) Suppression of p53 activity and p21WAF/CIP1 expression by vascular cell integrin αvβ3 during angiogenesis. J Clin Invest, 98, 426–433.
Google Scholar - Takahashi T and Shibuya M (1997) The 230 Da mature form of KDR/Flk‐1 (VEGF receptor‐2) activates the PLC‐γ pathway and partially induces mitotic sgnals in NIH3T3 fibroblasts. Oncogene, 14, 2079–2089.
Google Scholar - Terman BI, Carrion ME, Kovacs E, Rasmussen BA, Eddy RL and Shows TB (1991) Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene, 6, 1677–1683.
Google Scholar - Thiagarajan P, Shapiro SS, Levine E, De Marco L and Yalcin A (1985) A monoclonal antibody to human platelet glycoprotein IIIa detects a related protein in cultured human endothelial cells. J Clin Invest, 75, 869–901.
Google Scholar - Thomas KA (1996) Vascular endothelial growth factor, a potent and selective angiogenic agent. J Biol Chem, 271, 603–606.
Google Scholar - Toker A and Cantley LC (1997) Signalling through the lipid products of phosphoinositide‐3‐OH‐kinase. Nature, 387, 673–676.
Google Scholar - Vuori K and Ruoslahti E (1994) Association of insulin receptor substrate‐1 with integrins. Science, 266, 1576–1578.
Google Scholar - Waltenberger J, Claesson‐Welsh L, Siegbahn A, Shibuya M and Heldin CH (1994) Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem, 269, 26988–26995.
Google Scholar - Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE and Giancotti FG (1996) The adaptor protein Sch couples a class of integrins to the control of cell cycle progression. Cell, 87, 733–743.
Google Scholar - Woodard AS, Garcia‐Cardena G, Leong M, Madri JA, Sessa CW and Languino RL (1998) The synergistic activity of αvβ3 integrin and PDGF receptor increases cell migration. J Cell Sci, 111, 469–478.
Google Scholar - Yamada RM and Miyamoto S (1995) Integrin transmembrane signaling and cytoskeletal control. Curr Opin Cell Biol, 7, 681–689.
Google Scholar - Zhu X, Otsubo M, Bohmer R, Roberts JM and Assoian RK (1996) Adhesion dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E‐cdk2 and phosphoryation of the retinoblastoma proteins. J Cell Biol, 133, 391–403.
Google Scholar